Abstract
We hypothesized that peripheral tryptophan (Trp) and/or kynurenine (Kyn) levels would provide prognostic value for physicians planning to enroll glioblastoma multiforme (GBM) patients in immunotherapy. GBM is the most common form of malignant glioma in adults. Despite aggressive surgical resection, irradiation and chemotherapy, patients with GBM have a median survival of only 14.6 months postdiagnosis. This poor outcome has led to the search for more effective treatments, including immunotherapy. However, the identification of parameters that proactively stratify GBM patients who have the potential for therapeutic benefit has been challenging. Given recent observations demonstrating high indoleamine 2,3 dioxygenase 1 (IDO1) expression in GBM, the immunosuppressive impact of IDO1-mediated Trp catabolism, as well as active transport of Trp and the IDO1-downstream Trp catabolite, Kyn, across the blood brain barrier, we hypothesized that peripheral blood analysis of this pathway would provide diagnostic utility. When comparing individuals without tumors to GBM patients prior to surgical resection, or at the 48 hour (48h) and ≥ 10 week (10w+) postoperative time points, Trp levels were significantly decreased (p < 0.0001). Similarly, Kyn levels were decreased in the pre and 48h postoperative GBM patients (p < 0.0001), while there was no difference between individuals without tumors and 10w+ GBM patients. Interestingly, those 10w+ patients with a high Kyn/Trp ratio (≥ 9.5) had a mean overall survival (OS) of 23.6 ± a standard error of 6.8 months, compared to an OS of 38.7 ± 4.9 months for patients with lower Kyn/Trp values. Since the 10w+ blood draw and analyses occurred prior to patient enrollment in the heat shock protein peptide complex-96 clinical trial, these novel data suggest that the late Kyn/Trp index may be a relevant clinical benchmark, providing prognostic value for GBM patients who are enrolled in immunotherapeutic regimens.
Keywords: Brain tumor, Diagnostic, Glioblastoma multiforme, Immunosuppression, Kynurenine, Metabolism, Tryptophan
1. Introduction
Glioblastoma multiforme (GBM) is a universally fatal primary tumor of the central nervous system (CNS) with a median postdiagnosis lifespan of 14.6 months [1]. Surgical resection, irradiation and chemotherapy have generally failed to durably halt tumor progression. Therefore, immunotherapy has quickly become a preferred commensurate approach, with already proven effectiveness in cancers that have been previously characterized as incurable [2, 3]. Although immunotherapy for pediatric and adult brain cancer is actively being pursued [4–6], the characterization of parameters that prognostically identify responders versus non-responders, such as the cytokine profile of cytolytic T cells [7], is a rapidly developing area of investigation.
In humans, tryptophan (Trp) catabolism via the kynurenine (Kyn) pathway has been suggested to be mediated by three different enzymes: indoleamine 2,3 dioxygenase 1 (IDO1), indoleamine 2,3 dioxygenase 2 (IDO2) and tryptophan 2,3 dioxygenase (TDO2). Notably, upregulated IDO1 and TDO2 expression within the tumor is correlated with decreased overall survival (OS) of patients with glioma [8–10]. While the individual contribution of these two enzymes toward tumor-induced immunosuppression is yet to be quantified, there is a general consensus that the conversion of Trp into Kyn is a mechanism that GBM employs to circumvent the anti-tumor immune response [11, 12]. Given that both Trp and Kyn are actively transported across the blood brain barrier via the large amino acid transporter system [13, 14], we hypothesized that peripheral Trp and/or Kyn levels would provide prognostic value for physicians planning to enroll GBM patients in immunotherapy.
2. Materials and methods
2.1. Human samples
The control population consisted of individuals without any diagnosed malignancy. Patients diagnosed with a recurrent GBM were either enrolled at Northwestern University Medicine, Chicago, or at the University of California, San Francisco. For those GBM patients who elected to receive the heat shock protein peptide complex-96 (HSPPC-96) vaccine through a clinical trial (NCT00293423), the preoperative eligibility included the ability to resect > 90% of the tumor. Patients with a Karnofsky performance status of 60 or greater and a life expectancy greater than 8 weeks were eligible for the study. Patients could not be on corticosteroids at the time of surgical resection. Intraoperative tissue biopsies were confirmed for recurrent GBM prior to being sent for vaccine manufacturing at Agenus Inc. (Lexington, MA, USA).
