Abstract
Inflammation and asthma have both been reported in some children with autism spectrum disorder (ASD). To further assess this connection, peripheral immune cells isolated from young children with ASD and typically developing (TD) controls and the production of cytokines IL-17, −13, and −4 assessed following ex vivo mitogen stimulation. Notably, IL-17 production was significantly higher following stimulation in ASD children compared to controls. Moreover, IL-17 was increased in ASD children with co-morbid asthma compared to controls with the same condition. In conclusion, children with ASD exhibited a differential response to T cell stimulation with elevated IL-17 production compared to controls.
Keywords: Autism, IL-17, Asthma, Food allergies
Introduction
Autism spectrum disorder (ASD) is a group of heterogeneous, behaviorally defined disorders whereby affected individuals are characterized by impairments in social interaction, verbal and nonverbal communication, often with stereotypical behaviors and restricted interests. The current prevalence of ASD is 1:68 children, with 1:42 affecting boys (Corcoran et al., 2015; Magalhães et al., 2009). There are currently no biological markers common to all individuals with ASD. The causes of ASD have yet to be discovered, and it has been suggested that ASD comprises of a combination of multiple etiologies, including gene X environment interaction (Croen et al., 2005; Lawler, 2008; Lyall et al., 2013; Money et al., 1971). A growing body of research has demonstrated that the immune response may be a contributing factor in the etiology and/or ontogeny of ASD (Ashwood et al., 2006; Careaga et al., 2010; Comi et al., 1999; Croen et al., 2005; Lawler, 2008; Lyall et al., 2013; 2015; Menage et al., 1992; Money et al., 1971; Renzoni et al., 1995). Previous findings indicate that some children with ASD have dysregulated immune responses such as increased natural killer cell activity (Enstrom et al., 2009), the presence of autoantibodies directed against brain proteins (Goines et al., 2011a), and altered cytokine profiles (Ashwood et al., 2010a; 2011a; Corcoran et al., 2015). Studies have also suggested that several co-morbidities might be associated with autism including immune-mediated disorders such as asthma, allergy and skin disorders such as atopic dermatitis and eczema (Lawler, 2008; Magalhães et al., 2009; Sacco et al., 2012). In addition, there is an increased prevalence of immune-mediated disorders in family members of children with ASD (Ashwood et al., 2006; Careaga et al., 2010; Comi et al., 1999; Croen et al., 2005; Lyall et al., 2013; Money et al., 1971). However, there are conflicting reports among the research findings regarding the prevalence of clinically relevant immune-mediated conditions and whether they are an accurate or only indicate immune dysregulation in ASD (Enstrom et al., 2009; Lyall et al., 2013; Menage et al., 1992; Renzoni et al., 1995). For instance, reported levels of IgE antibodies, which play a key role in the allergic immune response, are not consistently different between ASD and typically developing (TD) controls (Bakkaloglu et al., 2008; Goines et al., 2011a; Heuer et al., 2008) and makes for a poor biomarker for immune dysregulation in ASD. Thus, in light of the current ambiguity in reported allergic conditions in ASD (Croen et al., 2005; Lyall et al., 2015; 2013) it is of great interest to better understand the relationship between the immune anomalies noted in some children with ASD and the presence of the co-morbid conditions such as asthma, allergy, and eczema. It is clear through multiple studies that children with ASD have altered cytokine/chemokine profiles when compared to age-matched TD controls, but few studies have attempted to link these critical immune mediators with the co-morbid features present in some children with ASD (Ashwood et al., 2006; Careaga et al., 2010; Onore et al., 2011). One immune factor that has been suggested to have contributed to immune-mediated disorders including asthma, allergies and autoimmunity, is the inflammatory T cell cytokine, IL-17 (Cosmi et al., 2011).
IL-17 is a cytokine produced by Th17 CD4+ cells, which are known to secrete IL-17, IL-17F, IL-21 and IL-22 (Bettelli et al., 2007; Korn et al., 2009). Plasma levels of IL-17 have been shown to be elevated in patients with asthma (Cosmi et al., 2011; Hashimoto et al., 2005). However, there is conflicting evidence regarding the levels of IL-17 in children with ASD, which is likely due to the differences in methodology, study sample size, or study design such as population-based studies compared to specialist clinic-based studies. Some reports indicated increased serum levels of IL-17 in individuals with ASD (Al-Ayadhi and Mostafa, 2012; Suzuki et al., 2011), while other studies indicated no statistically significant differences in levels of IL-17 in plasma or serum (Enstrom et al., 2008; Jyonouchi et al., 2012; Onore et al., 2009). One study also described no difference in production of IL-17; however, there was a trend involving increased frequency of IL-17 that produced specific Th17 cells in children with ASD when compared to age-matched controls (Onore et al., 2009).
