Abstract
Although the Monoamine Oxidase-A (MAOA) gene has been linked to spatial learning and memory in animal models, convincing evidence in humans is lacking. Performance on an ecologically-valid, virtual computer-based equivalent of the Morris Water Maze task was compared between 28 healthy males with the low MAOA transcriptional activity and 41 healthy age- and IQ-matched males with the high MAOA transcriptional activity. The results revealed consistently better performance (reduced heading error, shorter path length, and reduced failed trials) for the high MAOA activity individuals relative to the low activity individuals. By comparison, groups did not differ on pre-task variables or strategic measures such as first-move latency. The results provide novel evidence of MAOA gene involvement in human spatial navigation using a virtual analogue of the Morris Water Maze task.
Keywords: monoamine-oxidase, virtual maze, spatial navigation, spatial memory, learning, gene
1. Introduction
Differences in the variable number of tandem repeats (VNTR) of the Monoamine Oxidase A gene (MAOA) have been linked to a variety of psychiatric disorders including anxiety [1, 2], depression [3], or schizophrenia [4]. These disorders are typically associated with cognitive deficits, such as perturbations in spatial learning and memory in anxiety [5] and depression [6]. Therefore, variations in MAOA gene expression might also contribute to disease-related cognitive dysfunction such as executive attention [7], working memory [8] or decision-making [9]. This study examines this possibility, specifically in relation to spatial learning and memory.
Basic neuroscience studies support a role of MAOA in spatial abilities but have mostly measured MAO enzyme activity rather than transcriptional activity of genetic expression. In mice, the MAOA inhibitor moclobemide improved spatial performance and novel object exploration [10], whereas pinoline, a MAOA and serotonin-uptake inhibitor, reduced the number of arm entries and time spent in the open area of a plus maze [11]. Similarly, inhibition of MAOA increased locomotor activity in a water maze task but failed to facilitate spatial learning in adult rats [12]. While these data suggest that MAOA influences basic learning and memory processes, parallel evidence in humans remains limited.
To our knowledge, only one previous study in humans assessed the influence of platelet MAO on spatial abilities [13]. Their data [13] indicated an inspection time of a perceptual maze that was shorter in high-MAO activity males relative to low-MAO activity males, but similar in high-MAO activity males and low-MAO activity females. However, these earlier findings focused on the MAO enzymatic activity rather than MAOA genetic expression, and they were based on a paper-and-pencil maze, a small sample size, and a potential confound of gender. Improving on previous paper-and-pencil based tasks, ecologically-valid, computerized virtual mazes have become available to measure spatial navigation more precisely in humans. These virtual mazes have been shown to be sensitive to exposure to sex steroids [14], age-effects [15], and neurological damage [16].
The MAOA gene has a 30 base pair repeat in the promoter region (MAOA-LPR) that has been shown to affect transcriptional efficiency in vitro. Individuals with the long allele (3.5 repeats and 4 repeats) show greater transcriptional activity (high MAO-A gene activity) than individuals with the short allele (3 repeats)(low MAOA gene activity) [17]. Of note, the MAOA gene is located on the X-chromosome. Thus, women have two MAOA genes, whereas males have only one copy. While the comparison of high vs. low expressing gene is simple in males (single gene yielding only two genotypes, high vs. low expressing), it becomes more complex in females (two genes yielding 4 possible combinations of the low/high efficiency genes).
The present study evaluates the contribution of high and low monoamine oxidase gene expression by virtue of examining MAOA-LPR genotypes in human spatial learning and memory. As a first step, we limited the sample to males to exclude potential confounds of sex [18]. To circumvent issues with age-related cognitive declines in spatial abilities across the life-span [15] as well as age-dependent effects of MAOA on spatial performance [19], we specifically focused on young males. With regard to predictions, previous findings have been ambiguous. On the one hand, animal studies measuring enzyme activity suggest that subjects with low MAOA activity perform better on learning and memory tasks than subjects with high MAOA activity [10], supported by human genetic expression studies showing better performance in decision-making [9] and higher attainment of educational levels for low-activity individuals [20]. On the other hand, the reverse pattern, a better performance for individuals with the high-activity (long) allele relative to individuals with the low-activity (short) allele has been found in gene expression studies of executive attention [7] and working memory [8]. In addition, molecular work also shows higher transcriptional efficiency in carriers with the high-activity MAOA allele [17]. Thus, based on this mixed literature, we sought to compare long and short-allele carriers and expected significant differences between groups on spatial performance parameters on a virtual Morris Water Maze task [14, 21].
