Abstract
BACKGROUND
Pneumonia is associated with high risk of heart failure (HF) in the short-term (30 days) post-infection. Whether this association persists beyond this period is unknown.
METHODS
We studied 5613 elderly (≥65-year-old) adults enrolled in the Cardiovascular Health Study between 1989 and 1994 at 4 U.S. communities. Participants had no clinical diagnosis of HF at enrolment and they were followed through December 2010. Hospitalizations for pneumonia were identified using validated ICD-9 codes. A centralized committee adjudicated new-onset HF events. Using Cox regression, we estimated adjusted hazard ratios (HRs) of new-onset HF at different time intervals after hospitalization for pneumonia.
RESULTS
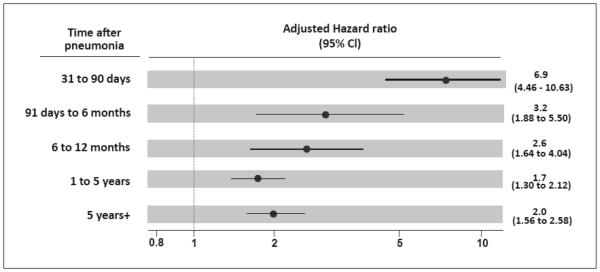
652 participants hospitalized for pneumonia during follow-up were still alive and free of clinical diagnosis of HF by day 30 post-hospitalization. Relative to the time of their hospitalization, new-onset HF occurred in 22 cases between 31 and 90 days (HR, 6.9; 95% CI, 4.46-10.63; p<0.001), 14 cases between 91 days and 6 months (HR, 3.2; 95% CI, 1.88-5.50; p<0.001), 20 cases between 6 months and 1 year (HR, 2.6; 95% CI, 1.64-4.04; p<0.001), 76 cases between 1 and 5 years (HR, 1.7; 95% CI, 1.30-2.12; p<0.001), and 71 cases after 5 years (HR, 2.0; 95% CI, 1.56-2.58; p<0.001). Results were robust to sensitivity analyses using stringent definitions of pneumonia and extreme assumptions for potential informative censoring.
CONCLUSION
Hospitalization for pneumonia is associated with increased risk of new-onset HF in the intermediate and long-term. Studies should characterize the mechanisms of this association in order to prevent HF in elderly pneumonia survivors.
Keywords: Heart failure, Pneumonia, Infection
INTRODUCTION
Heart failure (HF) affects ~2% of the western population and is a leading cause of morbidity and mortality in elderly adults (age ≥65 years).1 Because of the aging of our population, it has been estimated that by 2030, 1 in 33 Americans will have HF and the annual healthcare cost of this condition will be ~US$70 billion.1 Therefore, in order to design better preventive strategies, characterizing risk factors for the development of new-onset HF in elderly individuals is an important goal.
Pneumonia affects ~1.2% of the population in the northern hemisphere each year and it is the most common single diagnosis responsible for hospital admission in North America.2,3 Pneumonia is particularly common among elderly adults and two-thirds (66%) of pneumonia hospitalizations occur in this age-group.4,5 Contrary to other age-groups, the rates of pneumonia in elderly individuals continue to rise.2,4
On the basis of acutely increasing systemic metabolic demands, pneumonia has long been regarded as a trigger for HF.6 A recent meta-analysis showed that 14% of patients hospitalized for pneumonia develop new or worsening HF within 30 days of admission.7 Nonetheless, pneumonia survivors continue to have increased morbidity and mortality long-term after the infection and cardiovascular diseases are major contributors to this effect.8 Moreover, recent animal studies suggest that some pulmonary pathogens can produce persistent inflammatory damage to the myocardium event when the infection is resolved.9 These elements suggest that pneumonia can increase the risk of HF well beyond the short-term post-infection. Because of the increasing burden of HF and pneumonia in our aging population, such association would have important implications for HF prevention and the management of patients with pneumonia. However, this relationship has never been investigated.
