Abstract
In patients with multiple myeloma (MM) undergoing autologous hematopoietic cell transplantation (auto-HCT), peripheral blood progenitor cells (PBPCs) may be collected following mobilization with growth factor alone (GF) or cytotoxic chemotherapy plus GF ( (CC+GF). It is uncertain whether the method of mobilization affects post-transplant outcomes. We compared these mobilization strategies in a retrospective analysis of 968 patients with MM from the Center for International Blood and Marrow Transplant Research database who received an auto-HCT in the US and Canada between 2007 and 2012. The kinetics of neutrophil engraftment (≥ 0.5 × 109/L) was similar between groups (13 vs. 13 days, P=0.69) while platelet engraftment (≥ 20 × 109/L) was slightly faster with CC+GF (19 vs. 18 days, P=0.006). Adjusted 3-years PFS was 43% (95% C.I. 38–48) in GF and 40% (95% C.I. 35–45) in CC+GF, P=0.33. Adjusted 3-years OS was 82% (95% C.I. 78–86) vs. 80% (95% C.I. 75–84), P=0.43 and adjusted 5-year OS was 62% (95C.I. 54–68) vs. 60% (95% C.I. 52–67), P=0.76, for GF and CC+GF respectively. We conclude that MM patients undergoing auto-HCT have similar outcomes irrespective of the method of mobilization and found no evidence that the addition of chemotherapy to mobilization contributes to disease control.
INTRODUCTION
Multiple myeloma is currently the most common indication for autologous hematopoietic cell transplantation (auto-HCT) based on the prolongation of event-free and overall survival (OS) when compared to conventional chemotherapy alone.1–4 Currently 99% of auto-HCTs in adults utilize peripheral blood progenitor cells (PBPCs) as the graft source. PBPCs for transplantation may be mobilized either by hematopoietic growth factors (G-CSF and GM-CSF) alone (GF) or cytotoxic chemotherapy plus growth factor (CC+GF). However, the optimal method for mobilization of hematopoietic progenitor cells is unknown.
Proponents of CC+GF mobilization argue that the anti-myeloma activity of the chemotherapy agent contributes to long term disease control. In addition, CC+GF mobilization is associated with higher CD34+ yields than GF mobilization.5 Because induction therapy with lenalidomide has known detrimental effects on CD34+ yield,6–9 CC+GF mobilization has been proposed as preferred mobilization strategy for these patients due to the higher incidence of mobilization failure with GF.10, 11
The impact of chemotherapy in the mobilization regimen to disease control is controversial.12 Furthermore, CC+GF mobilization can be associated with significant morbidity with increased risks of infection and hospitalization, and increased costs.5, 12–16 In this study, we analyzed the CIBMTR database to compare the outcome of patients with MM receiving autologous HCT using PBSCs obtained by CC+GF mobilization versus GF mobilization.
METHODS
Data source
The CIBMTR® is a research collaboration between the National Marrow Donor Program® (NMDP)/Be The Match® and the Medical College of Wisconsin.. Established in 2004, it receives data from > 320 transplantation centers worldwide on allogeneic and autologous HCT. Data are submitted to the Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis, where computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed with approval of the institutional review boards of the National Marrow Donor Program and the Medical College of Wisconsin.
Study design
The primary objective of the study was to compare the progression-free survival (PFS) of patients receiving an auto-HCT after GF versus CC+GF mobilization for symptomatic MM. Secondary endpoints included OS, non-relapse mortality (NRM), and engraftment kinetics.
The study population consisted of all adult patients (age ≥ 18) who underwent their first auto-HCT following high dose melphalan (≥140 mg/m2) during the first year after diagnosis in the US or Canada and registered with CIBMTR between year 2007 and 2012. Patients who did not receive pre-transplant induction therapy with either thalidomide, lenalidomide or bortezomib, experienced disease progression prior to transplant, or in whom an allogeneic HCT was planned after auto-HCT were excluded. Due to limited numbers available for analysis, patients who received plerixafor for PBPC mobilization were also excluded.