2. Materials and Methods
2.1 Experimental subjects
Sixty-nine healthy male adolescents, mostly of Caucasian ethnicity, participated in this experiment. Of these, 28 males (mean age = 19.39 ± SD 10.91; mean IQ: 114.18 ± SD 12.46) were hemizygous for the low (3) activity variant and 41 males (mean age = 16.37 ± SD 6.86; mean IQ: 116.05 ± SD 13.18) were hemizygous for the high (4) activity variant. Both groups were similar in age (t(41.50)=1.30, p=.20) and IQ (t(64)=−.58, p=.56), which was measured with the Wechsler abbreviated scale of intelligence [22]. Adult participants and the parents of minors provided written consent, and adolescents provided written assent to participate in protocols approved by the Institutional Review Board of the National Institute of Mental Health (NIMH). Inclusion criteria consisted of an absence of medical or psychiatric problems, as determined by physical examination and structured psychiatric interviews (Kiddie-Schedule-for-Affective-Disorders–Present-and-Lifetime-version [23]) administered by an experienced clinician (inter-rater reliability k >.75), absence of psychotropic medications or drug use and an IQ > 80.
2.2 Materials
A virtual version of the Morris Water Maze [21] was used. It consisted of the display of a square room containing a circular pool of water. Four equally-sized abstract rectangular paintings that were distinguishable by their shape, colour, and placement on the walls surrounding the pool, served as navigational cues to aid orientation. Each of these cues was placed on a different wall of the room and stretched from the ceiling to the pool wall (see Figure 1). Participants navigated in the pool from a first-person perspective and moved around using the ‘up’, ‘left’ and ‘right’ arrow curser keys of the keyboard. Following previous authors’ task-rules [16], to more closely mirror typical rodent behaviour, the ‘back’ arrow key was disabled and participants were told they could not back up. If participants wanted to turn around, they had to spin 180 deg around their left or right axis using the left or right arrow keys.
Figure 1. Sample trial of virtual Morris Water Maze task.
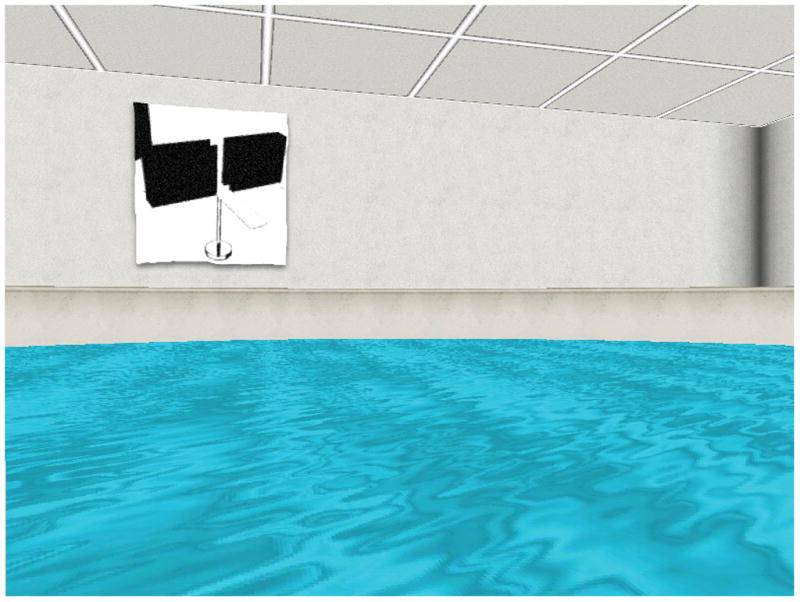
Sample screenshot of a typical trial in which the person must navigate through the water maze from a first-person perspective with the distal cue (e.g., the painting) visible in the background.