In this study, we tested the hypothesis that hospitalization for pneumonia is independently associated with increased risk of subsequent new-onset HF not only in the short-term, but also in the intermediate, and long-term in elderly adults.
METHODS
Study population and data collection
The Cardiovascular Health Study (CHS) enrolled 5,888 community-dwelling elderly adults (age≥65 years) from 4 US communities in California, Philadelphia, North Carolina and Maryland.10 The baseline evaluation (1989-1994) included a standardized physical examination, diagnostic and laboratory evaluation, and questionnaires on health status, medical history, lifestyle habits, and cardiovascular risk factors.10-12 Update of major exam components and surveillance of new cardiovascular events and hospitalizations occurred at twice-yearly contacts alternating between telephone calls and in-person clinic visits until 1999. Beyond 1999, ascertainment of vital status and surveillance for new cardiovascular events and hospitalizations continued through semiannual phone contacts and review of Medicare Utilization files and local newspaper obituaries.10 In our analyses, participants were followed through December 2010 (mean follow-up=11.9 years). The institutional review boards at each participating site approved the study. All participants gave informed consent.
Event assessment
Hospitalizations for pneumonia were adjudicated using a previously validated method based on the presence of International Classification of Disease Ninth Revision (ICD-9) codes for pneumonia in at least one of the first 5 discharge diagnosis fields of hospital discharge abstracts.4,13 Our review of a subset of medical records (158) identified by this approach found documentation of clinical and radiographic diagnoses of pneumonia in 89% and 88% of cases, respectively.14 We defined pneumonia as severe when ICD-9 codes suggestive of severe sepsis, septic shock or organ dysfunction were also present, as previously validated.15 For sensitivity analyses, we used a more stringent definition of pneumonia hospitalizations in which ICD-9 codes 481, 482, 485, or 486 (suggestive of bacterial pneumonia) were listed in the primary (first) discharge diagnosis field. This more stringent definition reduces the sensitivity of capturing all hospitalizations with pneumonia but maximizes the probability that pneumonia was indeed a reason for these admissions.13 We did this to account for the possibility of confounding due to unrecognized cases of HF misdiagnosed as pneumonia during a hospital admission. Using this definition, our review of a subset of medical records found documentation of clinical and radiographic diagnoses of pneumonia in 94% and 96% of cases, respectively.14
For adjudication of HF events, medical records of potential new events were directed to a centralized CHS Events Subcommittee.16 As per CHS protocol, diagnosis of HF required all of the following: (a) documentation of HF diagnosis by a treating physician; (b) HF symptoms (shortness of breath, fatigue, orthopnea, paroxysmal nocturnal dyspnea) and signs (edema, rales, tachycardia, gallop rhythm, displaced apical impulse), or supportive findings on echocardiography, contrast ventriculography, or chest radiography; and (c) medical therapy for HF (diuretics plus either digitalis or a vasodilator).17 New-onset HF was the first HF event during study follow-up in individuals without HF at enrolment.16
Covariables
These included age, sex, race, coronary heart disease (prevalent at study entry and incident during the study), atrial fibrillation, diabetes, heart valve disease, smoking status, body mass index, heart rate, systolic and diastolic blood pressures, serum low-density lipoprotein cholesterol and high-density lipoprotein cholesterol, glomerular filtration rate (estimated as per the Modification of Diet in Renal Disease Study equation),18 serum C-reactive protein, left ventricular hypertrophy by electrocardiogram, forced expiratory volume in 1 second (FEV1, measured by spirometry), use of anti-hypertensive drugs, use of aspirin, and use of statins. With the exception of FEV1, all covariables were regularly updated at study clinic visits (Supplementary material, Table S1).