We sought to determine the relationship between reported co-morbid features of asthma, allergies, eczema, and the cellular production of the cytokines IL-17, IL-13 and IL-4 in children with ASD. Towards this goal, we examined the cellular production of IL-17 following stimulation, as well as the allergy/asthma associated with Th2 cytokines, IL-4 and IL-13 (Brightling et al., 2002; Kim et al., 2013; Lambrecht and Hammad, 2013), from children with ASD, as well as age-matched TD controls.
2. Materials and Methods
2.1 Study participants
Consenting families were enrolled through the population-based case-control CHARGE (CHildhood Autism Risks from Genetics and Environment) study, which was previously associated with the Center for Children’s Environmental Health (CCEH) and the MIND (Medical Investigation of Neurodevelopmental Disorders) Institute at the University of California in Davis (Hertz-Picciotto et al., 2006).
For this study, we included children from CHARGE belonging to two groups: children with ASD and typically developing (TD) children. To be eligible for study participation, children needed to be between the ages of 24 and 60 months at the time of enrollment, living with at least one biological parent, English or Spanish speaking, and born in California. Residing catchment areas for the CHARGE study consisted of rural and urban areas in California. This study protocol followed the ethical guidelines of the most recent Declaration of Helsinki and was approved by the institutional review boards at the University of California, Davis, and the State of California Office of Human Research Protection. Informed consent was obtained prior to participation in the study. Recruitment, eligibility, and psychometric assessment protocols have been previously described (Ashwood et al., 2011b; 2010b; Hertz-Picciotto et al., 2006; Lyall et al., 2013).
The CHARGE study includes approximately 1,300 total participants. For this immune-based study, CHARGE blood samples were included in order of enrollment over the course of the study with no selection bias. A total of 114 participants with available information on immune-mediated conditions were examined in this study and the diagnoses of all enrolled children were confirmed at the UC Davis MIND Institute. The diagnosis of ASD (n=45) was based on DSM-IV criteria, the Autism Diagnostic Observation Schedule (ADOS), and the Autism Diagnostic Interview–Revised (ADI-R). The autism severity score was determined as described (Gotham et al., 2008). All controls were screened on the Social Communication Questionnaire (SCQ) and those scoring at or above the cutoff of 15 were then assessed using the ADI-R and ADOS. A diagnosis of typical development (TD) (n=69) was assigned to general population controls based on the SCQ and composite scores of 70 or higher on the Mullen Scales of Early Learning (MSEL) and the Vineland Adaptive Behavioral Scales (VABS). The presence of immune-mediated disorders in the child was collected from a clinician-administered medical history taken during enrollment using the CHARGE family medical history, child medical history, allergies and asthma survey, and environmental exposure questionnaire. The physician-administered medical history, including a family autoimmune survey, was used to assess the presence of autoimmune disease and immune-mediated disorders present in the child, biological parents, siblings, aunts, uncles, and grandparents. The child’s medical history collected detailed information about the child’s medical conditions, including asthma, allergies (food, skin, environmental – hay fever, seasonal allergies – and medication), eczema, and any immune-mediated conditions. Parental report on children’s medication used for immune-mediated conditions was determined during a telephone interview. All data sources were collected and a final dichotomous variable for all types of allergies, asthma, and eczema was generated (Lyall et al., 2015; 2013). If a disagreement between sources regarding immune conditions for a particular participant occurred, that individual’s immune data was excluded from the analysis. Of note, although our methods of dichotomous variable generation for immune-mediated disorders from CHARGE participants are the same as represented in Lyall et al. papers, the participants in this study are not necessarily representative of the larger CHARGE population.
2.2. Participant Demographics
ASD and TD participants in the study were maintained at a ratio of 4:1, boys to girls, which is consistent with the male to female ratio reported for ASD (Comi et al., 1999; Croen et al., 2005; Fombonne, 1999; Money et al., 1971)(Table 1). The majority of participants in this study were identified as white or white Hispanic (Table 1). The average age by year of the participants in this study was 3.57 years of age (Table 1).
Table 1.
Basic Demographic Characteristics of the Study Population
| ASD n=45 |
TD n=69 |
Total Participants n=114 |
|
|---|---|---|---|
| Age at blood draw (years) | 3.56 | 3.57 | 3.57 |
| Sex | |||
| Male | 37 (82.2%) | 60 (87.0%) | 97 (85%) |
| Female | 8 (17.8%) | 9 (13.0%) | 17 (15.0%) |
| Race | |||
| White | 19 (42.2%) | 34 (49.3%) | 53 (46.5%) |
| Black | 1 (2.2%) | 2 (2.9%) | 3 (2.62%) |
| Asian | 3 (6.7%) | 2 (2.9%) | 5 (4.39%) |
| White-Hispanic | 15 (33.3%) | 20 (29.0%) | 35 (30.7%) |
| Non-White Hispanic | 4 (8.9%) | 3 (4.34%) | 7 (6.14%) |
| Multi-racial | 3 (6.7%) | 8 (11.6%) | 11 (9.65%) |
Demographics of the study population.
