Abstract
The combination of photoredox catalysis and enamine catalysis has enabled the development of an enantioselective α-cyanoalkylation of aldehydes. This synergistic catalysis protocol allows for the coupling of two highly versatile yet orthogonal functionalities, allowing rapid diversification of the oxonitrile products to a wide array of medicinally relevant derivatives and heterocycles. This methodology has also been applied to the total synthesis of the lignan natural product (−)-bursehernin.
Keywords: photoredox catalysis, organocatalysis, enantioselective, alkylation, aldehyde, nitrile, total synthesis
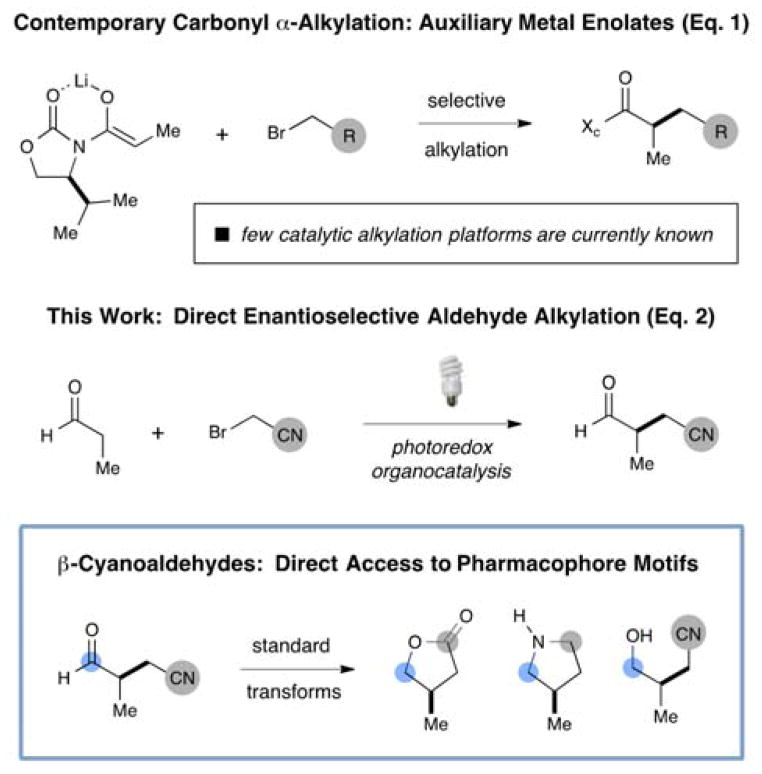
The enantioselective α-alkylation of carbonyl compounds with sp3-hybridized halide-bearing electrophiles has long been considered an elusive goal for practitioners of asymmetric catalysis.[1] Indeed, the most commonly employed strategy to achieve the stereoselective construction of α-alkyl carbonyls involves the coupling of auxiliary-based metal enolates with halo or tosyloxy alkanes.[2],[3] A critical issue for the development of catalytic variants of this venerable reaction has been the insufficient electrophilicity of alkyl halides towards silyl or alkyl enol ether π-nucleophiles (enolate equivalents that are broadly employed in asymmetric catalysis). This limitation has man-dated the use of lithium-, sodium-, or cesium-derived enolates for auxiliary controlled carbonyl α–functionalization at higher carbonyl oxidation states. Recently, however, the application of secondary amine organocatalysts has overcome several of these constraints via the direct use of aldehydes or ketones in a variety of chiral enamine α-functionalization reactions.[4] As one example, our laboratory disclosed the synergistic merger of enamine catalysis with visible-light photoredox catalysis, wherein a ruthenium photocatalyst is used to generate highly electrophilic alkyl radicals derived from simple α-bromoesters and ketones.[5] Since that time, the field of photoredox catalysis as applied to organic synthesis has received considerable attention[6] and we have disclosed its successful application to the enantioselective α-trifluoromethylation,[7] α-benzylation,[8] and α-amination[9] of aldehydes.
