Abstract
Objectives
The objectives were to review the discrepancy between numbers of people requiring weight loss treatment and results, and to assess the potential effects of pharmacological treatments (recently approved for obesity) and endoscopically deployed devices on quantitative gastrointestinal traits in development for obesity treatment.
Methods
We conducted a review of relevant literature to achieve our objectives.
Results
The 2013 guidelines increased the number of adults recommended for weight loss treatment by 20.9% (116.0 million to 140.2 million). There is an imbalance between efficacy and costs of commercial weight loss programs and drug therapy (average weight loss ~5 kg). The number of bariatric procedures performed in the United States has doubled in the past decade. The efficacy of bariatric surgery is attributed to reduction in the volume of the stomach, nutrient malabsorption with some types of surgery, increased postprandial incretin responses, and activation of farnesoid X receptor mechanisms. These gastrointestinal and behavioral traits identify sub-phenotypes of obesity based on recent research.
Conclusions
The mechanisms or traits targeted by drug and device treatments include centrally mediated alterations of appetite or satiation, diversion of nutrients, and alteration of stomach capacity, gastric emptying, or incretin hormones. Future treatment may be individualized based on quantitative gastrointestinal and behavioral traits measured in obese patients.
Keywords: obesity, medical devices, pharmacotherapy, gastrointestinal traits, behavioral traits
Introduction
Obesity is a complex chronic disease, which results from weight gain secondary to prolonged positive energy balance, that is, greater food intake over energy expenditure. The complexity of obesity goes beyond food intake, and this review does not address the hedonic aspects of energy intake or expenditure, or the strictly behavioral approaches to obesity therapy.
Compared with the 1998 guidelines, the 2013 guidelines (that were based on the National Health and Nutrition Examination Survey 2007-2012) increased the number of adults recommended for weight loss treatment by 20.9% from 116.0 million to 140.2 million, making 64.5% of non-pregnant, non-institutionalized U.S. adults candidates for weight loss treatment.1,2 The 2013 guidelines recommended treatment for a larger proportion of overweight people having only one risk factor or having a large waist circumference. With these recommendations, up to 53.4% of adults could be considered for pharmacologic therapy in addition to life style therapy, and up to 14.7% could be considered for bariatric surgery.
A recent review of 45 studies (of which 39 were randomized, controlled trials) involving commercial weight loss programs based on diet and behavioral modification showed that, at 12 months, the commercial diets achieved greater weight loss than control/education and counseling: Weight Watchers by >2.6%, Jenny Craig >4.9%, and very-low-calorie programs (e. g. Medifast and OPTIFAST) >4.0% (latter with some attenuation of effect beyond 6 months).3 These commercial programs incorporate group sessions (eg, Weight Watchers) or more expensive 1-on-1 counselling.
Despite the approval of novel pharmacological therapies for weight loss through medications that suppress patients’ appetites and make them feel full, there is a perception that the public and physicians have not embraced the opportunity to use these medications. Factors leading to the decision not to use medications for obesity include the safety issues with past diet drugs, significant costs or co-pays, and physician propensity to wait a year or longer after approval of each new diet drug before prescribing it, to allow for unforeseen safety issues to emerge.4
There is also moderate overall average weight loss of 3 to 8 kg after at least 12 weeks of treatment with the novel pharmacological agents: lorcaserin, GLP-1 agonists, phentermine-topiramate and bupropion-naltrexone.5-9 There is, thus, an imbalance between the perceived clinical need for weight loss, and the average efficacy and costs of commercial weight loss programs and drug therapy. There is a need for novel approaches to individualize therapy and enhance benefit to risk ratio with pharmacological approaches or therapeutic devices in development.
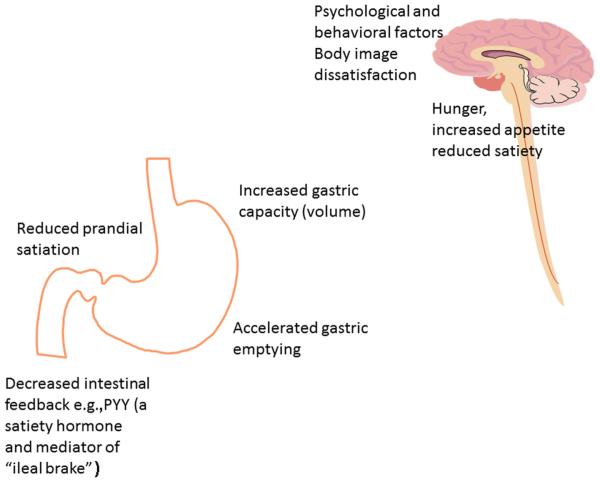
There has been significant progress in understanding the regulation of food intake by the brain gut axis and the central and peripheral regulation of appetite, and neural responses to macronutrients.10,11 The key principles of obesity pathophysiology are illustrated in Figure 1. However, current therapy is still based on the assumption that one-treatment-fits-all in obesity; this approach may explain the highly variable response to treatment with current pharmacological approaches.