Statistics
Patient-, disease- and transplant- related factors were compared between groups using the Chi-square test for categorical variables and the Wilcoxon two sample test for continuous variables. The probabilities of PFS and OS were calculated using the Kaplan-Meier estimator. Engraftment was compared using cumulative incidence estimates and considering death from any cause as competing risk. Cox proportional hazards regression was used to compare the two mobilization strategies. The assumption of proportional hazards for each factor in the Cox model was tested by adding a time-dependent covariate. A stepwise model selection approach was used to identify all significant risk factors for each of the time-dependent endpoints. Each step of model building contained the main effect for conditioning regimen types. The risk factors with significant level of p < 0.05 were included in the model. All computations were made with the statistical package SAS Version 9 (SAS Institute). All P values are 2-sided.
RESULTS
The analysis included 968 patients, 519 (from 62 centers) in GF and 449 (from 73 centers) in CC+GF. The median follow-up for survivors was 42 months in GF and 46 months in CC+GF. Baseline characteristics are displayed in Table 1. The two cohorts had similar characteristics. There were more patients in GF with only one prior line of therapy, more prior use of lenalidomide in GF and thalidomide in CC+GF, more patients in complete remission and fewer patients with HCT comorbidity index (HCT-CI) of 0 in the GF cohort.
Table 1.
Characteristics of patients included in the study
| Variable | GF (N=519) |
CC+GF (N=449) |
P | |
|---|---|---|---|---|
| Age at transplant | 0.63 | |||
| median (range) | 58 (25–78) | 58 (23–74) | ||
| 18–39 | 15 (3%) | 17 (4%) | ||
| 40–49 | 80 (15%) | 70 (16%) | ||
| 50–59 | 218 (42%) | 174 (39%) | ||
| 60–69 | 182 (35%) | 172 (38%) | ||
| ≥70 | 24 (5%) | 16 (4%) | ||
| Gender | 0.78 | |||
| Male | 304 (59%) | 259 (58%) | ||
| Female | 215 (41%) | 190 (42%) | ||
| Race | 0.07 | |||
| Caucasian | 411 (79%) | 335 (75%) | ||
| African American | 89 (17%) | 86 (19%) | ||
| Others, unknown | 19 (4%) | 28 (6%) | ||
| Karnofsky score | 0.30 | |||
| ≥90 | 283 (55%) | 261 (58%) | ||
| <90 | 193 (37%) | 161 (36%) | ||
| Missing | 43 (8%) | 27 (6%) | ||
| HCT-Comorbidity Index | 0.006 | |||
| 0 | 227 (44%) | 227 (51%) | ||
| 1–2 | 147 (29%) | 134 (30%) | ||
| >2 | 145 (28%) | 88 (20%) | ||
| Isotype | 0.36 | |||
| IgG | 375 (72%) | 333 (74%) | ||
| IgA | 21 (4%) | 19 (4%) | ||
| Light chain | 101 (19%) | 82 (18%) | ||
| Others, unknown | 22 (5%) | 15 (4%) | ||
| Stage III at diagnosis* | 160 (31%) | 162 (36%) | 0.22 | |
| Number of prior therapy regimens** | <0.001 | |||
| 1 | 382 (74%) | 265 (59%) | ||
| 2 | 113 (22%) | 136 (30%) | ||