Recently, we questioned whether this dual photoredox-organocatalysis platform could be translated to the asymmetric catalytic alkylation of aldehydes using α-bromo cyanoalkyls, a protocol that would generate β-cyanoaldehydes in one step. As a critical design element, we recognized that α-bromo cyanoalkylating reagents would not be suitable electrophiles for most catalytic enolate addition pathways; however, the corresponding open-shell radicals, derived via one-electron reduction of α-bromonitriles, should readily undergo coupling with transiently generated chiral enamines. In addition, the nitrile functional group offers rapid access to a large array of carbonyl, amine, or imidate motifs,[10] and as such, β-cyanoaldehydes can be readily translated to lactones, pyrrolidines, lactams, and cyanoalcohols – pharmacophore fragments that are ubiquitous in medicinal chemistry.[11] Herein we report the first enantioselective α-cyanoalkylation of aldehydes via the synergistic combination of photoredox and organocatalysis.[12] Furthermore, we demonstrate the application of this new dual catalysis platform to the rapid and stereoselective construction of cyclic and acyclic motifs of broad value to the chemistry of drug discovery.
Design Plan
We envisioned that our cyanoalkylation dual catalysis mechanism would proceed as depicted in Scheme 1. Single-electron reduction of the bromonitrile species by the strongly reducing Ru(bpy)3+ ion 1[13] (E1/2II/I = −1.33 V vs. SCE in CH3CN for Ru(bpy)+;[14] E1/2red = −0.69 V vs. SCE in DMF for bromoacetonitrile[15]) should provide the cyanoalkyl radical 3 and bromide ion after fragmentation. Simultaneously, the organocatalytic cycle would initiate by condensation of amine catalyst 4 with an aldehyde to generate a chiral enamine. Computational studies reveal that the lowest-energy conformation of the enamine DFT-5 is found to position the π-nucleophilic C=C system distal to the large tert-butyl group on the imidazolidinone catalyst framework. Effective shielding of the Re face of the enamine by the pendent methyl group of the organocatalyst requires coupling to the electron-deficient radical 3 via the enamine Si face, thereby generating α-amino radical 6, which is poised to re-engage the photoredox catalytic cycle. Photo-excitation of Ru(bpy)32+ generates the oxidizing species 2 (E1/2*II/I = +0.77 V vs. SCE in CH3CN),[14] which is well suited to perform a single-electron oxidation of α-amino radical 6 (E1/2red = −0.92 to −1.12 V vs. SCE)[16] thereby delivering iminium ion 7; hydrolysis thereafter provides the α-cyanoalkylated aldehyde product while regenerating amine 4, thus completing the organocatalytic cycle.
Scheme 1.
Catalytic Cycle for Aldehyde α-Cyanoalkylation.
We chose to initiate our α-cyanoalkylation studies by exposing octanal, α-bromoacetonitrile, Ru(bpy)3Cl2, and imidazolidinone catalyst 4 to a 26 W CFL light source.[6] To our great delight, the desired asymmetric bond formation was realized in 72% yield and with excellent enantioselectivity (Table 1, entry 1, 93% ee). Evaluation of a variety of high-dielectric solvents identified dimethyl sulfoxide (DMSO) as the optimal medium, while increasing the reaction concentration provided excellent levels of both yield and enantioselectivity (entry 7, 95% yield, 95% ee). Notably, high-throughput analysis of a 96-member organocatalyst library identified the novel tert-butyl-furyl substituted imidazolidinone 8 as a viable alternative to catalyst 4 (entry 6, 90% yield, 95% ee). Finally, reducing the aldehyde stoichiometry to 1–3 equivalents could be tolerated without significant impact on yield or enantiocontrol (entries 8–9, 74–90% yield, 95% ee).
Table 1.
Optimization of the Photoredox Organocatalytic Addition.