Figure 1.
Summary of key factors involved in obesity pathophysiology.
A testable hypothesis is that actionable quantitative traits in obesity may constitute more specific therapeutic targets and may predict enhanced weight loss in individual patients with obesity. Recently, we have identified quantitative traits12 that are regulated by the brain-gut axis, can be measured reliably in humans, and provide “actionable” approaches to control food intake. In a proof of concept, small, randomized, controlled trial, we demonstrated that phentermine-topiramate extended release resulted in significantly greater weight loss in patients who ingested >900 kcal at an ad libitum buffet meal.12
The objectives of this review are to examine “actionable” quantitative traits in the regulation of food intake in obesity; to identify pharmacological approaches that may be directed to these actionable traits in obesity; and to examine how the devices proposed for the treatment of obesity are directed to these traits, supporting the critical role of these actionable mechanisms in the treatment of obesity.
Food Intake: The Brain-Gut Axis
Food intake is regulated by a balance among appetite, satiation, and satiety, which is controlled by the brain-gut axis, whereby gastrointestinal signals inform brain centers about energy intake status (reviewed elsewhere13). Peripheral mechanisms in food intake regulation include the motor and sensory functions of the stomach such as the rate of emptying and gastric volume and accommodation, which result in signals of appetite in response to fasting and satiation in response to calorie and volume ingestion. In addition, the rich repertoire of peripherally released peptides and hormones (such as ghrelin, motilin, cholecystokinin, GLP-1, peptide YY and oxyntomodulin) provides feedback from the arrival of nutrients in different regions of the gut from where they are released to exert effects on satiation or regulate metabolism through their incretin effects. Ultimately, these peripheral factors provide input to the highly organized hypothalamic and vagal centers to influence energy intake during meal ingestion and the return of appetite and hunger during fasting. These peripheral mechanisms are discussed in greater detail elsewhere.10
Hypothalamic centers, involving several peptide receptors, also control food intake. The arcuate nucleus receives input from brainstem (eg, vagal) nuclei and from circulating hormones through an incomplete blood brain barrier. Neurons in the arcuate nucleus are either orexigenic [eg, contain neuropeptide Y via Y1 receptors or agouti-related peptide (AgRP)] or anorexigenic [eg, contain pro-opiomelanocortin (POMC)], cocaine- and amphetamine-related transcript (CART)]. POMC is a precursor of α melanocyte stimulating hormone (α-MSH). Ultimately, other regions of the hypothalamus (the paraventricular nucleus and lateral hypothalamus) and higher centers (such as amygdala, limbic system and cerebral cortex) are stimulated to influence the same hypothalamic nuclei to change feeding behavior.
Gastric Determinants of Postprandial Symptoms and Satiation
Satiation is the sense of feeling full during a meal, which induces meal termination; satiety is the degree of fullness that persists until the consumption of a subsequent meal after a period of fasting14,15 and regulates meal frequency, which is also influenced by learned habits. Satiation is appraised by the volume to fullness and maximum tolerated volume of Ensure nutrient drink (1 kcal/mL) ingested at a rate of 30 kcal/minute.16 Satiety is appraised by the total calorie intake at an ad libitum buffet meal17 after a standard period of fasting (eg, 4 hours) and a standard prior meal (eg, a 300 kcal liquid breakfast meal).
Studies based on hundreds of patients with dyspepsia convincingly showed that gastric motor functions (such as emptying and accommodation) and gastric sensation are important determinants of intra-prandial and postprandial symptoms.18 In studies conducted in patients with functional dyspepsia and in obese patients (in response to pharmacological perturbations) in a laboratory setting, it was demonstrated that, apart from body mass index (BMI), age, and gender, the following factors account for about 50% of the variance in postprandial symptoms such as bloating and satiation: smaller fasting gastric volume, accelerated gastric emptying at 1 hour, and delayed gastric emptying at 4 hours.19,20
These studies provided data to consider a role of the stomach and other gastrointestinal factors in association with brain centers such as the hypothalamus and vagal nuclei in the control of calorie intake. Some prior studies had indicated there were abnormalities of function, such as increased gastric volume, but these were only demonstrated in patients with binge eating disorder.21 Thus, the functions of the stomach in particular have not been systematically evaluated in overweight or obese patients until relatively recently.