| >2 | 24 (5%) | 48 (11%) | ||
| Prior therapy regimens** | <0.001 | |||
| Thalidomide + bortezomib +- corticosteroid | 74 (14%) | 92 (20%) | ||
| Lenalidomide+ bortezomib +- corticosteroid | 121 (23%) | 48 (11%) | ||
| Thalidomide +- corticosteroid | 85 (16%) | 106 (24%) | ||
| Bortezomib +- corticosteroid | 132 (25%) | 136 (30%) | ||
| Lenalidomide +- corticosteroid | 107 (21%) | 67 (15%) | ||
| Sensitivity to initial therapy# | 491 (95%) | 426 (95%) | 0.85 | |
| Overall cycles of chemotherapy | ||||
| N evaluable | 440 | 402 | ||
| Median (range) | 4 (1–42) | 4 (1–20) | 0.001 | |
| Prior radiation therapy | 110 (21%) | 101 (22%) | 0.63 | |
| Response to initial therapy# | 0.05 | |||
| CR | 84 (16%) | 48 (11%) | ||
| PR/VGPR | 407 (78%) | 378 (84%) | ||
| MR/NR/SD | 28 (5%) | 23 (4%) | ||
| Mobilization cytotoxic chemotherapy | ||||
| Cyclophosphamide | 338 (75%) | |||
| Etoposide | 55 (12%) | |||
| Cyclophosphamide + etoposide | 21 (5%) | |||
| VDT-PACE/similar | 35 (8%) | |||
| Year of transplant | 0.03 | |||
| 2007–2008 | 289 (56%) | 272 (61%) | ||
| 2009–2010 | 105 (20%) | 100 (22%) | ||
| 2011–2012 | 125 (24%) | 77 (17%) | ||
| Time from diagnosis to HCT | <0.001 | |||
| <6 months | 221 (43%) | 140 (31%) | ||
| 6–12 months | 298 (57%) | 309 (69%) | ||
| Melphalan dose for conditioning therapy | 0.40 | |||
| 140–180 mg/m2 | 71 (14%) | 70 (16%) | ||
| >180 mg/m2 | 448 (86%) | 379 (84%) | ||
| Number of transplants† | 0.02 | |||
| Single | 417 (80%) | 331 (74%) | ||
| Planned second auto-HCT | 47 (9%) | 69 (15%) | ||
| Salvage second transplant | 55 (10%) | 49 (11%) | ||
| Maintenance therapy | 0.29 | |||
| None | 321 (62%) | 273 (61%) | ||
| Thalidomide/lenalidomide +- corticosteroids | 147 (28%) | 134 (30%) | ||
| Thalidomide/lenalidomide + bortezomib +- corticosteroids | 32 (6%) | 30 (7%) | ||
| Bortezomib +- corticosteroids | 16 (3%) | 11 (2%) | ||
| Others | 3 (<1%) | 1 (<1%) | ||
Durie-Salmon or International Staging System.
Excludes cytotoxic chemotherapy administered for mobilization.
Assessed prior to mobilization.
Analysis is for first transplant only
Mobilization and transplant outcomes
Patients completed product collection over a shorter time in CC+GF than in GF (P<0.001). The proportion of patients completing collection in 1, 2 and > 2 days was 33%, 29% and 37% in GF vs. 52%, 21% and 26% in CC+GF respectively. Time from collection to transplant was slightly shorter in GF than in CC + GF (median 16 vs. 18 days, P<0.001). Characteristics of the product infused were available for 242 patients in GF (47%) and 147 patients in CC + GF (33%). Fewer CD34+ cells were infused in patients mobilized with GF (median 3.9 × 106/kg, interquartile range 3.1–4.8 × 106/kg) than in CC + GF (median 5.1 × 106/kg, interquartile range 3.5–6.9 × 106/kg, P < 0.001).