Statistical analyses
Because the outcome of our study was new-onset HF, patients with a clinical diagnosis of HF at baseline were excluded. The prevalence of missing values for covariables at baseline ranged from 0-5.3% (Supplementary material, Table S2). To account for this, we used multiple imputation (with additional predictors, Supplementary material, Table S2) to create 10 complete datasets of the covariables selected for analyses. To determine the risk of new-onset HF after pneumonia we used multivariable Cox regression. Patients were censored at time of death or loss to follow-up. The main exposure variable was a time-dependent indicator corresponding to 5 pre-specified time intervals after the first (index) hospitalization for pneumonia: 31-90 days, 91 days to 6 months, 6-12 months, 1-5 years, and >5 years. We included all pre-selected covariables (listed above) in our model. When covariables had >1 measurement during follow-up, we treated them as time-dependent and used their most recent value at any given event time. To account for changes in the hazard of pneumonia over time, we included an interaction term for pneumonia and age. We ran this model in each of the 10 imputed datasets and combined the results to produce our final estimates.19 We repeated these procedures with pneumonia defined by its severity.
Because time after pneumonia is a continuum, we did not arbitrarily exclude the immediate (0-30 days) post-pneumonia interval from our models. However, in CHS, all incident events diagnosed during one same hospitalization were adjudicated the date of that admission as the date of their occurrence (i.e. they were adjudicated the same date of occurrence). This prevented us from making any inference of precedence between pneumonia and HF events when they were diagnosed in the same hospitalization, rendering estimates of HF risk in the 0-30-day post-pneumonia interval unreliable. Therefore, these estimates are not reported. To evaluate informative censoring from death and loss to follow-up, we performed sensitivity analyses under 2 extreme assumptions: (1) That censored individuals experience a HF event immediately after censoring; and (2) That censored individuals have longer times to events than uncensored individuals 20. To explore potential confounding effects of recurrent episodes of pneumonia, we repeated our main analysis censoring patients who had >1 episode of pneumonia at the time of their second episode. To explore whether any association between hospitalization for pneumonia and subsequent new-onset HF is simply a function of individuals’ frailty and/or acute hospitalization per se (regardless of the cause), we repeated our main analyses with hospitalization for fractures (hip, ICD-9 code 820.00; vertebral, ICD-9 code 805.xx; wrist, ICD-9 codes 813.xx and 814.xx; and ribs, ICD-9 codes 807.0x and 807.1x), and not pneumonia, as the exposure of interest. We chose these fractures because they are (similar to pneumonia) also well-recognized markers of individuals’ aging and frailty, and a common cause of acute hospitalization; 21-24 however, they have no known intermediate or long-term biological effects that are shared with pneumonia.
All tests were 2-sided and a P value of less than .05 was considered statistically significant. Variables with a normal distribution were described using means and standard deviations (SDs), and those with a skewed distribution using medians and inter-quartile range (IQR). Comparisons were made using Pearson's chi-squared tests, two-sample Student t-tests, or Wilcoxon two-sample tests, as appropriate.
Analyses were implemented using SAS-v.9.2 or R statistical software.
RESULTS
Of 5,888 participants in CHS, 275 had clinical diagnosis HF at enrolment and were excluded. Our analysis included 5,613 participants. During follow-up, 1,315 (23.4%) participants were hospitalized with pneumonia at least once (median time to pneumonia=8.8 years; inter-quartile range [IQR]=5.3-12.5 years), whereas 1,868 participants developed new-onset HF (median time to new-onset HF=9.0 years; IQR=4.8-13.6 years). Participants that were hospitalized with pneumonia were slightly younger than the rest of participants but had a heavier burden of cardiovascular comorbidities and risk factors such as coronary heart disease, atrial fibrillation, diabetes and smoking (Table 1).
Table 1.