The preoperative exclusion criteria included a history of immunodeficiency or immunosuppressive drug use, excluding corticosteroids, other malignancies or other cancers within 5 years, active uncontrolled infection, and other serious medical conditions. Confirmation of whether the 7 g of tumor tissues could generate a vaccine took 2–4 weeks after surgery. The trial was approved by the Institutional Review Board at all participating sites. During treatment, the patients were evaluated for safety, toxicity, autoimmunity and injection site reactions. They underwent clinical and radiograph tumor assessments every 4 and 8 weeks, respectively, as per the standard of care (SOC) protocol. The primary endpoints were safety and feasibility [4]. The secondary endpoints were OS and the immune response to therapy [15].
2.2. High performance liquid chromatography
Serum was isolated from peripheral blood for Trp and Kyn analyses by reversed phase high performance liquid chromatography using a Coulochem III detector with a 5041 enhanced analytical cell containing a glassy carbon electrode (+600 mV; ESA, Chelmsford, MA, USA). The mobile phase (pH 4.6) consisted of 75 mM monosodium phosphate (NaH2PO4), 25 µM ethylenediaminetetraacetic acid (EDTA; disodium salt), and 100 µL/L triethylamine in acetonitrile (6:94 v:v with water). The chromatograms were integrated and quantified using EZChrom SI software (Agilent Technologies, Santa Clara, CA, USA).
Serum (30 µL) samples were processed in a blinded fashion and mixed with a 0.5% sodium dodecyl sulfate (30 µL) solution, incubated for 15 min at room temperature before the addition of 30 µL of 10% sulfosalicylic acid solution, and the proteins were allowed to precipitate on ice for at least 15 min. Following precipitation, the samples were centrifuged at 16,100 × g-force for 15 min at 4°C. The supernatant was extracted and loaded into a Costar Spin-X centrifuge tube filter (0.22 µM nylon; Corning Inc., Corning, NY, USA) and centrifuged at 12,000 × g-force for 6 min at 4°C. The samples were then diluted 1:10 in 0.02 normal perchloric acid (HClO4) prior to analysis.
A standard curve was generated daily from the concentrated Trp (9 mg/mL) and Kyn (1 mg/mL) standards, diluted in 0.02 normal HClO4 and held at 4°C until a 20 µL volume was injected into the system. The standards were made using a serial dilution technique so that the standard levels encompassed the expected levels in the serum samples. The standard curve was created using the system software, and samples were not analyzed unless a linear standard curve with r2 ≥ 0.995 was achieved.
2.3. Statistical analyses
The variables were compared among the controls and patients preoperatively, 48 hours and 12 weeks postoperatively, using analysis of variance and accounting for the repeated measures within the patient group, and for the variance between controls and patients, and through time within each patient. Post hoc testing was performed by two-tailed t-tests, adjusting for multiple testing. To adjust for multiple testing, the six pairwise comparisons among the four sets of data were considered statistically significant if p < 0.0083 (= 0.05/6; the Bonferroni correction). Progression-free survival and OS were compared between the subgroups determined by the kynurenine/tryptophan ratio, using Kaplan–Meier curves and log rank tests. The statistical analyses were performed using Prism software (version 6.05; GraphPad Software, San Diego, CA, USA). The data are presented as the mean ± standard error.