Participants completed the experiment on a laptop with a 17 inch monitor in a windowless room of the pediatric clinic of the NIH Clinical Center. The experiment was completed in one session without breaks and lasted about 15 minutes. Following previous investigators [24], the task consisted of 18 trials, including 2 initial practice trials and 16 experimental trials. On the first practice trial, participants had 30 seconds to explore the room and to learn to navigate comfortably in this environment. No platform was present during this first trial. The platform was introduced on the second practice trial. For this trial, participants were asked to simply “swim” towards the visible platform. Over the next 16 experimental trials, the platform was hidden but always located in the same position. However, the platform location was different from the second visible platform trial and participants had to ‘hunt’ for the platform on the first hidden trial. Participants were dropped in a pseudo-randomised order, which was fixed for all participants, across trials an equal number of times at four different locations on the side of the pool wall. For each trial, the task consisted of “swimming” directly to the hidden platform. Once participants successfully reached the platform, a neutral sound occurred. Participants remained on the platform for 2 seconds before the onset of the next trial. On each trial, participants were given 60 seconds to find the platform, after which the platform became visible and a written message appeared on the screen indicating the visibility of the platform and encouraging participants to move towards it. If such a trial occurred, it was counted as a failed trial.
2.3 Monoamine Oxidase A genotyping
Genomic DNA was prepared from saliva samples collected in the laboratory using Oragene·DNA kits (DNA genotek, Ottawa, Ontario, Canada). The MAOA-LPR genotyping was based on Ducci and colleagues [25]. The MAOA gene promoter VNTR polymorphism was amplified from 10 ng genomic DNA using the primer sequences: Forward 5′-(CCC AGG CTG CTC CAG AAA CAT G)-3′ and Reverse-5′(GTT CGG GAC CTG GGC AGT TGT G)-3′. Since GC content is high in the VNTR region, Invitrogen’s PlatinumTaq and PCR X Enhancer System kits (Invitrogen, Carlsbad, CA) were used for amplification, with 5 μM of each primer and 25 mM dNTPs in a total reaction volume of 15 μl. Amplifications were performed on a Perkin-Elmer 9700 thermocycler (Applied Biosystems, Foster City, CA) with one cycle at 96°C for 10 min followed by 35 cycles of 94°C for 15 s, 55°C for 15 s, 72°C for 30 s, and a final 3 min extension at 72°C. The fluorescent dye 6-FAM labeled the forward primer; amplicons were visualized with GeneScan-500 LIZ Size Standard (Applied Biosystems, Foster City, CA) and analyzed on an ABI 3730 capillary sequencer. Allele sizes (allele 2--183 bp; 3--213 bp; allele 3.5--232 bp; allele 4--244 bp; allele 5--272 bp) were determined using GeneMapper v4.0 (Applied Biosystems, Foster City, CA). Genotyping accuracy was determined empirically by duplicate genotyping of 25% of the samples selected randomly. The overall error rate was < 0.005, and the completion rate was > 0.98. As MAOA is an X linked gene and since our study only included males, genotypes were grouped by relative transcriptional activity of MAOA into two categories: high-activity (MAOA-H) (4 repeats) versus low-activity (MAOA-L)(3 repeats).
2.4 Statistical analysis
A 4 × 2 repeated measures ANOVA was run for each variable using a Block (1–4) by Genetic activity (low vs. high) design on correct-trials only. Five performance variables were used: (1) latency, i.e., the time (sec) spent to reach the platform; (2) path length, i.e., the distance (relative to the pool diameter) covered to reach the platform, (3) heading error (in deg), i.e., the angle between optimal heading direction and participant’s heading direction; (4) first-move latency, an indicator of how long subjects remained at the wall edge at the beginning of a trial before they started moving towards the platform. In addition, (5) an analysis was run on overall accuracy, i.e., number of failed attempts, in which the latency exceeded 60 sec. To examine learning patterns, the 16 experimental trials were binned into four blocks of four trials each, i.e., block 1: trials 1–4, block 2: trials 5–8, block 3: trials 9–12 and block 4: trials 13–16 (e.g.,[5]). In addition, we assessed pre-navigational motoric and search processes to rule out pre-task group differences. Accordingly, we used a multivariate F-test that included all 5 performance variables to examine the initial 2 trials, the visible platform trial and the first hidden trial in which participants had to search for the platform separately. Although both groups did not differ significantly in age (p = .20), because of a lower mean in the high vs. low subjects all analyses were re-run with age as a covariate of no interest to assess impact of this variable. To assess statistical power, measures of effect size (Cohen’s d) and post-hoc power (G*Power, [26]) were calculated.