Quantitative Gastrointestinal and Behavioral Traits in Obesity
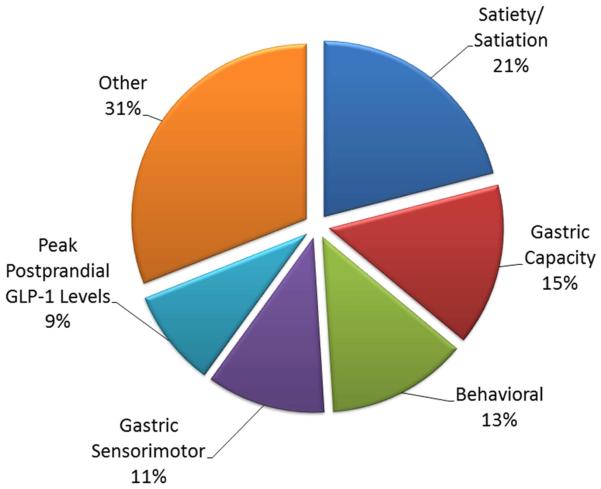
In a study involving 507 overweight, obese, or normal-weight participants,12 obesity was associated with larger fasting gastric volume, accelerated gastric emptying of solids and liquids, lower postprandial levels of one of the satiation-associated hormones, peptide tyrosine tyrosine (PYY), and higher volume of liquid calories ingested to achieve comfortable postprandial fullness and larger calorie intake in a buffet meal. Principal component analysis identified latent dimensions that accounted for approximately 81% of the variation among overweight and obese subjects, including satiety or satiation (21%), gastric motility (14%), behavioral factors (13%), and gastric sensorimotor factors (11%) (Fig. 2).
Figure 2.
Principal components analysis demonstrating the quantitative traits that contribute to obesity. Data derived from reference 12 (Acosta A, Camilleri M, et al. Gastroenterology 2015).
It was proposed that these quantitative traits may be specifically targeted by approved pharmacotherapy. Thus, the combination of phentermine and topiramate extended released caused significant weight loss, slowed gastric emptying, and decreased calorie intake; importantly, a baseline abnormal satiety test in the form of an ad libitum meal significantly predicted weight loss response to phentermine and topiramate extended released.12
These data suggested that quantitative traits are associated with high body mass index or high waist circumference; they can distinguish different obesity phenotypes and may serve as traits to individualize pharmacotherapy for obesity.
Lessons Learned from Bariatric Surgery
The number of bariatric procedures performed in the United States has virtually doubled in the last decade to about 180,000 operations per year, and the most frequently performed operations are Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy.22 At present, the best evidence for the validity of quantitative gastrointestinal and behavioral traits of obesity as “actionable” traits is based on the efficacy of bariatric surgery. Specifically, with partial gastrectomy in the RYGB procedure or vertical sleeve gastrectomy, there is early reduction in the volume of the stomach, induction of early satiation, and increased postprandial AUC GLP-1 response. These are 3 of the actionable quantitative traits because they are associated with obesity.12 In addition, some bariatric procedures divert nutrients and result in malabsorption, such as RYGB and biliopancreatic diversion with duodenal switch. These procedures also are associated with correction of glycemia even before the benefits of weight loss, and these effects are in part attributed to the earlier and higher postprandial incretin responses,23 to activation of farnesoid X receptor mediated mechanisms,24 and to changes in bile acids and microbiome.25
In addition to effects on weight, the long-term effects of bariatric surgery on health status and morbidity related to obesity are well documented and include long-term remission of type 2 diabetes mellitus (T2DM) with micro- and macro-vascular adverse events,26 more efficient than usual care in the prevention of T2DM in obese persons,27 better diabetes control with RYGB than medical care,28 better 3-year outcomes (such as HbA1c, BMI, number of medications for diabetes) with RYGB and vertical sleeve gastrectomy compared with intensive medical therapy for diabetes,29 lower unadjusted cumulative incidence of fatal and total cardiovascular events,30 improvement of cardiovascular risk factors with bilio-pancreatic diversion,31 reduced cancer incidence particularly in women, diabetics, and smokers,32 and reduced transminases proportionate to weight loss in patients with non-alcoholic steatohepatitis.33
The objectives of non-surgical weight loss therapies should include impact on the comorbidity of obesity in addition to effects on body weight.
Traits Associated with Food Intake, Postprandial Symptoms, and Obesity
Prior studies identified quantitative traits that are associated with postprandial symptoms and food intake. One example is intragastric pressure,34 but this requires invasive measurement with an intragastric manometric or barostatic sensor device. Our prospective studies12 identified the following quantitative traits in the brain-gut axis that are involved in food intake regulation and represent latent dimensions that contribute to the obesity trait, based on a principal components analysis (Fig. 2):
1) Appetite: Individuals with obesity have reported increased appetite (measured by visual analog scores or higher fasting plasma ghrelin levels);
2) Enlarged fasting gastric volume [measured by single photon emission computed tomography (SPECT)];
3) Reduced gastric accommodation (by SPECT, after a 300 kcal liquid nutrient meal);
4) Accelerated gastric emptying of solids and liquids (by scintigraphy of a dual radiolabeled meal);
5) Decreased satiation (assessed as an increased calorie intake to sensation of fullness by a laboratory-based test measuring liquid nutrient intake at constant rate of 30 mL/min);
6) Decreased satiety (assessed as an increased calories intake by ad-libitum meal 4 hours after standard breakfast);
7) Decreased peak postprandial GLP-1;
8) Eating behaviors and affect, based on validated questionnaires.