3. Results
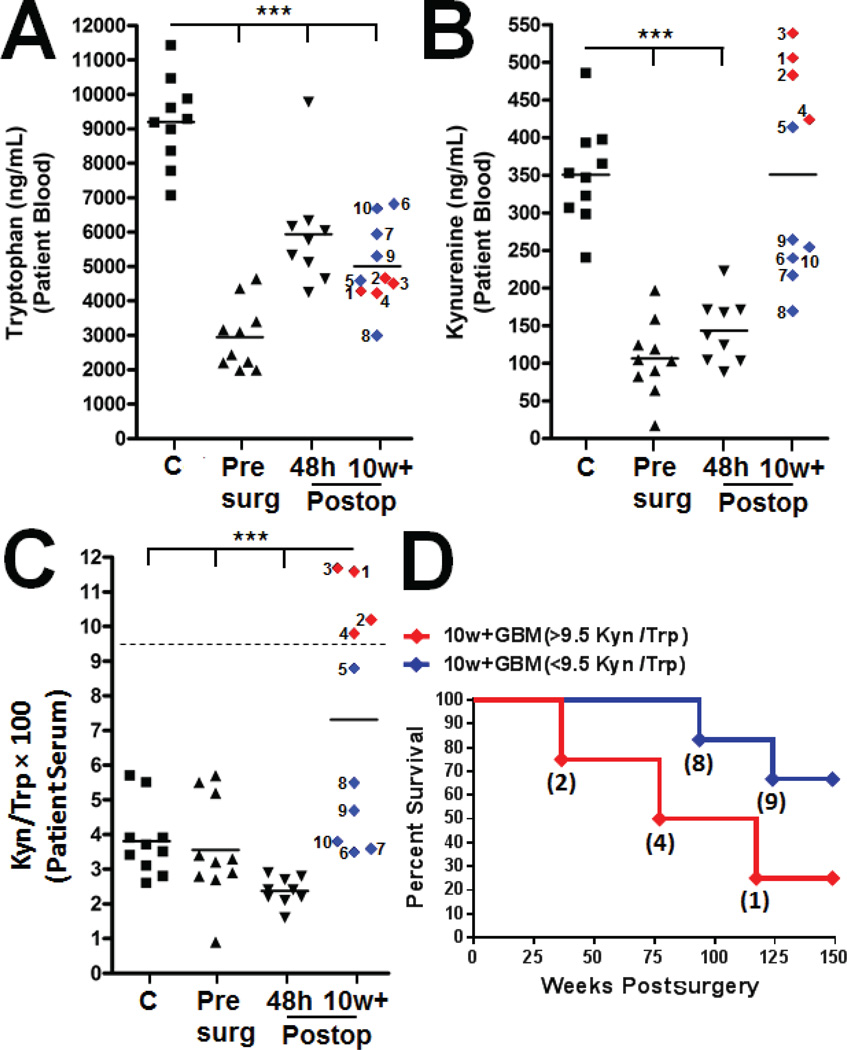
We tested for differences in Trp and Kyn levels between healthy individuals (no malignancy) compared to GBM patients, prior to and post tumor resection. Notably, all GBM patient blood analyses were of tissue that was isolated prior to enrolment in the HSPPC-96 vaccination protocol. The individuals without a diagnosed malignancy had a mean serum Trp level of 9201 ± 402 ng/mL (n = 10), which was significantly decreased to 2951 ± 304 preoperatively (n = 9), 5937 ± 534 at 48 hours postoperatively (n = 10) and 5007 ± 377 ng/mL at ≥ 10 weeks postoperatively in GBM patients (n = 10; p < 0.0001; F [3, 35] = 42.22; Fig. 1A). Notably, the 10w+ group included patient sera analysis just prior to HSPPC-96 enrollment, with the time of the clinical trial initiation identified in Supplementary Table 1. The serum Kyn levels were 350 ± 21 ng/mL in the controls. In the GBM patients, this was significantly decreased to 106 ± 16 preoperatively, and 143 ± 14 ng/mL at 48h (p < 0.0001; F [3, 35] = 24.43; Fig. 1B). In contrast, the Kyn levels in the GBM patients at 10w+ were 350 ± 43 ng/mL, which was not significantly different from that seen in the controls. The control, preoperative and 48h postoperative measures of serum Kyn/Trp ratios were 3.81 ± 0.33, 3.56 ± 0.47 and 2.37 ± 0.13 ng/mL, respectively, which significantly increased to 7.32 ± 1.1 at 10w+ in the GBM patients (p < 0.0001; F [3, 35] = 11.37; Fig. 1C). Only 25% of GBM patients with a Kyn/Trp ratio ≥ 9.5 at 10w+ were still alive at 149 weeks postoperatively, which was substantially decreased compared to the GBM patients who had lower Kyn/Trp ratios (associated with a patient OS of 67%; Fig. 1D).
Fig. 1.