Baseline characteristics of patients with and without pneumonia
| Baseline characteristics | Hospitalization for pneumonia | P value | |
|---|---|---|---|
| Yes (n=1,315) |
No (n=4,298) |
||
| Age (in years) | 72.5 (5.5) | 73.5 (5.6) | <0.001 |
| Male gender | 611 (46.5%) | 1,751 (40.7%) | <0.001 |
| Ethnicity | 0.024 | ||
| White | 1,130 (85.9%) | 3,585 (83.4%) | |
| African American | 174 (13.2%) | 690 (16.1%) | |
| Other | 11 (0.8%) | 23 (0.5%) | |
| Coronary heart disease | 283 (21.5%) | 696 (16.2%) | <0.001 |
| Atrial fibrillation | 38 (2.9%) | 80 (1.9%) | 0.023 |
| Diabetes | 245 (18.6%) | 625 (14.5%) | <0.001 |
| Valvular Heart Disease | 75 (5.9%) | 213 (5.1%) | 0.271 |
| Smoking | 774 (58.9%) | 2,231 (52.0%) | <0.001 |
| Body mass index in kg/m2 | 26.4 (4.6) | 26.8 (4.7) | 0.008 |
| Heart rate per minute | 66 (11) | 65 (11) | 0.002 |
| Systolic blood pressure in mm Hg | 137 (22) | 137 (22) | 0.618 |
| Diastolic blood pressure in mm Hg | 70 (12) | 71 (12) | 0.014 |
| Serum LDL cholesterol in mg/dL | 129 (37) | 131 (35) | 0.041 |
| Serum HDL cholesterol in mg/dL | 54 (16) | 55 (16) | 0.117 |
| Glomerular filtration rate in mL/min/1.73 m2 | 68.5 (18) | 68.7 (18) | 0.768 |
| Serum C-reactive protein in mg/L | 2·7 (1.3-4.8) | 2·4 (1.2-4.2) | 0.018 |
| LV hypertrophy by ECG | 66 (5.2%) | 176 (4.2%) | 0.138 |
| Percent of predicted FEV-1 | 85.9 (23.4) | 91.8 (21.6) | <0.001 |
| Use of anti-hypertensive medications | 655 (49.8%) | 1899 (44.3%) | <0.001 |
| Use of aspirin | 463 (35.2%) | 1406 (32.8%) | 0.100 |
| Use of statins | 30 (2.3%) | 97 (2.3%) | 0.965 |
Data are number (%), mean (SD), or median (IQR).
LDL denotes low-density lipoprotein. HDL denotes high-density lipoprotein. LV denotes left ventricle. ECG denotes electrocardiogram. FEV1 denotes forced expiratory volume in 1 second as measured by spirometry.
Of the 1,315 participants hospitalized for pneumonia, 315 were diagnosed with HF before their index pneumonia hospitalization and an additional 348 died (161), were lost to follow-up (1), or were diagnosed with new-onset HF (186) within the first 30 days of their pneumonia admission. Thus, there were 652 pneumonia survivors who were still free of HF diagnosis at 30 days post-hospitalization. Among these, the cumulative number of cases that experienced new-onset HF between 31 and 90 days, 91 days and 6 months, 6months and 1 year, 1 to 5 years, and >5 years post-infection were 22 (3.4%), 36 (5.5%), 56 (8.6%), 132 (20.2%), and 203 (31.1%), respectively.
Hospitalization with pneumonia was associated with increased risk of new-onset HF in the intermediate and long-term post infection. The magnitude and direction of HRs for new-onset HF after hospitalization for pneumonia were overall concordant in unadjusted analyses, adjusted analyses with covariables measured at study entry only, adjusted analyses using complete cases only (no imputed data), and adjusted analyses with the complete dataset after the imputation process and using time-updated covariables (Supplementary material, Table S3). In the latter, hazard ratios (HRs) for new-onset HF at 31 to 90 days, 91 days to 6 months, 6 months to 1 year, 1-5 years , and >5 years after pneumonia were 6.9 (95% confidence interval [CI], 4.46-10.63; p<0.001), 3.2 (95% CI, 1.88-5.50; p<0.001), 2.6 (95% CI, 1.64-4.04; p<0.001), 1.7 (95% CI, 1.30-2.12; p<0.001), and 2.0 (95% CI, 1.56-2.58; p<0.001), respectively (Figure 1). The magnitude of the association between pneumonia and intermediate and long-term risk of new-onset HF at each post-pneumonia interval was greater or similar to other well-established risk factors for HF (Supplementary material, Table S4).