In our patient cohort, these gastrointestinal “actionable” traits were largely independent of the behavioral traits. Indeed, among 169 patients with obesity, there were single trait abnormalities in subsets of the patients: 22 patients with exclusive acceleration of gastric emptying (GE T1/2 <85 minutes), 29 patients with fasting gastric volume >300 mL, and 24 patients with abnormal satiation (based on volume to fullness >800 mL [kcal] or >800 kcal ingested at buffet meal).12 In addition, 15 patients had large gastric volume and abnormal satiation/satiety, and another 15 patients had accelerated gastric emptying and abnormal satiation/satiety. Thus, in almost two-thirds of obese patients in our cohort, there was either unique or a combination of two alterations in quantitative traits that could constitute targets for therapy,
What Pharmacotherapies Might Be Directed at Different “Actionable” Obesity Traits?
Different “actionable” obesity traits have been identified based on quantifiable traits that differ in obesity compared with normal weight. These lead to hypotheses that can be tested in therapeutic trials and which should appraise patients with the abnormal function specified compared with patients with normal function.
Patients with accelerated gastric emptying: The class of amylin agonists (eg, pramlintide35) or GLP-1 agonists (eg, liraglutide, exenatide) might be preferred for this group of patients, as they retard gastric emptying.36-38
Patients with enlarged gastric volume: Ghrelin or motilin agonists may be preferred as they reduce gastric accommodation39,40; however, ghrelin agonists may not be the preferred class of medications because they increase appetite.41 The opioid antagonist, naltrexone, reduces gastric accommodation42; however, it is unclear whether the combination bupropion/naltrexone sustained released has any effect on gastric volume and accommodation. In contrast, GLP-1 agonists, which enhance gastric volume and postprandial accommodation,36 may not be the preferred medications for patients with enlarged gastric volume.
Patients with prominent psychological/depression scores: These patients may be amenable to treatment with centrally acting medications that may also impact affect, such as lorcaserin (a selective serotonin 5-HT2C agonist which activates hypothalamic centers to reduce food intake) or the combination of bupropion and naltrexone. The mechanisms of action of these medications are not completely understood; however, they have proven efficacious in reducing body weight. Bupropion appears to reduce food intake by acting on adrenergic and dopaminergic receptors in the hypothalamus, whereas naltrexone is an opioid receptor antagonist with minimal effect on weight loss that might block inhibitory influences of opioid receptors activated by the β-endorphin that is released in the hypothalamus and stimulates feeding.43 Although lorcaserin and bupropion/naltrexone may have an effect on satiation, their main effects may be mediated through higher brain centers that regulate feeding behavior and cravings.
Patients with low levels of satiation or satiety: Centrally acting medications that act on the hypothalamic nuclei of satiation, such as phentermine/topiramate ER, may be the drugs selected for patients with low levels of satiation or satiety who tend to consume more calories. Phentermine acts to reduce appetite through increasing norepinephrine in the hypothalamus, and topiramate may reduce appetite through its effect on γ-aminobutyric acid (GABA) receptors. Interestingly, phentermine/topiramate ER produced a decrease of 160 kcal in a satiety test, and individuals with abnormal satiation (who consumed more than 800 kcal before reaching “usual fullness” or 1400 kcal before reporting “maximal tolerated volume”) lost twice as much weight in a pilot 2-week trial compared with obese individuals with normal satiation.12
Novel Devices Proposed in Obesity Treatment and their Potential Effects on Gastrointestinal Traits Associated with Obesity
In general, the devices proposed or in development for treatment of obesity can be grouped into 3 categories: those that divert nutrients, those that occupy space in the stomach, and those that alter gastric emptying or capacity. The principal findings on efficacy and mechanisms of action will be described briefly here (extensively reviewed elsewhere44).
Devices that divert nutrients
AspireAssist Aspiration Therapy System essentially diverts food out of the stomach after a time elapsed in the postprandial period; this results in diversion of food away from absorption in the small bowel, and mean weight loss of 15 kg is reported at 6 months. Adverse effects arise from electrolyte losses such as reduced serum sodium and potassium; on the other hand, blood glucose and HbA1C are improved.45 This device clearly impacts those patients whose calorie consumption cannot be reduced in any other way.