| |||||
|---|---|---|---|---|---|
| entry | solvent | concentration | catalyst | yield | ee[a] |
| 1 | DMF | 0.5 M | 4 | 72% | 93% |
| 2 | CH3CN | 0.5 M | 4 | 62% | 92% |
| 3 | CH3NO2 | 0.5 M | 4 | 28% | 94% |
| 4 | DMSO | 0.5 M | 4 | 84% | 95% |
| 5 | DMSO | 0.5 M | 8 | 85% | 95% |
| 6 | DMSO | 4 M | 8 | 90% | 95% |
| 7 | DMSO | 4 M | 4 | 95%[b] | 95% |
| 8 | DMSO | 4 M | 4 | 90%[c] | 95% |
| 9 | DMSO | 4 M | 4 | 74%[d] | 95% |
Yield and enantiomeric excess determined by chiral GLC analysis of the aldehyde product using p-methoxyphenylacetone as internal standard. Reactions were performed with 5 equivalents of octanal unless otherwise noted.
Yield of isolated product.
3 equivalents of octanal.
1 equivalent of octanal.
Reaction Scope
Using the optimal conditions identified in Table 1, we have demonstrated that our alkylcyanation reaction is quite general in scope with respect to the aldehyde component (Table 2). For example, aldehydes bearing aryl rings and significant steric bulk were well tolerated (11–16, 18, 79–97% yield, 92–96% ee). Interestingly, intramolecular radical cyclization was not observed when aldehydes bearing pendent π-bonds were used (10 and 17, 68–90% yield, 91–95% ee). Finally, p-methoxyphenylacetaldehyde represents an intriguing addition to the scope of aldehydes in the transformation, as the alkylcyano product contains a tertiary α-formyl benzylic stereocenter, a motif typically prone to facile racemization (18, 80% yield, 90% ee).
Table 2.
Enantioselective α-Cyanoakylation: Aldehyde Scope.

|
Yield of isolated aldehyde product. Enantiomeric excess determined by chiral GLC, HPLC, or SFC analysis on the aldehyde or the corresponding alcohol (see Supporting Information).
Reaction performed at −20 °C. Product isolated as the corresponding alcohol after NaBH4 reduction of the crude reaction mixture.
We next investigated the scope of the α-bromonitrile alkylating component in this new dual catalysis bond-forming reaction. While bromoacetonitrile is an ideal coupling partner, we were also delighted to find that bromocyano systems bearing diverse substitution patterns couple in high efficiency and excellent enantioselectivity (Table 3, 73–88% yield, 93–98% ee). It should be noted that imidazolidinone 8 was the preferred amine catalyst when substituted cyanoalkyls were employed.[17] Notably, fully substituted bromonitriles coupled effieicntly, generating all-carbon quaternary stereocenters with excellent enantiocontrol albeit with modest diastereoselectivity (25 and 26, >93% ee).
Table 3.
Evaluation of the Cyanobromide Coupling Partner.

|
Yield of isolated product. Diastereomeric ratios (dr) 1–3:1, determined by 1H NMR analysis, see Supporting Information. Products were isolated as the aldehyde and further derivatized to determine enantiomeric excess (see Supporting Information).
Catalyst added as the solid free amine; 20 mol% 2,6-lutidinium triflate was added as a source of the acid co-catalyst.
Having examined the scope of both reaction partners, we next explored the breadth of pharmacophore fragments that could be accessed readily using these enantioenriched cyanoaldehyde building blocks. As illustrated in Table 4, alcohols, ethers, lactones, aldehydes, ketones, amides, amines, and lactams could be constructed in a straightforward manner in under three steps.[18] Intriguingly, we found that the nitrile could be hydrogenated under Raney Nickel® conditions and achieve in situ cyclization of the amine on the pendent aldehyde moiety; further reduction of the resulting iminium ion generates (R)-3-benzylpyrrolidine (35) in a single step in 81% yield.[19] Most important, no erosion of enantiopurity (<1%) was observed in any of these synthetic elaboration studies.
Table 4.
Elaboration of Cyanoalkylation Products to Useful Motifs.