Correlation of the kynurenine/tryptophan (Kyn/Trp) ratio with overall survival in glioblastoma multiforme (GBM) patients prior to enrolling in heat shock protein peptide complex-96 immunotherapy. Peripheral blood was collected from non-tumor bearing individuals as controls (C; squares), GBM patients prior to surgical resection (Presurg; upright triangles), or 48 hour (48h; upside down triangles) and ≥ 10 week (10w+; diamonds) postoperative resected GBM patients. The serum was analyzed for systemic (A) Trp, (B) Kyn and the (C) Kyn/Trp ratio. Each patient of the 10w+ GBM cohort was assigned a tracking number from 1–10 (A–D). (D) Overall survival of the 10w+ GBM patient cohort stratified by a ≥ 9.5 (red line) or ≤ 9.5 (blue line) Kyn/Trp ratio. The numbers in parentheses correspond to the assigned patient number. ***p < 0.0001.
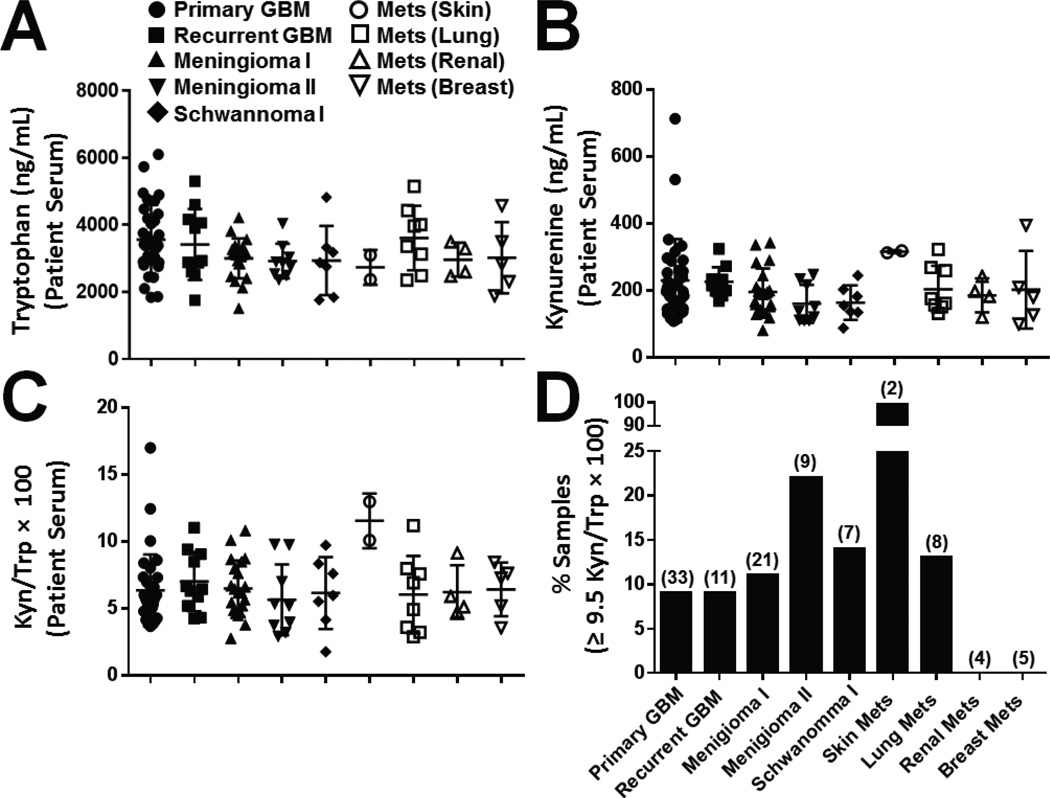
We also tested for differences in Trp and Kyn levels between patients with newly diagnosed and recurrent GBM, as well as other forms of primary brain tumors and brain metastases, in the serum of peripheral blood drawn at the time of surgery. The Trp and Kyn levels for newly diagnosed GBM patients (n = 33) were 3562 ± 180 and 230 ± 21 ng/mL, respectively, which were not significantly different from the levels identified in individuals diagnosed with recurrent GBM (n = 11), meningioma Grade I (n = 21)/Grade II (n = 9), schwanoma Grade I (n = 7), or metastatic melanoma (n = 2), lung (n = 8), kidney (n = 4) or breast cancer (n = 5; Fig. 2A, B). Concordantly, there was no significant difference in the Kyn/Trp ratio of serum isolated from newly diagnosed GBM patients (6.4 ± 0.47 ng/mL) compared to all other groups of patients with CNS tumors (Fig. 2C). When the frequency of patients with a high Kyn/Trp ratio was compared, defined by a level ≥ 9.5, fewer than 10% of newly diagnosed and recurrent GBM patients were distinguished (Fig. 2D). Similarly, for all groups, except those diagnosed with CNS infiltrating metastatic melanoma, fewer than 25% of the samples had a high Kyn/Trp ratio.