Figure 1. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia.
CI denotes confidence interval. All estimates are adjusted for age, gender, race, coronary heart disease (prevalent at study entry and incident during the study), atrial fibrillation, diabetes, heart valve disease, smoking status, body mass index, heart rate, systolic and diastolic blood pressures, serum low-density lipoprotein (LDL) cholesterol, serum high-density lipoprotein (HDL) cholesterol, glomerular filtration rate, serum C-reactive protein, left ventricular hypertrophy by electrocardiogram, percent of predicted forced expiratory volume in 1 second (FEV1, as measured by spirometry), use of anti-hypertensive drugs, use of aspirin, and use of statins. With the exception of age, gender, race, prevalent coronary heart disease at study entry, and percent of predicted FEV1; all covariables that were regularly updated during study follow-up were treated as time-dependent in the model.
The HRs for new-onset HF after pneumonia did not substantially differ between severe and non-severe cases, except for the period of >5 years after pneumonia (HR, 3.4; 95% CI: 2.47 to 4.72 in severe pneumonia; and HR, 1.3; 95% CI: 0.89 to 1.86 in non-severe pneumonia; Table 2).
Table 2.
Intermediate and long-term risk of new-onset heart failure after hospitalization for severe and non-severe pneumonia.
| Time after hospitalization for pneumonia |
Severe pneumonia | Non-severe pneumonia | ||
|---|---|---|---|---|
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| 30-90 days | 6.2 | 2.74 to 13.88 | 7.2 | 4.33 to 11.85 |
| 91 days-6 months | 3.0 | 1.13 to 8.15 | 3.3 | 1.75 to 6.16 |
| 6-12 months | 1.7 | 0.64 to 4.58 | 2.9 | 1.76 to 4.83 |
| 1-5 years | 1.9 | 1.25 to 2.77 | 1.6 | 1.16 to 2.09 |
| 5 years+ | 3.4 | 2.47 to 4.72 | 1.3 | 0.89 to 1.86 |
CI denotes confidence interval.
All estimates are adjusted for age, gender, race, coronary heart disease (prevalent at study entry and incident during the study), atrial fibrillation, diabetes, heart valve disease, smoking status, body mass index, heart rate, systolic and diastolic blood pressures, serum low-density lipoprotein (LDL) cholesterol, serum high-density lipoprotein (HDL) cholesterol, glomerular filtration rate, serum C-reactive protein, left ventricular hypertrophy by electrocardiogram, percent of predicted forced expiratory volume in 1 second (FEV1, as measured by spirometry), use of anti-hypertensive drugs, use of aspirin, and use of statins. With the exception of age, gender, race, prevalent coronary heart disease at study entry, and percent of predicted FEV1; all covariables that were regularly updated during study follow-up were treated as time-dependent in the model.
Only a minority (14%) of new-onset HF events after hospitalization for pneumonia were preceded by coronary heart disease (Supplementary material, Tables S5).
Sensitivity analyses and analysis with fracture as the exposure of interest
Sensitivity analysis using the more stringent definition of pneumonia produced estimates largely consistent with our main analyses (Supplementary material, Tables S6). Sensitivity analyses also indicated robustness against informative censoring (Supplementary material, Table S7). When patients who had ≥2 episodes of pneumonia were censored at the time of their second pneumonia episode, the HRs for new-onset HF after pneumonia remained significant up to one year and >5 years post infection; however, although the HR for the 1 to 5 years post-pneumonia interval also remained elevated at 1.2, its 95% CI was not statistically significant (0.91 to 1.67; Supplementary material, Table S8).