3. Results
Differences in performance between the 2 genotype groups emerged on several variables. A significant effect of path length indicated a shorter path length for the MAO-H vs. MAO-L gene variant (F(1,67) = 4.17, p = .04, d = .51, power = .74). A significant main effect of block (F(3,201) = 7.41, p < .001) with a significant linear trend (F(1,67) = 19.54, p < .001) indicated a learning effect across trials (Figure 2a).
Figure 2. Performance data.
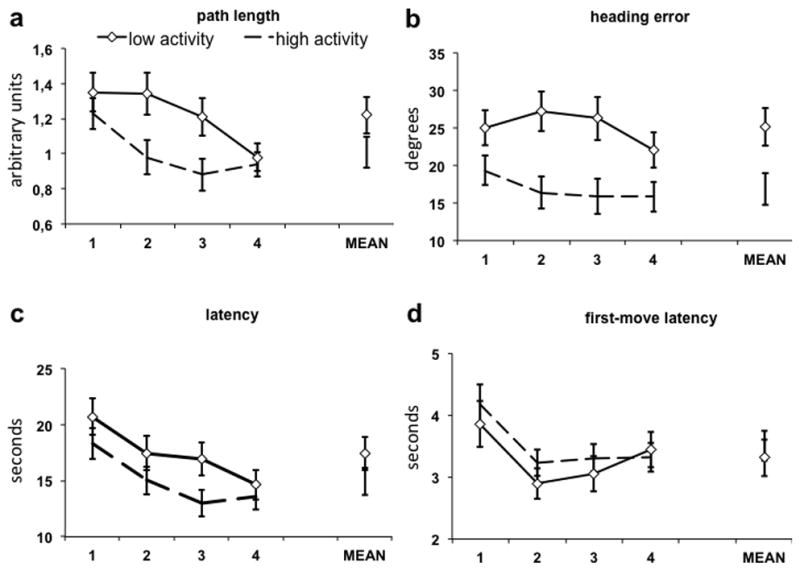
Figure shows performance data of males with the high-activity MAOA (n = 41, no marker) and with the low-activity MAOA (n = 28, diamond marker) variant for path length (a), heading error (b), latency to reach the platform (c), and first-move latency (d).
A similar main effect of group was also found on heading error showing less heading error for MAO-H relative to MAO-L individuals (F(1,67) = 9.91, p = .002, d = .74, power = .98)(Figure 2b).
Although there was no significant difference between genotype groups in the latency to reach the platform (F(1,67) = 2.63, p=.11, d = .39), a significant main effect of block (F(3,201) = 12.31, p < .001) indicated learning over the course of the experiment, manifest as a significant linear effect (F(1,67) = 27.20, p < .001) (Figure 2c).
The first-move latency albeit showing a significant effect of block (F(3,201) = 10.93, p < .001) with significant linear trend (F(1,67) = 7.24, p = .009) did not show a group difference (F(1,67) = .44, p = .51), suggesting that latency to disengage from the arena wall did not contribute to group differences (Figure 2d).
When accuracy, as measured by the amount of completed trials, was assessed, MAO-L males failed to complete more trials (Mean = 0.30 +/− SEM = 0.06) than MAO-H males (Mean = 0.12 +/− SEM = 0.05)(F(1,67) = 5.57, p =.02, d = .54, power = .85), showing also a significant main effect of block across groups (F(3,201) = 2.67, p < .05).