Note: ASD = autism spectrum disorder; TD = typically developing children in the general population.
2.2. PBMC (Peripheral Blood Mononuclear Cells) Isolation and Immune Challenge
Blood was collected in acid-citrate-dextrose Vacutainer tubes (BD Biosciences, San Jose, CA) by a trained phlebotomist at the MIND Institute. Samples were centrifuged for 2,300 rpm and the plasma was removed. The buffy coat layer was brought up to 20 ml with Hank’s Balanced Salt Solution (HBSS) (VWR, West Chester, PA), layered over 15 ml of Histopaque (Sigma; St. Louis, MO) and centrifuged at 1,700 rpm for 30 minutes for isolation of PBMC. Isolated PBMC were washed with HBSS twice and viable PBMC were counted using Trypan Blue (Sigma, St. Louis, MO).
PBMC were adjusted to a concentration of 3 × 106 live cells ml−1 with a solution of serum-free X-Vivo media (Lanzo, Waldersville, MD) supplemented with 0.2% T-stim (BD, Franklin Lakes, NJ). One hundred microliters of PBMC suspension were plated into 100µl of 10µl/ml phytohemagglutinin (PHA) (Sigma, St. Louis, MO) or 100µl of media into a 96-well tissue culture plate (Corning, Corning, NY). Cells were incubated at 37°C for 48 hours, after which the supernatants were harvested and stored at −80°C until cytokine analysis.
2.3 Cytokine Measurements
Supernatant levels of cytokines/chemokines were analyzed by a Luminex-based multiplex platform using a Multi-Plex bead set (Millipore, Saint Charles, MO) that included IL-17, IL-13 and IL-4. Briefly, analyte-specific antibody conjugated beads were incubated with a 25 µl assay buffer, 25µl of X-vivo, and 25µl of the cell culture supernatant overnight at 4°C on an orbital shaker in the dark. The following day, the plate was allowed to warm to room temperature and the fluid was gently aspirated by vacuum manifold aspiration and the beads washed. Detection antibodies (25 µl) were then added to each well and incubated on an orbital shaker for 1 hour at room temperature. Subsequently, 25µl of Streptavidin-Phycoerythrin was added to each well and incubated on a plate shaker for 30 minutes at room temperature. The plate was then washed 3 times, 150µl of 1× Sheath fluid (Bio-Rad Laboratories Inc., Hercules, CA) was added to each well, and the fluorescence measured on a Bio-Plex 200 System (Bio-Rad Laboratories Inc., Hercules, CA).
2.4 Statistical Analysis
Fisher’s exact test was used to determine if there was a significant difference in the prevalence of immune-mediated condition between ASD and TD groups. Medians and interquartile ranges of cytokine concentrations (pg/ml) were graphed on a log10 Y-axis due to the skewedness of the data. Cytokine concentrations between ASD and TD groups were conducted using a two-tailed Mann-Whitney U test. For cytokines values that fell below the limit of detection (LOD), we assigned the value of LOD/2 as described (Goines et al., 2011b). Graphs and statistical analysis comparisons were first performed across diagnostic groups, ASD, and TD for IL-17, IL-13 and IL-4. This was followed by analysis within and between diagnosis groups with and without immune-mediated for IL-17, IL-13 and IL-4 production levels. The spearman correlation coefficient (r) was calculated for a correlation between PHA-stimulated IL-17, IL-13 and IL-4 cytokines levels, and autism severity scores. P-values were determined by a two-tailed Mann-Whitney U t-test. (*p<0.05).
4. Results
4.1 Immune-Mediated Disorders in Study Participants
In this study population, the reported prevalence of asthma in children with ASD (26.7%) was significantly higher when compared to the TD group (7.3%; p= 0.016) (Table 2). Outside of asthma, there were no significant differences in the prevalence of other immune-mediated conditions evaluated between participants in this study (Table 2).
Table 2.