DuodenoJejunal Bypass Liner mimics Roux-en-Y gastric bypass, inducing weight loss and improved metabolic control through malabsorption and release of incretin hormones (GLP-1, PYY), with improved glycemic control and delay in gastric emptying.46 This device delivers food to the jejunum and ileum without proper mixing with pancreaticobiliary juices, delays gastric emptying,46 and may stimulate the release of GLP-1,47 with resulting weight loss and improved control of type 2 diabetes.48
Devices that occupy space in the stomach
We have shown that the proximal stomach is not the only part of the organ that has capacity to store food; the antrum also accommodates food,49 and it appears that gastric capacity interventions must include antrum to be effective in the long term. This may explain the disappointing results with surgical vertical banded gastroplasty or gastric banding, or the endoscopic transoral gastroplasty (TOGA).
In 12-month follow-up of the TRIM trial of TOGA conducted at 2 centers, gastric volume reduction procedure using the RESTORe Suturing System device proved to be safe, well tolerated, and technical success was achieved. However, modest decreases in weight, BMI, and waist circumference were observed and, despite some overall positive clinical findings, the applications were not durable, and the effects of the procedure varied widely among the study participants.50
Different balloon devices are in development,51 and they may induce fullness, induce symptoms such as discomfort or nausea that reduce food intake, or accelerate or delay gastric emptying. Examples are the transpyloric shuttle which consists of a large spherical bulb connected to a smaller cylindrical bulb by a flexible tether that passes freely into the duodenum to position the device across the pylorus; this may delay gastric emptying, reducing caloric intake and enabling weight loss. It is associated with nausea, vomiting, pain, and GERD, especially in the first 30 days after deployment.52
A second example is the ReShape Intragastric Duo-Balloon, which attempts to occupy space in proximal and distal stomach, and results in weight loss with shorter duration (<7 days) of symptoms such as nausea, vomiting and retching.53
A third example is a balloon device which can be left in the stomach for >6 months and results in weight loss with an average reduction of 4.5 kg/m2. 54 After first removal, this type of balloon has been repeatedly placed into the stomach and resulted in cycling loss of weight that was regained after removal; however, the beneficial effects on type 2 diabetes mellitus, blood pressure, and obstructive sleep apnea are retained over time, despite “cycling” weight regain.54
It is currently assumed that the action of these occupying space devices placed in the stomach will limit the size of the stomach available to accommodate food and result in satiation with lower volume intake. There is some evidence that such devices, as balloons, may delay gastric emptying and reduce plasma ghrelin,55 although this effect on ghrelin is not consistently demonstrated at different times after insertion of the device.56,57
Devices that alter gastric emptying or capacity
Delay in gastric emptying results in increased satiation and, potentially, in cessation of food intake. Examples include reversible vagal block (VBLOC), antral injection of botulinum toxin (BOTOX), and endoscopic sleeve gastroplasty.
VBLOC was efficacious in retarding gastric emptying, inducing earlier satiation, and reducing hunger, which resulted in weight loss, especially in patients in whom there was effective vagal inhibition demonstrated by inhibition of the increase in plasma pancreatic polypeptide in response to sham feeding.58 VBLOC was associated with significantly greater weight loss compared with sham block;59 in addition, there are other beneficial effects of vagal block that may be independent of weight loss, such as a 1% mean reduction in HbA1c at 12 months of treatment compared with baseline, mean 28 mg/dL reduction in fasting blood glucose in obese patients with type 2 diabetes mellitus, and reduced blood pressure among those with hypertension.60 The beneficial effect on glycemia is incompletely understood, although, in rodents, interruption of hepatic afferent vagal pathways prevented steroid-induced insulin resistance shown, for example, by the reduced hepatic glucose production and improved glucose tolerance on challenge with dexamethasone.61
Gastric antral injection of BOTOX appeared promising, as it also delayed gastric emptying and induced satiation in an open-label trial62; however, in a 4-month trial, despite documentation of delay in gastric emptying, there was no significant reduction in body weight.63 A recently published meta-analysis and meta-regression of 4 randomized, controlled trials evaluating antral BOTOX injection suggested overall benefit (Hedges’ g −0.521; 95% CI, −0.845 to −0.040, P = .031), but 3 of the 4 trials were individually nonsignificant. In addition, wide-area injection was associated with weight loss (Hedges’ g, −0.890; 95% CI, −1.522 to −0.258; P = .006), though antrum only injection did not show significant efficacy (Hedges’ g, −0.192; 95% CI, −0.788 to 0.404; P = .528). In the overall analysis, there was considerable heterogeneity (I2 55.5%) among studies.64
Gastric electrical stimulation may induce antegrade or retrograde stimulation. Overall, results with several devices are associated with variable degrees of weight loss even with the same device.65 The mechanisms of action include changes in gastric emptying or gastric accommodation and induction of satiety.66,67
Endoscopic sleeve gastroplasty results in significant weight loss68 which is thought to result from reduced gastric capacity, inducing fullness (early satiation) and, possibly, altered gastric emptying. Based on effects of surgical vertical sleeve gastrectomy,69 it is expected that there will be acceleration of gastric emptying. Results of 1-year follow-up and effects on satiation and gastric emptying were preliminarily reported with >50% reduction in maximum tolerated volume in a satiation drink test and about 80% increase in gastric emptying t1/2.70
Adverse effects from devices and future needs
There are adverse effects associated with deployment of the endoscopic devices for obesity which are not addressed in great detail here. The adverse effects include those that are associated with procedures such as the potential for leaks leading to intra-abdominal abscess associated with gastroplasty involving deep suturing; leaks around devices associated with aspiration; mucosal irritation by intragastric balloons; dumping syndrome or gastric retention associated with changes in gastric capacity or motor function; and malabsorption of nutrients as a result of dumping or a duodenal by-pass liner. However, experience with the current devices proves the principle of efficacy and should encourage the development of safer and more readily available devices.