Finally, when we repeated our main analysis with hospitalization for fractures as the exposure of interest, there was a significant increase in the risk of HF in the first 6 months post-hospitalization but no obvious association after that (Table 3). Details of the baseline characteristics of the 767 patients hospitalized with fracture in CHS and their rates and timing of new-onset HF events after hospitalization are provided in the Supplementary material, Tables S9 and S10.
Table 3.
Intermediate and long-term risk of new-onset heart failure after hospitalization for fracture.
| Time after hospitalization for fracture |
Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| 30-90 days | 2.3 | 1.09 to 5.01 | 0.029 |
| 90 days-6 months | 2.3 | 1.19 to 4.34 | 0.012 |
| 6-12 months | 1.0 | 0.48 to 2.01 | 0.960 |
| 1-5 years | 1.2 | 0.85 to 1.56 | 0.336 |
| 5 years+ | 1.2 | 0.86 to 1.72 | 0.260 |
CI denotes confidence interval.
All estimates are adjusted for age, gender, race, coronary heart disease (prevalent at study entry and incident during the study), atrial fibrillation, diabetes, heart valve disease, smoking status, body mass index, heart rate, systolic and diastolic blood pressures, serum low-density lipoprotein (LDL) cholesterol, serum high-density lipoprotein (HDL) cholesterol, glomerular filtration rate, serum C-reactive protein, left ventricular hypertrophy by electrocardiogram, percent of predicted forced expiratory volume in 1 second (FEV1, as measured by spirometry), use of anti-hypertensive drugs, use of aspirin, and use of statins. With the exception of age, gender, race, prevalent coronary heart disease at study entry, and percent of predicted FEV1; all covariables that were regularly updated during study follow-up were treated as time-dependent in the model.
DISCUSSION
In a large community-based prospective sample of elderly adults without HF at baseline, hospitalization for pneumonia was independently associated with a pronounced increase in the risk of new-onset HF in the intermediate and long-term. This association was evident for cases with both severe and non-severe pneumonia. Our results did not substantially change even when stringent case definitions for pneumonia were used or under extreme assumptions for potential informative censoring. The association between hospitalization for pneumonia and new-onset HF remained significant after removing the potential contribution from repeated episodes of pneumonia. Even in the very long-term (>5 years after hospitalization for pneumonia), the magnitude of the relative risk increase of new-onset HF associated with pneumonia was comparable to or greater than the risk associated with well-established risk factors for HF. The increase in HF risk after pneumonia hospitalization was independent of traditional risk factors including coronary heart disease and the large majority (86%) of new-onset HF events post-pneumonia were not preceded by this diagnosis. Hospitalization for fracture was also associated with increased risk of new-onset HF in the first six-months after admission; however, in contrast to pneumonia, hospitalization for fracture was not associated with HF risk in the long-term..
Previous studies have typified the immediate short-term (≤30 days) post-pneumonia as a high-risk period for the development of HF.6,25 However, to the best of our knowledge, our study is the first to describe an independent association between pneumonia and new-onset HF beyond the short-term. The strength and magnitude of this association suggests that there is an opportunity for preventing a substantial number of new-onset HF events in this population. Moreover, because pneumonia survivors have increased long-term morbidity and mortality, prevention HF post-pneumonia could also improve these outcomes. Finally, because of the burden of pneumonia in elderly adults, recognizing this association may also contribute to improve the assessment of HF risk in the general elderly population and it should be taken into account when estimating the cost and benefit of interventions (i.e. vaccination) aimed at preventing pneumonia in this population.