|
Conditions: (a) NaBH4, MeOH, CH2Cl2, 84%, 93% ee; (b) MeOH, conc. HCl, 100 °C, 88%, 93% ee; (c) NaH, BnBr, DMF, 85%, 93% ee; (d) DIBAL-H, Et2O, 83%, 93% ee; (e) Mg, I2, PhBr, Et2O, then THF, 1M HCl, 88%, 92% ee; (f) NaClO2, NaH2PO4, THF, t-BuOH, 2-methyl-2-butene, H2O, product not isolated; (g) BnNH2, HOBt•H2O, NMM, EDCI•HCl, THF, 48%, 93% ee for two steps; (h) BnNH2, NaBH(OAc)3, DCE, 77%, 93% ee; (i) 1 mol% Parkins’ catalyst, diglyme, 160 °C, 93%, 93% ee; (j) p-TsOH•H2O, Raney Nickel® 2400, H2, EtOH, 81%, 93% ee.
Finally, to demonstrate the utility of our novel alkylcyanation technology, we undertook a short synthesis of (−)-bursehernin (37), a lignan natural product of proven biological activity.[20] Employing our standard photoredox-amine catalysis protocol, β-exposure to sodium borohydride and cyclization under basic conditions provided the lactone 36. The four-step synthesis of (−)-bursehernin was then completed via a highly selective α-alkylation using 3,4-dimethoxybenzyl bromide, generating the natural product in 80% yield overall from bromoacetonitrile.
Supplementary Material
Figure 1.
Photoredox Organocatalysis α-Cyanoalkylation of Aldehydes.
Scheme 2.
Total Synthesis of (−)-Bursehernin.
Footnotes
The authors are grateful for financial support provided by the NIH General Medical Sciences (Grant NIHGMS (R01 GM093213-01) and kind gifts from Merck, AbbVie, and Bristol Myers Squibb.
Supporting information for this article is given via a link at the end of the document.
References
- 1.Vesely J, Rios R. Chem Cat Chem. 2012;4:942–953. [Google Scholar]
- 2.a) Enders D, Eichenauer H. Tetrahedron Lett. 1977;18:191–194. [Google Scholar]; b) Enders D, Eichenauer H. Chem Ber. 1979;112:2933–2960. [Google Scholar]; c) Evans DA, Ennis MD, Mathre DJ. J Am Chem Soc. 1982;104:1737–1739. [Google Scholar]; d) Dolling UH, Davis P, Grabowski EJJ. J Am Chem Soc. 1984;106:446–447. [Google Scholar]; e) Myers AG, Yang BH, Chen H, Gleason JL. J Am Chem Soc. 1994;116:9361–9362. [Google Scholar]; f) Oppolzer W, Moretti R, Thomi S. Tetrahedron Lett. 1989;30:5603–5606. [Google Scholar]; g) Imai M, Hagihara A, Kawasaki H, Manabe K, Koga K. J Am Chem Soc. 1994;116:8829–8830. [Google Scholar]; h) Job A, Janeck CF, Bettray W, Peters R, Enders D. Tetrahedron. 2002;58:2253–2329. [Google Scholar]; i) Doyle AG, Jacobsen EN. J Am Chem Soc. 2005;127:62–63. doi: 10.1021/ja043601p. [DOI] [PubMed] [Google Scholar]; j) Brak K, Jacobsen EN. Angew Chem Int Ed. 2013;52:534–561. doi: 10.1002/anie.201205449. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2013;125:558–588. [Google Scholar]
- 3.For a Suzuki-Miyaura coupling approach using α-bromo amides see: Fischer C, Fu GC. J Am Chem Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509.
- 4.MacMillan DWC. Nature. 2008;455:304–308. doi: 10.1038/nature07367.Mukherjee S, Yang JW, Hoffmann S, List B. Chem Rev. 2007;107:5471–5569. doi: 10.1021/cr0684016.Erkkilä A, Majander I, Pihko PM. Chem Rev. 2007;107:5416–5470. doi: 10.1021/cr068388p.For early reports of aldehyde α-alkylation reactions, see: Vignola N, List B. J Am Chem Soc. 2004;126:450–451. doi: 10.1021/ja0392566.Enders D, Wang C, Bats JW. Angew Chem Int Ed. 2008;47:7539–7542. doi: 10.1002/anie.200802532.Angew Chem. 2008;120:7649–7653.List B, Čorić I, OGrygorenko O, Kaib PSJ, Komarov I, Lee A, Leutzsch M, Chandra Pan S, Tymtsunik AV, van Gemmeren M. Angew Chem Int Ed. 2014;53:282–285. doi: 10.1002/anie.201306037.Angew Chem. 2014;126:286–289.