Conclusion
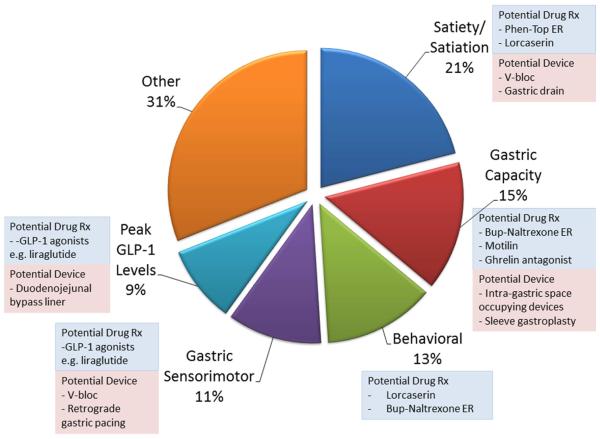
Understanding different obesity phenotypes, which may be relatively easily measured with gastric emptying, satiation and satiety assessments with validated tests, provides opportunity to personalize treatment of obesity using both pharmacotherapies and devices, as summarized in Figure 3. Although further validation is clearly necessary, these insights lead to the conclusion that gastroenterologists have a pivotal role to play in the future treatment of patients with obesity.
Figure 3.
Potential application of medications and devices directed at quantitative traits associated with obesity.
Acknowledgments
Grant support: R01-DK67071 from National Institutes of Health to Dr. Camilleri
Abbreviations used
- GLP1
glucagon-like peptide 1
- AgRP
agouti-related peptide
- POMC
pro-opiomelanocortin
- CART
cocaine- and amphetamine-related transcript
- α-MSH
α melanocyte stimulating hormone
- PYY
peptide tyrosine tyrosine
- RYGB
Roux-en-Y bypass
- T2DM
type 2 diabetes mellitus
- TOGA
transoral gastroplasty
- VBLOC®
vagal block
- BOTOX®
botulinum toxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Camilleri has served as an advisor to Enteromedics (St. Paul, MN) and ReShape Medical (San Clemente, CA). Dr. Acosta has ownership interest in Gila Therapeutics, Inc. (Rochester, MN).
Author contributions: Dr. Camilleri: article concept; acquisition of data; co-authored the article
Dr. Acosta: acquisition of data; co-authored the article
References
- 1.Stevens J, Oakkar EE, Cui Z, et al. US adults recommended for weight reduction by 1998 and 2013 obesity guidelines, NHANES 2007-2012. Obesity (Silver Spring) 2015;23:527–531. doi: 10.1002/oby.20985. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Donato KA, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22(Supplement 2):S1–S410. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 3.Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. 2015;162:501–512. doi: 10.7326/M14-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. http://www.wsj.com/articles/weight-loss-drugs-seek-acceptance-from-patients-and-physicians-1426526252.