The mechanisms by which pneumonia may trigger HF in the short-term have been discussed elsewhere.6,25 These mechanisms are mostly explained by the acute physiological stress associated with the acute illness and also by processes and procedures related to patients’ transfer of care and inpatient management (transient interruption of stable therapeutic regimens for pre-existing cardiovascular conditions, administration of large sodium loads and/or infusion volumes from commonly used intravenous medications, transfer of care back to community primary care providers, etc.). These mechanisms are not specific to pneumonia and their effect could linger after hospitalization. This could explain the highest HRs for new-onset HF in the first few months after pneumonia hospitalization and also the higher risk in the first few months after hospitalization for fracture. However, the persistent higher risk of new-onset HF in the long-term after hospitalization for pneumonia suggests more complex biological interactions. Even when patients recover from the acute infection, pneumonia survivors seem to continue exhibiting elevated systemic inflammatory activation. This is important because chronic inflammatory activation has been implicated in the development and progression of HF in the general population.26 Although >80% of patients hospitalized for pneumonia clinically recover by one week, >50% still show heightened markers of systemic inflammation.27,28 Hansson et al29 reported mean circulating C- reactive protein (CRP) levels of 5 mg/L (95% CI, 4 to 6) in 95 pneumonia survivors (mean age 70 years) at 6 months after their infection. Levels of CRP ≥3.0 mg/L have been associated with high risk of coronary artery disease and HF in non-pneumonia settings.30,31 Moreover, elevated levels of interleukin-6 in blood at hospital discharge in pneumonia survivors are predictive of 1-year cardiovascular mortality 32. Another potential mechanism is that acute insults to the cardiovascular system that occur during the acute infection have long-lasting effect on vascular or myocardial function. A detailed autopsy study of 67 patients with lobar pneumonia showed pathological evidence of myocarditis in 39% of cases 33. Moreover, markers of myocardial cell injury (i.e. troponins) are frequently elevated at hospital presentation in patients with pneumonia without acute coronary syndromes 34. Therefore, it is possible that myocardial injury during pneumonia causes enduring decline in myocardial function. More recent animal studies also suggest that certain pneumonia-producing bacteria such as S. pneumoniae can invade the myocardium during severe infections and leave lingering foci of myocardial inflammation even when the infection has resolved after antibiotic therapy.9 In addition, infection can also induce acute inflammation of the arterial wall,35 potentially leading to lasting impairment of the elastic properties of arteries, which in turn may result in lasting increments in the pulsatile left ventricular afterload.36 Finally, acute kidney injury is common during pneumonia and its progression to chronic kidney disease could also increase HF risk.37 A more accurate characterization of these mechanisms should help informing the design and testing of any future strategies for the prevention of HF in elderly pneumonia survivors.
Strengths of this study include its large, community-based population, its prospective design, long follow-up, detailed and longitudinal evaluation of cardiovascular risk factors, and comprehensive methodology for surveillance and adjudication of incident HF events. Limitations include our reliance on hospital discharge ICD-9 codes to ascertain hospitalizations for pneumonia. Although this method has been previously validated,4,13 we performed an internal independent confirmation of its applicability to our sample,14 and completed a sensitivity analysis using a most stringent definition of pneumonia; we cannot completely rule out misclassification. Similarly, since we ascertained severity of pneumonia based on the presence specific ICD-codes in discharge abstracts, and although this strategy has also been formerly validated,15 potential misclassification is possible. Because we only ascertained index (first-time) hospitalizations for pneumonia, our analyses did not account for the potential cumulative effect of recurrent pneumonia events. In CHS, baseline abnormalities in participants’ LV function were uncommon38 and this did not allow for meaningful stratified analyses by this factor. In addition, we did not have information regarding left-ventricular function at the time of diagnosis of HF events and therefore, we could not explore differential effects of pneumonia on new-onset HF with preserved or reduced ejection fraction. Finally, because our analyses were restricted to individuals over age 65 and cases of pneumonia requiring hospital admission, our results should not be generalized beyond those boundaries.
CONCLUSION
In this large community-based sample of elderly adults, hospitalization for pneumonia was associated with subsequent increase in the risk of new-onset HF in the intermediate and long-term post-infection. Future studies are needed to clearly elucidate the mechanisms of this association in order to design targeted preventive strategies.