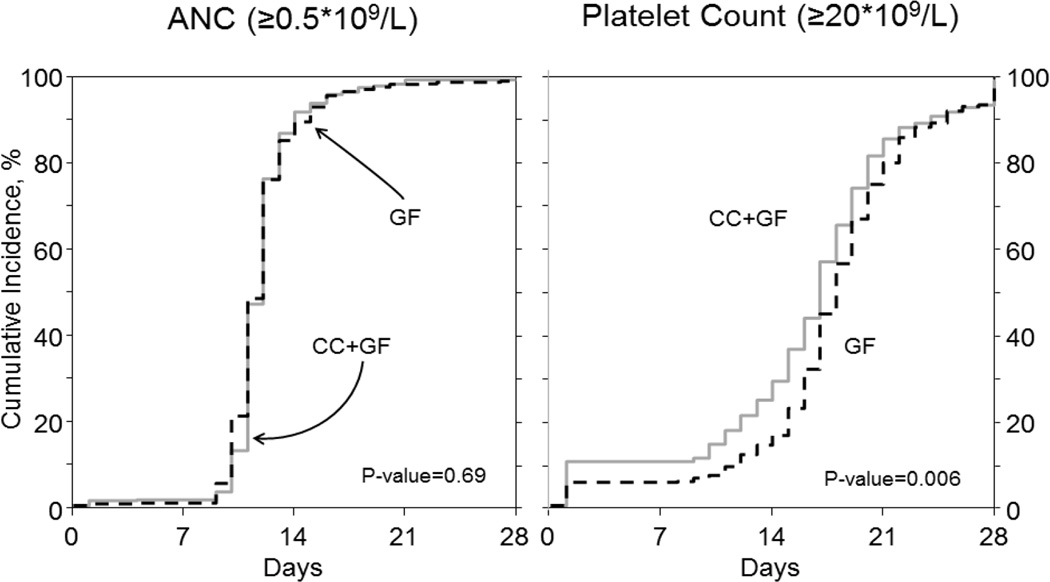
Neutrophil engraftment (≥ 0.5 × 109/L) was similar between groups (median 13 vs. 13 days, P=0.69) while platelet engraftment (≥ 20 × 109/L) was faster in CC+GF (median 19 vs. 18 days, P=0.006, Figure 1). Number of inpatient days during first 100 days after transplant, a surrogate of short term morbidity, did not differ between the two groups (median 14 days, P=0.70). NRM at 1 year also was not affected by mobilization strategy (2% in GF vs. 1% if CC+GF, P=0.42)
Figure 1.
Engraftment kinetics according to the method of mobilization.
Disease control and survival outcomes
In the univariate analysis, PFS at 1 year, 3 years and 5 years from auto-HCT were 77%, 43%, 19% in GF and 79%, 40%, 26% in CC+GF respectively (P=0.76). The OS at 1 year, 3 years and 5 years from auto-HCT were 95%, 82% and 63% for GF and 92%, 80% and 60% for CC+GF respectively (P=0.20).
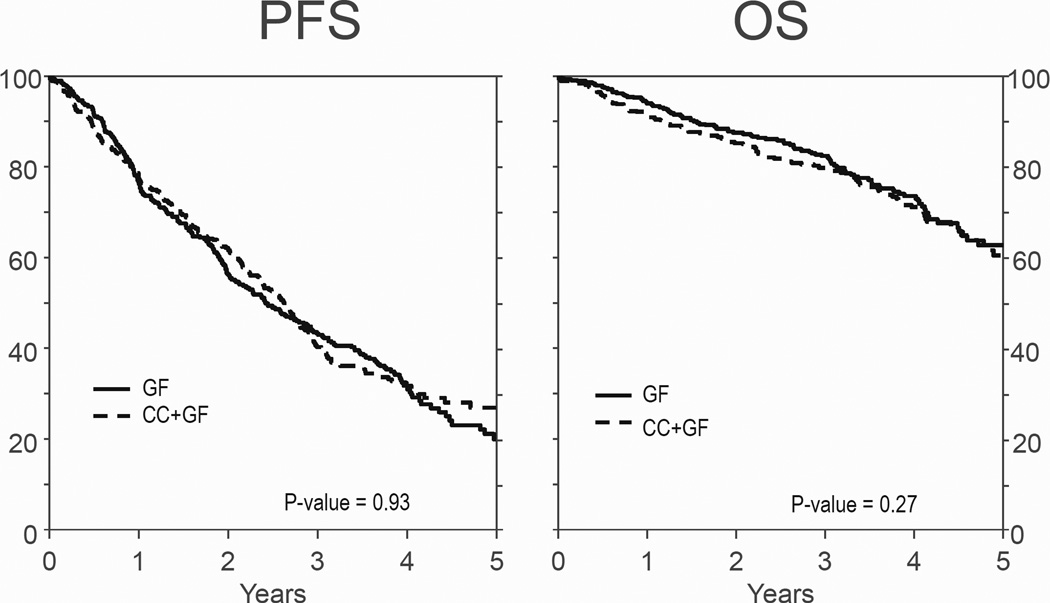
In the multivariate analysis, Karnofsky score at transplant and stage at diagnosis affected PFS. Neither MM isotype, number of prior regimens of therapy, response to therapy prior to auto-HCT, use of planned second auto-HCT or HCT-CI contributed to the final PFS model (Table 2). Mobilization strategy was also not predictive of PFS (P=0.93, Figure 2 and Table 3). The factors associated with OS were MM isotype, stage at diagnosis and HCT-CI. Mobilization strategy was not associated with OS (P=0.27, Figure 2 and Table 3). There were 107 deaths in GF and 116 in CC+GF. MM was the main cause of death in both GF and CC+GF (79% and 78% respectively). Second malignancies (4% vs. 2%), infection (3% vs. 3%) and organ failure (3% vs. 6%) accounted for similar proportions of deaths in both cohorts.