Importantly, the examination of potential pre-task differences revealed no group differences of any of the performance parameters from either the visible platform trial (e.g., latency MAOA-L: mean = 13.51 sec +/− SEM = 1.35; latency MAOA-H: mean = 13.52 +/− SEM = 1.11) or the first hidden trial (e.g., latency MAOA-L: mean = 26.92 +/− SEM 3.72; latency MAOA-H: mean = 30.38 +/− SEM 3.07)(all F(1,67) < 1.10, all p > .30). These data suggest that performance differences were unique to spatial navigation and not associated with motoric ability or ability to locate the platform on the first trial. Despite absence of significant differences in age, analyses were re-run with age as a covariate of no interest. All between-group effects remained significant (path length: F(1,66) = 4.64, p = .035, heading error: F(1,66) = 10.31, p = .002 and fail to complete: F(1,66) = 5.96, p = .02).
4. Discussion
This study provides the first evidence for the involvement of MAOA gene expression in human spatial navigation. Males with the low MAOA activity allele (MAO-L) performed consistently worse on all navigational indices than males with the high-activity allele (MAO-H). Critically, potential age-effects or pre-task differences did not influence these findings.
Males with the high MAOA transcriptional activity of gene expression performed more efficiently on several spatial performance measures when trying to locate the hidden platform relative to males with the low transcriptional activity. Importantly, no differences were found on the visible platform trial or the first ‘hidden’ trial, when participants had to locate the platform for the first time and no memory set had been established at that point. Absence of effects on these measures suggests no pre-task differences related to search efficiency or motor ability. Moreover, both groups were well-matched on IQ.
Work on the molecular contribution to spatial learning and memory is still in its infancy. A recent study implicated BDNF, a neurotrophic gene hypothesized to be strongly involved in episodic memory and hippocampal function [27], and with a link to anxiety disorders [28], in spatial orientation [29]. The current findings complement and extend this earlier work to genes involved in deamination of neurotransmitters implicated in prefrontal function such as dopamine, serotonin, and noradrenaline [17]. This is of particular interest as some authors have suggested a prefrontal contribution to spatial navigation [30, 31]. Their hypothesis was supported by fMRI reports on PFC activity during spatial tasks [15, 32]. Interestingly, the high-activity variant of the MAOA gene has been associated with increased activity in the medial PFC [33], an area involved in memory with tight connections to the striatum [34]. Moreover, regarding executive processes known to rely on PFC function, individuals with the high-activity variant performed significantly faster than individuals with the low-activity variant on a working memory task [8]. Speculatively, MAOA could thus possibly influence spatial cognition indirectly by impacting catecholamine function in PFC and/or striatum. Higher transcription of high-activity MAOA gene in males provide larger production of MAOA with amplified catecholamine deamination and, in turn, faster clearance of neurotransmitters from the synaptic cleft allowing for a faster turnover of available monoamines. This would be consistent with the idea that individuals with the low-activity variant might have higher levels of homovanillic acid (HVA), a major metabolite of catecholamines in the CNS [25], but show worse performance on executive tasks [7, 8]. However, these findings remain to be clarified at the behavioural level given contrary evidence that individuals with low MAO-A gene expression make better financial decisions [9] and accomplish higher educational levels given similar IQs [20]. Together with prior work, the current findings imply the involvement of multiple genes in the neurochemistry of spatial cognition and may implicate different neuroanatomical foci. For example, it is conceivable that BDNF might modulate spatial navigation through its action on the hippocampus, whereas MAOA might do so at the prefrontal level. Yet, the precise directionality of the findings remains to be confirmed.
In fact, the current data offer exciting new hypotheses to test in animal knock-in/knock-out genetic models using similar navigational tasks to further isolate effects of MAOA (and/or BDNF) on spatial abilities. Such research endeavour would further our understanding of the molecular basis of spatial navigation. Indeed, the virtual analogue of the Morris Water Maze is an ideal task for translational work, since it allows to assess the same performance measures across both humans [14] and rodents [35].