Fig. 2.
Quantification of peripheral tryptophan (Trp) and kynurenine (Kyn) in presurgical patients with tumors of the central nervous system. Peripheral blood was collected from patients with primary glioblastoma multiforme (GBM; closed circle), recurrent GBM (closed square), meningioma Grade I (closed upright triangle), meningioma Grade II (closed upside down triangle), schwannoma Grade I (closed diamond), or central nervous infiltration of metastatic (Mets) melanoma (open circle), Mets lung (open square), Mets kidney (open upright triangle) and Mets breast (upside down triangle) cancer. The serum was analyzed for (A) Trp, (B) Kyn and the (C) Kyn/Trp ratio. (D) The frequency of patients from each cohort who possessed a high (≥ 9.5) Kyn/Trp ratio is plotted, and the total number of patients from each group is shown in parentheses above each respective bar.
4. Discussion
Collectively, these preliminary data suggest that the Kyn/Trp ratio is a potentially important prognostic biomarker to predict improved OS for patients treated with surgery followed by HSPPC-96 immunotherapy. These data also demonstrate that the majority of patients with primary brain tumors or metastatic disease of the CNS are not significantly different from one another with respect to peripheral circulating Trp and Kyn levels prior to surgical intervention.
The Kyn/Trp index has previously been shown to be a useful prognostic indicator for patients with lung cancer progression [16], HIV [17], and is increased in individuals with endometrial, ovarian and vulvar cancers [18]. Several studies have suggested that the increased Kyn/Trp level is attributable to the enzymatic function of IDO1 [19], with a consequent tolerogenic impact on T cells [20]. However, what is yet to be established is the exact levels of Kyn and Trp that are required to physiologically promote immunosuppression. In contrast to the original Trp starvation-induced T cell tolerizing hypothesis [21], in vitro cultures of IDO1-expressing dendritic cells with T lymphocytes lead to anergy, even when Trp is in excess [22]. Recent work has demonstrated that the Trp catabolite, Kyn, activates the aryl hydrocarbon receptor on naive CD4-positive T cells to promote the expression of the master regulatory T cell transcription factor, FoxP3 [23]. These data suggest that the accumulation of Trp catabolites, rather than tryptophan depletion alone, is a mechanism that is utilized during IDO1-mediated immunosuppression.
Previous work has demonstrated that human GBM cell lines do not normally express IDO1 in vitro, but are induced with exogenous interferon-gamma, resulting in the catabolism of Trp to Kyn [24]. In vivo, human GBM expresses IDO1 prolifically [25], which may be the result of inflammation caused by tumor outgrowth [26]. Importantly, both an increased level of IDO1 mRNA and protein expression has been correlated with decreased OS in patients with malignant glioma [8, 9]. Since both Trp and Kyn are actively transported across the blood brain barrier via the large neutral amino acid transporter [14], we hypothesized that increased IDO1 expression in GBM may induce a Trp sink effect, thereby depleting Trp while increasing Kyn levels from and into the circulation, both simultaneously and respectively. This hypothesis is not supported by the data in Figure 1 and 2. Instead, we found that when compared to non-tumor bearing individuals, both pre and early postoperative GBM patients have significantly decreased Trp and Kyn levels. Unexpectedly, we also identified a bimodal stratification of Kyn levels in the surgically-resected GBM patients at 10w+, prior to treatment with the HSPPC-96 immunotherapeutic treatment modality. Although there was a strong trend among those individuals with a high Kyn/Trp ratio of ≥ 9.5 for decreased OS compared to patients with ≤ 9.5, the sample size that was analyzed (n = 10) was too small to reach statistical significance (p = 0.1).