Table 2.
Multivariate analysis for progression-free survival and overall survival
| Progression-free survival | ||||
|---|---|---|---|---|
| Factor | HR | 95% C.I. | P | |
| Mobilization | 0.93 | |||
| GF | 1.00 | |||
| CC+GF | 0.99 | 0.84–1.18 | ||
| Karnofsky score, % | 0.004 | |||
| ≥90 | 1.00 | |||
| <90 | 1.21 | 1.01–1.45 | 0.04 | |
| Missing | 1.61 | 1.18–2.20 | 0.003 | |
| Stage at diagnosis (ISS or DSS) | 0.046 | |||
| III | 1.00 | |||
| I or II | 0.80 | 0.67–0.96 | 0.01 | |
| Unknown | 0.93 | 0.64–1.36 | 0.71 | |
| Overall Survival | ||||
| Factor | HR | 95% C.I. | P | |
| Mobilization | 0.27 | |||
| GF | 1.00 | |||
| CC+GF | 1.16 | 0.89–1.52 | ||
| Isotype | 0.01 | |||
| IgG/IgA/Others | 1.00 | |||
| Light chain/Non-secretory | 0.62 | 0.43–0.88 | 0.009 | |
| Unknown | 0.21 | 0.03–1.50 | 0.12 | |
| Stage at diagnosis (ISS or DSS) | <0.001 | |||
| III | 1.00 | |||
| I or II | 0.54 | 0.41–0.71 | <0.001 | |
| Unknown | 0.51 | 0.26–0.97 | 0.04 | |
| HCT-CI | 0.01 | |||
| 0–2 | 1.00 | |||
| >2 | 1.48 | 1.09–2.03 | 0.01 | |
Figure 2.
Adjusted probability of PFS and OS according to the method of mobilization
Table 3.
Adjusted probabilities of outcomes
| GF* | CC+GF* | P | ||
|---|---|---|---|---|
| Non relapse mortality | ||||
| 1–year | 2 (1–4)% | 1 (1–3)% | 0.42 | |
| 2–year | 3 (1–4)% | 2 (1–3)% | 0.32 | |
| 3–year | 3 (1–4)% | 2 (1–4)% | 0.54 | |
| 4–year | 4 (2–6)% | 3 (2–6)% | 0.64 | |
| 5–year | 4 (2–6)% | 3 (2–6)% | 0.64 | |
| Progression-free survival | ||||
| 1–year | 77 (73–81)% | 79 (74–82)% | 0.62 | |
| 2–year | 57 (52–61)% | 62 (57–67)% | 0.13 | |
| 3–year | 43 (38–48)% | 40 (35–45)% | 0.33 | |
| 4–year | 31 (26–37)% | 31 (26–36)% | 0.85 | |
| 5–year | 19 (13–25)% | 26 (20–32)% | 0.11 | |
| Overall survival | ||||
| 1–year | 95 (92–96)% | 92 (89–94)% | 0.10 | |
| 2–year | 88 (84–90)% | 85 (82–89)% | 0.37 | |
| 3–year | 82 (78–86)% | 80 (75–84)% | 0.43 | |
| 4–year | 73 (68–78)% | 71 (65–76)% | 0.54 | |
| 5–year | 62 (54–68)% | 60 (52–67)% | 0.76 | |
Percentage ( 95% confidence interval)
Many individual centers reported patients who were mobilized using both strategies raising the possibility of selection bias (Supplementary Table 1), i.e. centers could have assigned patients with more aggressive or refractory disease to CC+GF. To address this concern we compared PFS and OS between the two cohorts restricting analysis to centers that reported >70% of patients in one or the other mobilization strategy. Additionally we compared patients receiving CC+GF as the non-preferred strategy (i.e. CC+GF from centers that had >70% GF based mobilization) versus those receiving it as the preferred center strategy. These analyses did not indicate any difference between cohorts in PFS (Supplemental Table 1 and supplemental figures)
DISCUSSION
In this report, we compare the outcomes of patients with MM undergoing auto-HCT following high dose melphalan according to mobilization strategy. Previous studies of mobilization strategy primarily examined the impact on CD34+ yields and engraftment kinetics and were comprised of patients who received alkylator based induction therapy. In contrast, this study was limited to indivduals who received immunomodulatory agents (IMiDs) or proteasome inhibitors during induction therapy. Our results are consistent with previous reports in which mobilization with the combination of chemotherapy and G-CSF was associated with fewer collection days and more CD34+ cells infused than G-CSF alone.5,13 Neutrophil and platelet engraftment were comparable between the two groups.16, 17,19