Immune-mediated disorders in study population
| ASD | TD | ASD vs. TD | |||
|---|---|---|---|---|---|
| Condition | n/N | % | n/N | % | p-value |
| Asthma | 12/45 | 26.7 | 5/69 | 7.3 | 0.016 |
| Any allergy* | 17/45 | 37.8 | 26/69 | 37.7 | 1.000 |
| Environmental (hay fever/seasonal allergies) | 13/45 | 28.9 | 22/69 | 31.9 | 0.845 |
| Food allergy | 4/45 | 8.9 | 8/69 | 11.6 | 0.765 |
| Medication allergy | 2/45 | 4.4 | 3/69 | 4.4 | 1.000 |
| Skin allergy (excl. eczema) | 1/45 | 2.2 | 1/69 | 1.5 | 1.000 |
| Eczema | 15/45 | 33.3 | 24/69 | 34.8 | 1.000 |
| Any immune-mediated condition | 28/45 | 62.2 | 39/69 | 56.5 | 0.757 |
Percentage of immune-mediated disorders found in study population. P-values were determined using a two-tailed Fisher’s Exact Test.
Any allergy includes the following types of allergies: environmental, food, medication or skin; eczema was examined separately.
Note: ASD = autism spectrum disorder; TD = typically developing children in the general population.
4.2 Increased Level of Stimulated IL-17 in ASD Participants
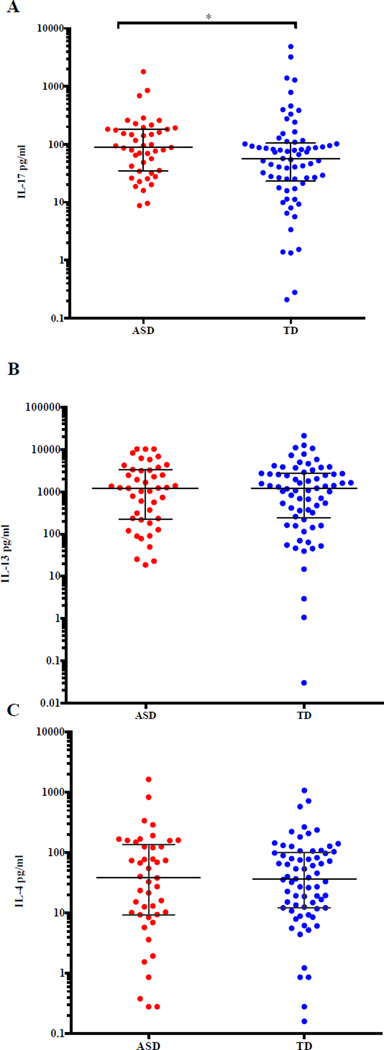
T cells isolated from peripheral blood can differentiate into Th2 and Th17 cells when activated with the broad T cell activator, PHA, producing IL-4 and IL-13 (Th2) or IL-17 (Th17) (Fournié et al., 2001). Thus, for this project, we sought to determine if there were any differences in production of IL-17, IL-13, or IL-4 from activated PBMC between ASD and TD subjects using Luminex technology. Baseline levels (media alone) of IL-17, IL-13, and IL-4 demonstrated no significant differences between the ASD and TD groups (data not shown). However, a significant increase in IL-17 production by supernatants from PBMC cultures stimulated with PHA was observed from children with ASD (median (interquartile range) (88.35 pg/ml, 8.78 pg/ml to 1,790 pg/ml) compared to TD children (56.99 pg/ml, 0.210 pg/ml to 4,860 pg/ml; p=0.047) (Figure 1A). For both of the Th2 cytokines, IL-13 and IL-4, there were no statistically significant differences in production between ASD and TD participants (Figure 1B and 1C).
Figure 1.
Cytokine expression of IL-17, IL-13 and IL-4 in PHA-stimulated cell culture supernatant from ASD and TD participants. A) There is a significant difference in PHA-stimulated IL-17 levels in ASD cases compared to TD controls (p=0.047). B) No significant difference was observed from PHA-stimulated IL-13 levels from cell culture supernatants between groups. C) No significant difference was observed from PHA-stimulated IL-4 levels from cell culture supernatants between groups. P-values were determined by a two-tailed Mann-Whitney U t-test. (*p<0.05, **p<0.001). Bars represent the median and interquartile range on a log10 Y-axis.