- 5.Bray GA. Medical treatment of obesity: the past, the present and the future. Best Pract Res Clin Gastroenterol. 2014;28:665–684. doi: 10.1016/j.bpg.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015;313:1213–1214. doi: 10.1001/jama.2015.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilsbøll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond) 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri M. Peripheral mechanisms in appetite regulation. Gastroenterology. 2015;148:1219–1233. doi: 10.1053/j.gastro.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulloch AJ, Murray S, Vaicekonyte R, et al. Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterology. 2015;148:1205–1218. doi: 10.1053/j.gastro.2014.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148:537–546. doi: 10.1053/j.gastro.2014.11.020. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta A, Abu Dayyeh BK, Port JD, et al. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut. 2014;63:687–695. doi: 10.1136/gutjnl-2013-306235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blundell J, Rogers P, Hill A. Evaluating the satiating power of foods: implications for acceptance and consumption. In: Solms J, Booth DA, Pangborn RM, Raunhardt O, editors. Food Acceptance and Nutrition. Academic Press; London: 1987. pp. 205–219. [Google Scholar]
- 15.Gibbons C, Finlayson G, Dalton M, et al. Metabolic Phenotyping Guidelines: studying eating behaviour in humans. J Endocrinol. 2014;222:G1–G12. doi: 10.1530/JOE-14-0020. [DOI] [PubMed] [Google Scholar]
- 16.Chial HJ, Camilleri C, Delgado-Aros S, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil. 2002;14:249–253. doi: 10.1046/j.1365-2982.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Tack J, Bisschops R. Mechanisms underlying meal-induced symptoms in functional dyspepsia. Gastroenterology. 2004;127:1844–1847. doi: 10.1053/j.gastro.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and post-meal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Delgado-Aros S, Camilleri M, Castillo EJ, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacological study. Clin Gastroenterol Hepatol. 2005;3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 21.Geliebter A, Yahav EK, Gluck ME, et al. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. 2004;81:735–740. doi: 10.1016/j.physbeh.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 22. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
- 23.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 24.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghow R. Ménage-à-trois of bariatric surgery, bile acids and the gut microbiome. World J Diabetes. 2015;6:367–370. doi: 10.4239/wjd.v6.i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311:2297–2304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 27.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 28.Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 31.Piché MÈ, Martin J, Cianflone K, et al. Changes in predicted cardiovascular disease risk after biliopancreatic diversion surgery in severely obese patients. Metabolism. 2014;63:79–86. doi: 10.1016/j.metabol.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 33.Burza MA, Romeo S, Kotronen A, et al. Long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study. PLoS One. 2013;8(3):e60495. doi: 10.1371/journal.pone.0060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen P1, Verschueren S, Tack J. Intragastric pressure as a determinant of food intake. Neurogastroenterol Motil. 2012;24:612–615. doi: 10.1111/j.1365-2982.2012.01911.x. [DOI] [PubMed] [Google Scholar]
- 35.Samsom M, Szarka LA, Camilleri M, et al. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol. 2000;278:G946–951. doi: 10.1152/ajpgi.2000.278.6.G946. [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Aros S, Kim D, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested and postprandial symptoms in humans. Am J Physiol. 2002;282:G424–G431. doi: 10.1152/ajpgi.2002.282.3.G424. Y. [DOI] [PubMed] [Google Scholar]
- 37.Cervera A, Wajcberg E, Sriwijitkamol A, et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab. 2008;294:E846–E852. doi: 10.1152/ajpendo.00030.2008. [DOI] [PubMed] [Google Scholar]
- 38.van Can J, Sloth B, Jensen CB, et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:784–793. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuomo R, Vandaele P, Coulie B, et al. Influence of motilin on gastric fundus tone and on meal-induced satiety in man: role of cholinergic pathways. Am J Gastroenterol. 2006;101:804–811. doi: 10.1111/j.1572-0241.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 41.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 42.Janssen P1, Pottel H, Vos R, et al. Endogenously released opioids mediate meal-induced gastric relaxation via peripheral mu-opioid receptors. Aliment Pharmacol Ther. 2011;33:607–614. doi: 10.1111/j.1365-2036.2010.04557.x. [DOI] [PubMed] [Google Scholar]
- 43.Bray GA, Ryan DH. Update on obesity pharmacotherapy. Ann NY Acad Sci. 2014;1311:1–13. doi: 10.1111/nyas.12328. [DOI] [PubMed] [Google Scholar]
- 44.ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee. Abu Dayyeh BK, Edmundowicz SA, et al. Endoscopic bariatric therapies. Gastrointest Endosc. 2015;81:1073–1086. doi: 10.1016/j.gie.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Forssell H, Norén E. A novel endoscopic weight loss therapy using gastric aspiration: results after 6 months. Endoscopy. 2015;47:68–71. doi: 10.1055/s-0034-1378097. [DOI] [PubMed] [Google Scholar]