Our work confirms that GBM patients possess altered Trp and Kyn levels at the time of surgery compared to controls [27]. Notably, and to our knowledge, this is the first time that differences in the Kyn/Trp ratio have been found among postoperative resected recurrent GBM patients, with a trend predicting the patient survival time after surgical and HSPPC-96 vaccine treatment. Given that this profile became evident at a time point well after surgery and SOC treatment, but prior to treatment with immunotherapy, the mechanism underlying this phenomenon is yet to be elucidated. However, some of the factors that may account for these observations include: IDO1 peripheral induction associated with immune activation during GBM recurrence, increased GBM circulation transport of Kyn during recurrence, and/or recruitment of the tryptophan catabolic enzymes (IDO1/IDO2/TDO2) induced by SOC treatments, including irradiation, temozolomide and/or decadron. In the future, it will be important to increase the sample size and scope of this analysis to include additional postresection time points, patients who do not elect HSPPC-96 immunotherapy, as well as individuals with CNS malignancies other than GBM. Ultimately, this work provides promising preliminary evidence that suggests a better understanding of the mechanisms governing tryptophan catabolism may lead to more effective prognostic and predictive biomarkers for patients with GBM who are treated with surgery and immunotherapy.
Supplementary Material
Highlights.
We discuss the complex link between IDO1, Trp and Kyn in GBM patients.
We identify a potential new prognostic tool for GBM patient stratification.
We found that, differences in the Kyn/Trp ratio appear ≥ 10 weeks post-surgery.
We introduce novel physiologically-relevant clinical data from GBM patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch O, Crane CA, Fuks Y, Kaur R, Aghi MK, Berger MS, et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro-oncology. 2014;16:274–279. doi: 10.1093/neuonc/not203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuster J, Lai RK, Recht LD, Reardon DA, Paleologos NA, Groves MD, et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-oncology. 2015 doi: 10.1093/neuonc/nou348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasky JL, 3rd, Panosyan EH, Plant A, Davidson T, Yong WH, Prins RM, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy for pediatric patients with newly diagnosed or recurrent high-grade gliomas. Anticancer Res. 2013;33:2047–2056. [PMC free article] [PubMed] [Google Scholar]
- 7.Everson RG, Jin RM, Wang X, Safaee M, Scharnweber R, Lisiero DN, et al. Cytokine responsiveness of CD8(+) T cells is a reproducible biomarker for the clinical efficacy of dendritic cell vaccination in glioblastoma patients. Journal for immunotherapy of cancer. 2014;2:10. doi: 10.1186/2051-1426-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsuka K, Kawataki T, Satoh E, Asahara T, Horikoshi T, Kinouchi H. Expression of indoleamine 2,3-dioxygenase and correlation with pathological malignancy in gliomas. Neurosurgery. 2013;72:1031–1038. doi: 10.1227/NEU.0b013e31828cf945. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 10.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 11.Zhai L, Lauing KL, Chang AL, Dey M, Qian J, Cheng Y, et al. The role of IDO in brain tumor immunotherapy. J Neurooncol. 2014 doi: 10.1007/s11060-014-1687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3:e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. Journal of neurochemistry. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 15.Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, et al. Individual patient-specific immunity against high-grade glioma after vaccination with autologous tumor derived peptides bound to the 96 KD chaperone protein. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:205–214. doi: 10.1158/1078-0432.CCR-11-3358. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung cancer. 2010;67:361–365. doi: 10.1016/j.lungcan.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clinical chemistry. 1998;44:858–862. [PubMed] [Google Scholar]
- 18.de Jong RA, Nijman HW, Boezen HM, Volmer M, Ten Hoor KA, Krijnen J, et al. Serum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2011;21:1320–1327. doi: 10.1097/IGC.0b013e31822017fb. [DOI] [PubMed] [Google Scholar]
- 19.Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1989;1012:140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 20.Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, et al. Tryptophan catabolism and T cell responses. Advances in experimental medicine and biology. 2003;527:27–35. doi: 10.1007/978-1-4615-0135-0_3. [DOI] [PubMed] [Google Scholar]
- 21.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunology today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 22.Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H, et al. Inhibition of Allogeneic T Cell Proliferation by Indoleamine 2,3-Dioxygenase–expressing Dendritic Cells: Mediation of Suppression by Tryptophan Metabolites. The Journal of experimental medicine. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T, et al. Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. J Neurosurg. 2009;111:230–237. doi: 10.3171/2008.10.JNS081141. [DOI] [PubMed] [Google Scholar]
- 25.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature medicine. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 26.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams S, Teo C, McDonald KL, Zinger A, Bustamante S, Lim CK, et al. Involvement of the kynurenine pathway in human glioma pathophysiology. PLoS One. 2014;9:e112945. doi: 10.1371/journal.pone.0112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.