Cytokine Levels in Participants with Asthma
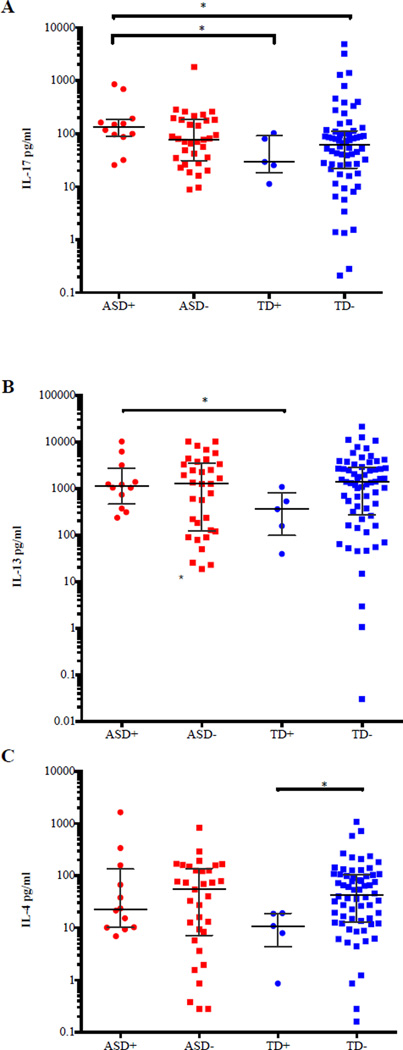
We next sought to determine if there was any association between IL-17, IL-13 and IL-4 production in children with ASD and TD controls and immune-mediated conditions such as asthma, allergies, and eczema. The category of allergies was further divided into environmental (hay fever/seasonal and other exposures such as mold), food, medication, skin (excluding eczema) and eczema. We also evaluated diagnostic groups with any allergy or any immune-mediated condition. Medical history availability was the primary basis for our selection of these particular immune-mediated disorders. In addition, age was also important for the determination of medical history since other inflammatory conditions that could be of interest, such as autoimmune diseases, are very rare in a pediatric population of this age range. Children with ASD who also had asthma produced significantly higher levels of IL-17 following stimulation (132.4 pg/ml, 25.54 pg/ml to 848.5 pg/ml) compared to TD children with asthma (29.17 pg/ml, 11.25 pg/ml to 102.2 pg/ml; p=0.019) and TD participants without asthma (61.84 pg/ml, 0.210pg/ml to 4,860 pg/ml; p=0.018), but did not significantly differ from ASD children without reported asthma (Figure 2A). As with IL-17, a significant increase in production of IL-13 following PHA-stimulation was observed between ASD with asthma (1,122 pg/ml, 234.3 pg/ml to 10,206 pg/ml) compared to TD participants with asthma (359.3 pg/ml, 39.13 pg/ml to 1,080 pg/ml; p=0.047) (Figure 2B). TD participants without reported asthma produced significantly higher levels of IL-4 (42.74 pg/ml, 0.160 pg/ml to 1,077 pg/ml) compared to TD participants with asthma (10.85 pg/ml, 0.860 pg/ml to 19.14 pg/ml; p=0.022) (Figure 2C). Finally, there was no significant difference in IL-4 production between ASD children and TD children (with or without reported asthma) Figure 2C).
Figure 2.
IL-17, IL-13 and IL-4 expression in ASD and TD participants with and without asthma. A) There is a highly significant difference in PHA-stimulated IL-17 levels from ASD cases with asthma compared to TD controls with asthma (p=0.019) and TD controls without asthma (p=0.018). B) There is a significant difference in PHA-stimulated IL-13 levels between ASD cases with asthma compared to TD cases with asthma (p=0.047). C) There is a significant difference in PHA-stimulated IL-4 levels between TD cases with and without asthma where those typically developing children without asthma produce higher amounts of IL-4 (p=0.022) respectively. P-values were determined by a two-tailed Mann-Whitney U test. (*p<0.05, **p<0.001). (+) with asthma; (−) asthma. Bars represent the median and interquartile range on a log10 Y-axis.
Cytokine Levels in Participants with Food Allergies
Groups of participants with allergy sub-categories other than asthma, including skin (excluding eczema) and medication allergies, were too small to perform individual statistical analysis on (Table 2). Therefore, these categories are not included in this report. We did evaluate the cytokine profile in the context of “food” and “environmental allergies” and whether or not the participant had any reported allergy or eczema (as a total allergy group). No significant difference in production of IL-17, IL-13, or IL-4 was noted between diagnostic groups for the categories of environmental allergies or any reported allergies, including eczema.