Supplementary Material
Acknowledgements
CHS was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Dr. Corrales-Medina is supported by a Recruitment Award from the Department of Medicine of The Ottawa Hospital and the Ottawa Hospital Research Institute in Ottawa, Ontario, Canada.
Dr. Yende is supported by K23GM083215.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of Investigators
V.F.C-M., M.T., S.Y., R.K., G.D.; M.S.V.E. and J.A.C. designed the study; the data was gathered by R.K.; A.B.N. and M.F.L.; M.T. performed the statistical analyses; V.F.C-M., M.T. and J.A.C. vouch for the data and analysis; all authors provided critical input to the interpretations of the results; V.F.C-M. wrote the first manuscript draft; all authors approved the final version. The authors had full access to data and full control of the decision to publish.
Financial Disclosures or Conflicts of Interest
None.
References
- 1.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeb M. Community-acquired pneumonia. Clin Evid (Online) 2010 [Google Scholar]
- 3.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;26:1–20. 24. [PubMed] [Google Scholar]
- 4.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 5.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008;5:1–20. [PubMed] [Google Scholar]
- 6.Corrales-Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 7.Corrales-Medina VF, Suh KN, Rose G, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone J, Eurich DT, Majumdar SR, Jin Y, Marrie TJ. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine (Baltimore) 2008;87:329–334. doi: 10.1097/MD.0b013e318190f444. [DOI] [PubMed] [Google Scholar]
- 9.Brown AO, Mann B, Gao G, et al. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog. 2014;10:e1004383. doi: 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 11.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 12.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 13.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003-2009. JAMA. 2012;307:1405–1413. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 14.Yende S, Alvarez K, Loehr L, et al. Epidemiology and Long-term Clinical and Biologic Risk Factors for Pneumonia in Community-Dwelling Older Americans: Analysis of Three Cohorts. Chest. 2013;144:1008–1017. doi: 10.1378/chest.12-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 17.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 18.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Multiple Imputation for Nonresponse in Surveys. J. Wiley & Sons; New York: 1987. [Google Scholar]
- 20.Allison PD. Survival Analysis Using SAS: A Practical Guide. Second North Carolina SAS Press; Cary: 2010. [Google Scholar]
- 21.van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol. 2012;8:163–172. doi: 10.1038/nrrheum.2011.217. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68:1236–1242. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 23.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248–1250. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 25.Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125:773–781. doi: 10.1161/CIRCULATIONAHA.111.040766. [DOI] [PubMed] [Google Scholar]
- 26.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 27.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–1457. doi: 10.1001/jama.279.18.1452. [DOI] [PubMed] [Google Scholar]
- 29.Hansson LO, Hedlund JU, Ortqvist AB. Sequential changes of inflammatory and nutritional markers in patients with community-acquired pneumonia. Scand J Clin Lab Invest. 1997;57:111–118. doi: 10.1080/00365519709056378. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 31.Araujo JP, Lourenco P, Azevedo A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Card Fail. 2009;15:256–266. doi: 10.1016/j.cardfail.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Yende S, D'Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saphir O, Amromin GD. Myocarditis in instances of pneumonia. Ann Intern Med. 1948;28:963–970. doi: 10.7326/0003-4819-28-5-963. [DOI] [PubMed] [Google Scholar]
- 34.Chang CL, Mills GD, Karalus NC, et al. Biomarkers of cardiac dysfunction and mortality from community-acquired pneumonia in adults. PLoS One. 2013;8:e62612. doi: 10.1371/journal.pone.0062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madjid M, Vela D, Khalili-Tabrizi H, Casscells SW, Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34:11–18. [PMC free article] [PubMed] [Google Scholar]
- 36.Chirinos JA, Kips JG, Jacobs DR, Jr., et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2012;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mujib M, Desai R, Levitan EB, et al. Prospective population studies of incident heart failure without data on baseline left ventricular ejection fraction. Arch Med Sci. 2010;6:686–688. doi: 10.5114/aoms.2010.17081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.