In addition, previous studies were primarily single center studies with limited follow up and were not designed to analyze the impact of mobilization strategy on long term disease control. In the multivariate analysis presented here, we did not detect any differences in PFS or OS based on mobilization strategy. 5, 18, 19 A more recent, single-center retrospective analysis also showed no benefit of chemotherapy mobilization on long term outcomes.20
The treatment landscape for MM has changed dramatically over the past decade with the introduction of the immunomodulatory agents (IMiDs) and the proteasome inhibitors. Incorporation of these less toxic and highly effective agents into two and three drug induction regimens has resulted in an increased rate and depth of response prior to transplant with improved OS.21 Furthermore, the increasing use of maintenance therapy has improved PFS and possibly OS post-transplant.22, 23 For this reason, the need for additional cytoreduction with cytotoxic chemotherapeutic agents such as cyclophosphamide in the mobilization regimen may no longer be necessary or relevant for disease control in myeloma.
The other potential benefit of CC+GF mobilization is increased CD34 yields compared to GF alone. However, others have observed that the mobilization failure rates did not differ substantially between GF and CC+GF.17 In addition, the development of the CXCR4 antagonist, plerixafor, can improve the CD34 yields and decrease rates of mobilization failures without using cytotoxic chemotherapy.24
Our study has some important limitations. Because of a limited sample size and markedly different patient characteristics, we were unable to analyze patients who received plerixafor in this study. Patients included in the present study received auto-HCT before or shortly after approval of plerixafor by the U.S. Food and Drug Administration and much of the early adoption of plerixafor may have been for patients who had already failed another mobilization modality or who were perceived to be at risk of failure, further impairing a fair comparison with GF and CC+GF. We believe, however, that the present work is still relevant in a scenario where plerixafor is an established option for upfront mobilization as the combination of plerixafor + GF is not expected to have an effect on disease control dissimilar to GF alone.
The CIBMTR database only collects data on the product infused at transplant (including data on mobilization) but not on products that are stored for later use, even if collected during the same mobilization cycle. Therefore we were unable to assess actual collection targets and yields and do not have any information on patients who were mobilized but not transplanted (“collect and store” or “mobilization failures”).
The other potential limitation of this study is that the decision to use CC + GF mobilization may be based on the presence of either high-risk disease or a high myeloma disease burden prior to auto-HCT. In our dataset, the two groups were very similar in both baseline characteristics which included both initial stage, number of prior lines of therapy, and response to induction therapy, factors that were all included in the multivariate analysis. In addition, an exploratory analysis of the dataset suggests that in the majority of cases, the method of mobilization is largely based on center preference rather than disease characteristics. Because of the nature of this database, comprehensive information on certain prognostic factors including fluorescent in situ hybridization, conventional cytogenetics or plasma cell labeling index was not available. We cannot exclude the possibility that a subset of patients with high disease burden or who are refractory to initial IMiD and proteasome inhibitor therapy may benefit from CC + GF mobilization since very few of these cases were available for analysis in the CIBMTR database (<5% of the population).