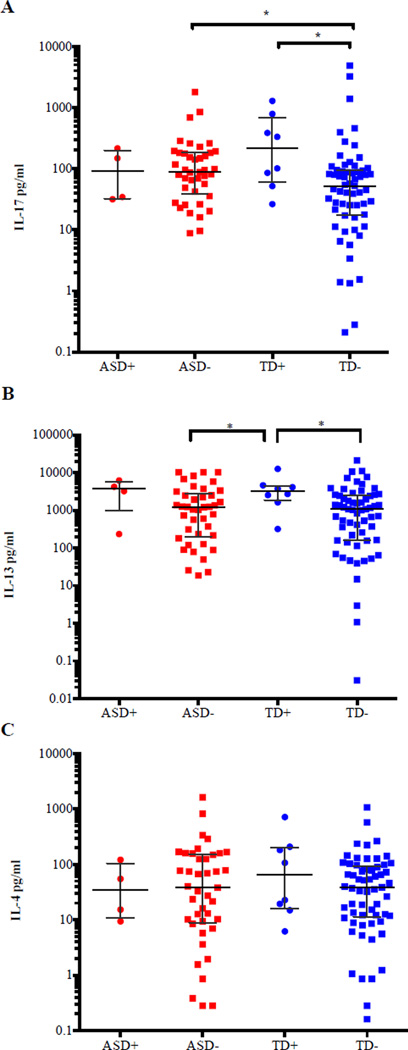
We did note differences between cytokine production levels following stimulation for participants with food allergies (Figures 3). While no significant differences were observed within the ASD diagnostic group for food allergy and IL-17, IL-13, and IL-4, differences were observed between diagnostic groups (Figure A-C). ASD participants without reported food allergies (88.35 pg/ml, 8.780 pg/ml to 1,790 pg/ml) and TD participants with reported food allergies (216.8 pg/ml, 26.35 pg/ml to 1283 pg/ml) produced higher levels of IL-17 compared to TD participants without reported food allergies (52.20 pg/ml, 0.210 pg/ml to 4,860 pg/ml; p=0.011; p=0.021, respectively) (Figure 3A). However, it was the TD children with a food allergy that had the highest overall production of IL-17 (Figure 3A). As for IL-13, TD children with reported food allergies produced significantly higher levels of IL-13 (3,231 pg/ml, 318.3 pg/ml to 12,600 pg/ml) compared with TD (1,072 pg/ml, 0.030 pg/ml to 21,115 pg/ml, p=0.013) and ASD (1,205 pg/ml, 18.60 pg/ml to 10, 206 pg/ml, p=0.030) participants without a reported food allergy (Figure 3B). No significant difference was observed for IL-4 between either study group, with or without food allergies (Figure 3C).
Figure 3.
IL-17, IL-13 and IL-4 expression in ASD, TD participants with and without reported food allergies. A) There was a significant difference in PHA-stimulated IL-17 levels from ASD cases without reported food allergies compared to TD cases without reported food allergies (p=0.011). In addition, there was a significant difference in PHA-stimulated IL-17 levels between TD cases with and without food allergies (p=0.021). B) There was a significant difference in PHA-stimulated IL-13 levels from TD cases with reported food allergy compared to ASD cases without any reported food allergy (p=0.030) and TD cases without a reported allergy (p=0.013). C) No significant difference was observed for PHA-stimulated IL-4 levels from cell culture supernatants between groups. P-values were determined by a two-tailed Mann-Whitney U test. (*p<0.05, **p<0.001). (+) with reported food allergy ; (−) without any reported food allergy. Bars represent the median and interquartile range on a log10 Y-axis.
5. Discussion
In this study, we evaluated peripheral blood immune cells from children with ASD and TD controls for the cellular production of the Th17 cytokine, IL-17, as well as the Th2 cytokines, IL-13, and IL-4, each of which are thought to be associated with allergic immune-mediated conditions. We found that following ex vivo PHA-stimulation, cells from children with ASD had increased production of IL-17 compared to TD controls. It is now well documented that individuals with ASD display altered immune profiles (Onore et al., 2011) that are correlated with deficits in behavioral profiles (Ashwood et al., 2011b; Goines et al., 2011a). The current study further suggests that the increased production of IL-17 in some children with ASD represents an immune hyperactivity, perhaps reflecting underlying inflammatory conditions in these children. While this immune profile is likely remote from pathogenicity at the time of blood sampling at 2–5 years of age, our findings could suggest chronic immune dysfunction that could lead to further immune-mediated conditions later in life. Longitudinal studies would be necessary to address this question.
Previous studies presented conflicting information regarding IL-17 production in ASD participants. In an earlier study by Onore, et al., the authors examined IL-17 production following PHA-stimulation in children with ASD and TD controls from the CHARGE study and found no significant differences in IL-17 production (Onore et al., 2009). Another study by Enstrom et al. evaluated IL-23 and IL-17 levels in plasma from CHARGE children and the authors reported a decrease in plasma levels of IL-23 for children diagnosed with ASD compared to TD control children with no statistically significant differences in plasma levels of IL-17 (Enstrom et al., 2008). There are several factors that could have contributed to the differences between the prior studies by Onore et al., Enstrom et al., and the current report including sample size, cytokine detection techniques, and cell stimulation time-frame. Although all three studies utilized samples from the CHARGE study, there was no overlap in participants for any of the studies. The data reported herein shed light on immune dysregulation in ASD that might occur during signaling for the cellular differentiation of T cell subsets (Onore et al., 2011).