Some limitations require discussion. First, we should acknowledge the relatively small sample size, given that this may constrain interpretability of findings in behavioural genetic studies (c.f.[36] for review). However, power analyses of the current study indicated good statistical power to obtain effects of a medium-to-large magnitude. Second, because little is known about sex differences regarding function or distribution of MAOA, we limited our analyses to males in order to rule out complex sex by genotype interaction effects and to preserve degrees of freedom. Replication in females is needed to generalize these findings across sex.
4.1 Conclusions
In summary, these novel data provide a window into the neurochemical basis of human spatial navigation and implicate involvement of the MAOA gene. Along with other studies, such data are important for guiding behavioural neuroscience research, specifically the neurochemistry of behaviour. Future, translational work might be able to probe more specifically how and where in the brain MAOA activity influences cognitive abilities.
Highlights.
Lacking evidence of monoamine-oxidase (MAO-A) involvement in human spatial memory
Participants performed a virtual Morris Water Maze task
High MAO-A activity males performed consistently better than low MAO-A activity males
Findings not due to pre-task differences or age
Acknowledgments
This research was supported, in part, by the Intramural Research Programme of the NIMH, NIH. We would also like to acknowledge the support of Ghent University (Multidisciplinary Research Partnership: “The integrative neuroscience of behavioural control”).
Footnotes
None of the authors has a conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tadic A, Rujescu D, Szegedi A, Giegling I, Singer P, Moller HJ, et al. Association of a MAOA gene variant with generalized anxiety disorder, but not with panic disorder or major depression. Am J Med Gen Part B. 2003;117B:1–6. doi: 10.1002/ajmg.b.10013. [DOI] [PubMed] [Google Scholar]
- 2.Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Human Mol Gen. 1999;8:621–4. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- 3.Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson EG, Norton N, Forslund K, Mattila-Evenden M, Rylander G, Asberg M, et al. Association between a promoter variant in the monoamine oxidase A gene and schizophrenia. Schizophr Res. 2003;61:31–7. doi: 10.1016/s0920-9964(02)00224-4. [DOI] [PubMed] [Google Scholar]
- 5.Mueller SC, Temple V, Cornwell B, Grillon C, Pine DS, Ernst M. Impaired spatial navigation in pediatric anxiety. J Child Psychol and Psychiatry. 2009;50:1227–34. doi: 10.1111/j.1469-7610.2009.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould NF, Holmes MK, Fantie BD, Luckenbaugh DA, Pine DS, Gould TD, et al. Performance on a virtual reality spatial memory navigation task in depressed patients. Am J Psychiatry. 2007;164:516–9. doi: 10.1176/ajp.2007.164.3.516. [DOI] [PubMed] [Google Scholar]
- 7.Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks. BMC neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enge S, Fleischhauer M, Lesch K-P, Reif A, Strobel A. Serotonergic modulation in executive functioning: Linking genetic variations to working memory performance. Neuropsychologia. 2011;49:3776–85. doi: 10.1016/j.neuropsychologia.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Frydman C, Camerer C, Bossaerts P, Rangel A. MAOA-L carriers are better at making optimal financial decisions under risk. Proc Royal Soc B. 2011:278. doi: 10.1098/rspb.2010.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steckler T, Rammes G, Sauvage M, van Gaalen MM, Weis C, Zieglgansberger W, et al. Effects of the monoamine oxidase A inhibitor moclobemide on hippocampal plasticity in GR-impaired transgenic mice. J Psych Res. 2001;35:29–42. doi: 10.1016/s0022-3956(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 11.Pahkla R, Harro J, Rago L. Behavioural effects of pinoline in the rat forced swimming, open field and elevated plus-maze tests. Pharmacol Res. 1996;34:73–8. doi: 10.1006/phrs.1996.0066. [DOI] [PubMed] [Google Scholar]
- 12.Barbelivien A, Nyman L, Haapalinna A, Sirvio J. Inhibition of MAO-A activity enhances behavioural activity of rats assessed using water maze and open arena tasks. Pharmacol Toxicol. 2001;88:304–12. [PubMed] [Google Scholar]
- 13.Klinteberg B, Levander SE, Oreland L, Asberg M, Schalling D. Neuropsychological correlates of platelet monoamine oxidase (MAO) activity in female and male subjects. Biol Psychol. 1987;24:237–52. doi: 10.1016/0301-0511(87)90005-6. [DOI] [PubMed] [Google Scholar]