- 46.de Moura EG, Lopes GS, da Costa Martins B, et al. Effects of duodenal-jejunal bypass liner (EndoBarrier®) on gastric emptying in obese and type 2 diabetic patients. Obes Surg. 2015 Feb 18; doi: 10.1007/s11695-015-1594-x. [Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 47.de Jonge C, Rensen SS, Verdam FJ, et al. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes Surg. 2013;23:1354–1360. doi: 10.1007/s11695-013-0921-3. [DOI] [PubMed] [Google Scholar]
- 48.Koehestanie P, de Jonge C, Berends FJ, et al. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann Surg. 2014;260:984–992. doi: 10.1097/SLA.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 49.Bouras EP, Delgado-Aros S, Camilleri M, et al. SPECT imaging of the stomach: comparison with barostat and effects of sex, age, body mass index, and fundoplication. Gut. 2002;51:781–786. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brethauer SA, Chand B, Schauer PR, et al. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis. 2012;8:296–303. doi: 10.1016/j.soard.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Abu-Dayyeh BK, Sarmiento R, Rajan E, et al. Endoscopic treatments of obesity and metabolic disease: are we there yet? Rev Esp Enferm Dig. 2014;106:467–476. [PubMed] [Google Scholar]
- 52.Marinos G, Eliades C, Raman Muthusamy V, et al. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg Obes Relat Dis. 2014;10:929–934. doi: 10.1016/j.soard.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Ponce J, Quebbemann BB, Patterson EJ. Prospective, randomized, multicenter study evaluating safety and efficacy of intragastric dual-balloon in obesity. Surg Obes Related Dis. 2013;9:290–295. doi: 10.1016/j.soard.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Genco A, Maselli R, Cipriano M, et al. Long-term multiple intragastric balloon treatment--a new strategy to treat morbid obese patients refusing surgery: prospective 6-year follow-up study. Surg Obes Relat Dis. 2014;10:307–311. doi: 10.1016/j.soard.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 55.Mion F, Napoléon B, Roman S, et al. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes Surg. 2005;15:510–516. doi: 10.1381/0960892053723411. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Brocca MA, Belda O, Parejo J, et al. Intragastric balloon-induced satiety is not mediated by modification in fasting or postprandial plasma ghrelin levels in morbid obesity. Obes Surg. 2007;17:649–657. doi: 10.1007/s11695-007-9109-z. [DOI] [PubMed] [Google Scholar]
- 57.Mion F, Gincul R, Roman S, et al. Tolerance and efficacy of an air-filled balloon in non-morbidly obese patients: results of a prospective multicenter study. Obes Surg. 2007;17:764–769. doi: 10.1007/s11695-007-9141-z. [DOI] [PubMed] [Google Scholar]
- 58.Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 59.Ikramuddin S, Blackstone RP, Brancatisano A, et al. Effect of reversible intermittent intra-abdominal vagal nerve blockade on morbid obesity: the ReCharge randomized clinical trial. JAMA. 2014;312:915–922. doi: 10.1001/jama.2014.10540. [DOI] [PubMed] [Google Scholar]
- 60.Shikora S, Toouli J, Herrera MF, et al. Vagal blocking improves glycemic control and elevated blood pressure in obese subjects with type 2 diabetes mellitus. J Obes. 2013;2013:245683. doi: 10.1155/2013/245683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernal-Mizrachi, Xiaozhong L, Yin L, et al. An afferent vagal nerve pathway links hepatic PPARalpha activation to glucocorticoid-induced insulin resistance and hypertension. Cell Metab. 2007;5:91–102. doi: 10.1016/j.cmet.2006.12.010. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Topazian M, Camilleri M, De La Mora-Levy J, et al. Endoscopic ultrasound-guided gastric botulinum toxin injections in obese subjects: a pilot study. Obes Surg. 2008;18:401–407. doi: 10.1007/s11695-008-9442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Topazian M, Camilleri M, Enders FT, et al. Gastric antral injections of botulinum toxin delay gastric emptying but do not reduce body weight. Clin Gastroenterol Hepatol. 2013;11:145–150. doi: 10.1016/j.cgh.2012.09.029. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bang CS, Baik GH, Shin IS, et al. Effect of intragastric injection of botulinum toxin A for the treatment of obesity: a meta-analysis and meta-regression. Gastrointest Endosc. 2015;81:1141–1149. doi: 10.1016/j.gie.2014.12.025. e7. [DOI] [PubMed] [Google Scholar]
- 65.Cha R, Marescaux J, Diana M. Updates on gastric electrical stimulation to treat obesity: Systematic review and future perspectives. World J Gastrointest Endosc. 2014;6:419–431. doi: 10.4253/wjge.v6.i9.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao S, Ke M, Wang Z, et al. Retrograde gastric pacing reduces food intake and delays gastric emptying in humans: a potential therapy for obesity? Dig Dis Sci. 2005;50:1569–1575. doi: 10.1007/s10620-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Du S, Fang L, et al. Retrograde gastric electrical stimulation suppresses calorie intake in obese subjects. Obesity (Silver Spring) 2014;22:1447–1451. doi: 10.1002/oby.20664. [DOI] [PubMed] [Google Scholar]
- 68.Sharaiha RZ, Kedia P, Kumta N, et al. Initial experience with endoscopic sleeve gastroplasty: technical success and reproducibility in the bariatric population. Endoscopy. 2015;47:164–166. doi: 10.1055/s-0034-1390773. [DOI] [PubMed] [Google Scholar]
- 69.Melissas J, Leventi A, Klinaki I, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258:976–982. doi: 10.1097/SLA.0b013e3182774522. [DOI] [PubMed] [Google Scholar]
- 70.Abu Dayyeh B, Acosta A, Topazian M, et al. One-year follow-up and physiological alterations following endoscopic sleeve gastroplasty for treatment of obesity. Gastroenterology. 2015;148(Suppl. 1):S11–S12. [Google Scholar]