In the current investigation, the prevalence of reported asthma was notably more prevalent among children affected with ASD than TD children. This was slightly different from published findings from a larger population of children from the CHARGE study with ASD where no significant difference was noted between ASD and TD children for parent-reported asthma (Lyall et al., 2015; 2013). With regard to the comparison of the differences in IL-17 production after PHA-stimulation with the other published reports, it is possible that the study population included by Onore, et al. and Enstrom, et al. contained a smaller proportion of ASD cases with asthma, which could have reduced the magnitude of the differences observed in IL-17 levels between ASD cases and TD controls.
Asthma and allergy are frequently diagnosed in young children, and perhaps more so in children with ASD as parents and physicians are often more aware of potentially treatable co-morbidities. Thus, the threshold for diagnosis of asthma or allergy, as well as level of severity, might vary considerably in the study population. Other reports using the CHARGE sample collection described an increased prevalence of asthma and allergies occurrences as the child ages (Lyall et al., 2015). The average age of children in the current study was 3.57 years of age. Therefore, some children might not have developed asthma and/or allergies at the time of the sample collection. Thus, it would be of great interest to examine IL-17 production and asthma/allergies longitudinally.
Recent reports have indicated Th17-positive cells may undergo transdifferentiation into regulatory T-cells under certain conditions, such as elevated TGF-β levels and through the aryl hydrocarbon receptor (AhR) (Gagliani et al., 2015). Gagliani et al. also suggested that plasticity of Th17 cells is a potential therapeutic opportunity for inflammatory conditions such as asthma and allergies. It should be noted here that in previous studies of TGF-β in blood from children enrolled in the CHARGE study, children with ASD had significantly lower plasma TGF-β levels compared to the typically developing controls and compared to children with developmental disabilities other than ASD (Ashwood et al., 2008). Thus, the dysregulated immune system noted in some children with ASD appears to skew their response towards an inflammatory phenotype with a reduced capacity for transdifferentiation based on their TGF-β levels. Although, we did not measure Th17 cells in this report, Onore et al. previously reported elevated intracellular IL-17 levels in T cells by flow cytometry. That study described a trend toward the increased frequency of Th17 positive cells in peripheral blood on children with ASD versus typically developing controls (Onore et al., 2009).
In a report by Al-Ayahi and Mostafa, increased serum levels of IL-17 in ASD participants versus controls were observed. While the authors studied serum levels rather than activated cell supernatants, these findings are in line with those in the current report (Al-Ayadhi and Mostafa, 2012). In the noted study and others published by the Al-Ayadhi group, the subject population studied was enriched for children with autoimmune conditions with the majority having autoantibodies to various antigens (Mostafa et al., 2014). In one intriguing report from Al-Ayadhi and Mostafa et. al, the authors noted that children with severe autism had elevated levels of IL-17 compared to those with mild-moderate autism. In this current investigation, we correlated autism severity scores and PHA-stimulated IL-17, IL-13, and IL-4 production in ASD participants with and without asthma and food allergies. There was no observed correlation between autism severity scores and cytokine production for ASD participants with or without asthma and food allergies. These data suggest that increased production of PHA-stimulated IL-17, IL-13, and IL-4 in ASD children with asthma and/or food allergies does not correlate with severity of autism symptoms.
It is important to understand the potential role of IL-17 in the etiology of ASD since uncontrolled and unregulated immune pathology during critical time points of neurogenesis may lead to a disruption in neurodevelopment and neural connectivity (Goines and Ashwood, 2013; Stamou et al., 2013). For example, elevated IL-17 levels in a mouse model were shown to impair the integrity of the blood-brain barrier (Huppert et al., 2010) by disruption of tight junction proteins on the central nervous system (CNS) endothelial cells (Kebir et al., 2007). Since IL-17 has been suggested to contribute to the immunopathology of various immune-mediated conditions (Okada and Khoury, 2012), and IL-13 and IL-4 are both associated with allergy or atopy, we sought to determine if IL-17, IL-13 and IL-4 levels differed between children with and without immune-based conditions such as asthma, allergies, and eczema. While we did not observe any significant differences between diagnostic groups with reported cases of eczema, we did note differences for children with reported cases of asthma and food allergies. For those children with asthma, our results indicated a strong association between IL-17 production and asthma in the ASD subjects compared to TD controls with the same condition. Asthma is an immune-mediated disorder characterized by an inappropriate inflammatory response in the lungs. A skewed Th2 cytokine profile, (IL-4, IL-5 and IL-13) is generally found in allergic asthma with increased numbers of eosinophils, mast cells, and neutrophils present in lung tissue (Bettelli et al., 2007; Bradding et al., 2006; Cosmi et al., 2011; Korn et al., 2009; Lloyd and Hessel, 2010). An association between IL-17 and asthma has also been previously demonstrated as indicated in studies showing that local production of IL-17 was increased in asthmatic airways and its concentration in the sputum was correlated with bronchial hyper-responsiveness (Barczyk et al., 2003; Bettelli et al., 2007). Several studies have demonstrated that patients with severe asthma have a marked increase in IL-17 levels in lung biopsy specimens, which was also associated with disease severity (Cosmi et al., 2011; Hashimoto et al., 2005; Iwakura et al., 2011). Such findings suggest that Th17 cells are important in the etiology of asthma and that the increased production of IL-17 by peripheral blood cells from ASD children with asthma in the current study further supports this notion. As noted above, it was of particular interest that such an association was limited to the ASD population, and that the TD children with asthma did not have elevated levels of IL-17 compared to those without asthma. This suggests that asthma in children with ASD might have a more robust inflammatory underpinning with a greater response to T cell stimulation compared to age-matched typically developing children.