- 14.Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–80. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moffat SD, Kennedy KM, Rodrigue KM, Raz N. Extrahippocampal contributions to age differences in human spatial navigation. Cereb Cortex. 2007;17:1274–82. doi: 10.1093/cercor/bhl036. [DOI] [PubMed] [Google Scholar]
- 16.Skelton RW, Bukach CM, Laurance HE, Thomas KGF, Jacobs WJ. Humans with traumatic brain injuries show place-learning deficits in computer-generated virtual space. J Clin Exp Neuropsychol. 2000;22:157–75. doi: 10.1076/1380-3395(200004)22:2;1-1;FT157. [DOI] [PubMed] [Google Scholar]
- 17.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Gen. 1998;103:273–9. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Morgan M, Hutchison K, Calhoun VD. A study of the influence of sex on genome wide methylation. PloS one. 2010;5:e10028. doi: 10.1371/journal.pone.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebastian CL, Roiser JP, Tan GC, Viding E, Wood NW, Blakemore SJ. Effects of age and MAOA genotype on the neural processing of social rejection. Genes Brain Beh. 2010;9:628–37. doi: 10.1111/j.1601-183X.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 20.Kiive E, Laas K, Akkerman K, Comasco E, Oreland L, Veidebaum T, et al. Mitigating aggressiveness through education? The monoamine oxidase A genotype and mental health in general population. Acta Neuropsychiatrica. 2014;26:19–28. doi: 10.1017/neu.2013.34. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton DA, Johnson TE, Redhead ES, Verney SP. Control of rodent and human spatial navigation by room and apparatus cues. Behav Proc. 2009;81:154–69. doi: 10.1016/j.beproc.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, Tx: Harcourt Assessment; 1999. [Google Scholar]
- 23.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adol Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Duncko R, Cornwell B, Cui L, Merikangas KR, Grillon C. Acute exposure to stress improves performance in trace eyeblink conditioning and spatial learning tasks in healthy men. Learn & Mem. 2007;14:329–35. doi: 10.1101/lm.483807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, Goldman D. A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry. 2006;11:858–66. doi: 10.1038/sj.mp.4001856. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Beh Res Meth. 2007;39:175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 27.Egan M, Kojima M, Callicott J, Goldberg T, Kolachana B, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 28.Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, Ernst M. Gray Matter Volume in Adolescent Anxiety: An Impact of the Brain-Derived Neurotrophic Factor Val66Met Polymorphism? J Am Acad Child Adol Psychiatry. 2013;52:184–95. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banner H, Bhat V, Etchamendy N, Joober R, Bohbot VD. The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced functional magnetic resonance imaging activity in the hippocampus and increased use of caudate nucleus-dependent strategies in a human virtual navigation task. Eur J Neurosci. 2011;33:968–77. doi: 10.1111/j.1460-9568.2010.07550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiers HJ. Keeping the goal in mind: prefrontal contributions to spatial navigation. Neuropsychologia. 2008;46:2106–8. doi: 10.1016/j.neuropsychologia.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolbers T, Hegarty M. What determines our navigational abilities? Trends Cogn Sci. 2010;14:138–46. doi: 10.1016/j.tics.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Gron G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci. 2000;3:404–8. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. PNAS U S A. 2003;100:7406–11. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Euston DR, Gruber AJ, McNaughton BL. The role of medial prefrontal cortex in memory and decision making. Neuron. 2012;76:1057–70. doi: 10.1016/j.neuron.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grissom EM, Hawley WR, Bromley-Dulfano SS, Marino SE, Stathopoulos NG, Dohanich GP. Learning strategy is influenced by trait anxiety and early rearing conditions in prepubertal male, but not prepubertal female rats. Neurobiol Learn & Mem. 2012 doi: 10.1016/j.nlm.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Beh. 2006;5:311–28. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]