- 5.Nicewicz D, MacMillan DWC. Science. 2008;322:77–80. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tucker JW, Stephenson CRJ. J Org Chem. 2012;77:1617–1622. doi: 10.1021/jo202538x. [DOI] [PubMed] [Google Scholar]
- 7.Nagib DA, Scott ME, MacMillan DWC. J Am Chem Soc. 2009;131:10875–10877. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih HW, Vander Wal MN, Grange RL, MacMillan DWC. J Am Chem Soc. 2010;132:13600–13603. doi: 10.1021/ja106593m.For a related α-benzylation of aldehydes using organocatalysis, see: Arceo E, Jurberg ID, Álvarez-Fernández A, Melchiorre P. Nature Chem. 2013;5:750–756. doi: 10.1038/nchem.1727.
- 9.Cecere G, König CM, Alleva JL, MacMillan DWC. J Am Chem Soc. 2013;135:11521–11524. doi: 10.1021/ja406181e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Brown BR, editor. The Organic Chemistry of Aliphatic Nitrogen Compounds. Oxford University Press; Oxford: 1994. p. 217.p. 342. [Google Scholar]; b) Ghaffar T, Parkins A. Tetrahedron Lett. 1995;36:8657–8660. [Google Scholar]; c) Ghaffar T, Parkins A. J Mol Catal A. 2000;160:249–261. [Google Scholar]
- 11.a) Roughley SD, Jordan AM. J Med Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]; b) Vitaku E, Smith DT, Njardarson JT. J Med Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 12.For an enantioselective cyanomethylation of an oxindole derivative see: Lee TBK, Wong GSK. J Org Chem. 1991;56:872–875.
- 13.Ru(bpy)3+ is generated by single-electron oxidation of a sacrificial equivalent of enamine. This mechanism of cycle initiation has been invoked in previous photoredox organocatalytic transformations and has been supported by fluorescence quenching experiments; see Ref. 5.
- 14.Bock CR, Connor JA, Gutierrez AR, Meyer TJ, Whitten DG, Sullivan BP, Nagle JK. J Am Chem Soc. 1979;101:4815–4824. [Google Scholar]
- 15.The reduction potential of α-bromoacetonitrile in acetonitrile has not been accurately measured; however, the strong overpotential implies that SET reduction of bromoacetonitrile by Ru(bpy)3+ will be thermodynamically favorable. Isse AA, Genarro A. J Phys Chem A. 2004;108:4180–4186.Cardinale A, Isse AA, Gennaro A, Robert M, Savéant JM. J Am Chem Soc. 2002;124:13533–13539. doi: 10.1021/ja0275212.
- 16.Wayner DDM, Dannenberg JJ, Griller D. Chem Phys Lett. 1986;131:189–191. [Google Scholar]
- 17.One possible explanation is differential equilibrium constants for enamine formation between the two catalysts; see Supporting Information for details.
- 18.Cobley CJ, van den Heuvel M, Abbadi A, de Vries JG. Tetrahedron Lett. 2000;41:2467–2470. Also see ref. 10e. [Google Scholar]
- 19.The crude pyrrolidine was protected as its benzyl carbamate prior to isolation.
- 20.a) Chang CC, Lien YC, Liu KCSC, Lee SS. Phytochem. 2003;63:825–833. doi: 10.1016/s0031-9422(03)00371-6. [DOI] [PubMed] [Google Scholar]; b) Itoh T, Chika JI, Takagi Y, Nishiyama S. J Org Chem. 1993;58:5717–5723. [Google Scholar]; c) Baran PS, DeMartino MP. Angew Chem Int Ed. 2006;45:7083–7086. doi: 10.1002/anie.200603024. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2006;118:7241–7244. [Google Scholar]; d) Tomioka K, Mizuguchi H, Koga K. Chem Pharm Bull. 1982;30:4304–4313. doi: 10.1248/cpb.33.609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.