In summary, our data indicates that for patients with MM who received either an IMiD or bortezomib-based induction regimen, similar outcomes are observed post auto-HCT irrespective of the method of mobilization and found no evidence that the addition of chemotherapy to mobilization contributes to disease control.
Supplementary Material
ACKNOWLEDGMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA December 6–9, 2014
Conflict of Interest Statement: There are no relevant conflicts of interest to disclose.
REFRENCES
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. The New England journal of medicine. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92(9):3131–3136. [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. The New England journal of medicine. 2014;371(10):895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 5.Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R, et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant. 1997;20(3):211–217. doi: 10.1038/sj.bmt.1700867. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 7.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22(6):1280–1281. doi: 10.1038/sj.leu.2405035. author reply 1281-2. [DOI] [PubMed] [Google Scholar]
- 8.Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008;22(6):1282–1284. doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]
- 9.Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15(6):718–723. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazha A, Cook R, Vogl DT, Mangan PA, Gardler M, Hummel K, et al. Stem cell collection in patients with multiple myeloma: impact of induction therapy and mobilization regimen. Bone Marrow Transplant. 2011;46(1):59–63. doi: 10.1038/bmt.2010.63. [DOI] [PubMed] [Google Scholar]
- 11.Mark T, Stern J, Furst JR, Jayabalan D, Zafar F, LaRow A, et al. Stem cell mobilization with cyclophosphamide overcomes the suppressive effect of lenalidomide therapy on stem cell collection in multiple myeloma. Biol Blood Marrow Transplant. 2008;14(7):795–798. doi: 10.1016/j.bbmt.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6(5):384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 13.Shaughnessy P, Islas-Ohlmayer M, Murphy J, Hougham M, Macpherson J, Winkler K, et al. Cost and Clinical Analysis of Autologous Hematopoietic Stem Cell Mobilization with G-CSF and Plerixafor compared to G-CSF and Cyclophosphamide. Biol Blood Marrow Transplant. 2011;17(5):729–736. doi: 10.1016/j.bbmt.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Costa LJ, Miller AN, Alexander ET, Hogan KR, Shabbir M, Schaub C, et al. Growth factor and patient-adapted use of plerixafor is superior to CY and growth factor for autologous hematopoietic stem cells mobilization. Bone Marrow Transplant. 2011;46(4):523–528. doi: 10.1038/bmt.2010.170. [DOI] [PubMed] [Google Scholar]
- 15.Schwartzberg LS, Birch R, Hazelton B, Tauer KW, Lee P, Jr, Altemose R, et al. Peripheral blood stem cell mobilization by chemotherapy with and without recombinant human granulocyte colony-stimulating factor. J Hematother. 1992;1(4):317–327. doi: 10.1089/scd.1.1992.1.317. [DOI] [PubMed] [Google Scholar]
- 16.Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16(4):1547–1553. doi: 10.1200/JCO.1998.16.4.1547. [DOI] [PubMed] [Google Scholar]
- 17.Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98(7):2059–2064. doi: 10.1182/blood.v98.7.2059. [DOI] [PubMed] [Google Scholar]
- 19.Desikan KR, Barlogie B, Jagannath S, Vesole DH, Siegel D, Fassas A, et al. Comparable engraftment kinetics following peripheral-blood stem-cell infusion mobilized with granulocyte colony-stimulating factor with or without cyclophosphamide in multiple myeloma. J Clin Oncol. 1998;16(4):1547–1553. doi: 10.1200/JCO.1998.16.4.1547. [DOI] [PubMed] [Google Scholar]
- 20.Tuchman SA, Bacon WA, Huang LW, Long G, Rizzieri D, Horwitz M, et al. Cyclophosphamide-based hematopoietic stem cell mobilization before autologous stem cell transplantation in newly diagnosed multiple myeloma. Journal of clinical apheresis. 2014 doi: 10.1002/jca.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 24.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.