While the cause of the elevated production of IL-17 in children with ASD is currently unclear, it has been suggested that the environment might play a role in dysregulating immune activation. A recent study found that diesel exhaust particle exposure contributed to severe asthma by enhancing IL-17 production and that increased IL-17 might be a marker of severity in asthmatic children (Brandt et al., 2013). Asthma has also been associated with proximity to traffic in children (Gauderman et al., 2005) and freeway proximity in adults (Ehrenstein et al., 2014). This connection is of particular interest as previous studies from our research group indicate that children with autism are more immune-sensitive to environmental exposure (Ashwood et al., 2009). Further studies have also demonstrated a relationship between proximity to freeways during early development and ASD (Volk et al., 2011; 2013). Thus, differences in cytokine profiling in the context of asthma could be a product of particular environmental exposures.
Both IL-4 and IL-13 play a key role in allergic asthma. In addition to IL-17, we also observed an association between asthma and IL-13 in children with ASD. The CD4+ cells that dominate the airways of asthmatic patients produce both IL-4 and IL-13, which drives B-cell production of IgE further. This then leads to increased expression of cell adhesion molecules, eosinophil recruitment, and mucus hyper-secretion (Cosmi et al., 2011). Thus, we anticipated that IL-4 and IL-13 production levels would be higher in subjects with asthma and we found such an association with IL-13, but not with IL-4 for ASD cases. However, we did observe increased levels of IL-4 in TD children without asthma compared to those TD with asthma. This seems counterintuitive, but we only evaluated IL-4 and IL-13 production from isolated PBMC following PHA-stimulation. Perhaps a more direct evaluation of IL-4 levels in individuals with asthma might be to measure IL-4 levels plasma, serum, or bronchoalveolar lavage. Also of interest were the lower levels of PHA-stimulated IL-4 production in TD children with asthma compared to those without reported asthma. While this could reflect T-cell exhaustion, this might simply be due to the fact that there are too few TD subjects with asthma in the current sample set to accurately evaluate this relationship. A final possibility is that those cells producing IL-4 and IL-13 have been recruited to the tissues and are thus present with lower frequency in circulation.
We also evaluated cytokine levels for IL-17, IL-13, and IL-4 cases with reported allergic conditions. Allergies are a hypersensitivity disorder of the immune system and, in the current study, the category of “allergies” was broad and included several sub-categories of skin allergies, as well as environmental allergies, medication, food, or any reported allergies. Herein, we found significant differences between diagnostic groups only in the context of food allergies. No significant differences in the prevalence of any of the other allergy related sub-categories were found between the diagnostic groups. Some reports have previously indicated a strong association between ASD and a family history of allergies (Croen et al., 2005; Lyall et al., 2015; Sacco et al., 2012) while others have not (Jyonouchi et al., 2005). Thus, there remain some discrepancies in the association between allergies and ASD. The current study was limited in sample size and thus was not fully representative of the general pediatric population. However, the data presented herein suggest that, for the populations studied, there were differences in the immune profile of children with coincident ASD and asthma/food allergy and that of children who are typically developing. This difference is not limited to ASD. A correlation between cytokine/chemokine production levels and seasonal changes (Singh et al., 2011) has been indicated especially for individuals with allergies. It should be noted here that the population derived from the CHARGE study is geographically controlled in that all participants reside within a catchment area of a specified list of regional centers within California (Hertz-Picciotto et al., 2006). This restricted sample area controls for environmental exposures and the samples chosen for this study were also controlled for the season of collection across all of the study participants. Thus, the neurobiological differences between ASD and TD participants is more likely due to differences in immune regulation between the two study populations rather than seasonal exposure.
An association between allergies and ASD has previously been described showing that children with ASD have more severe allergies to food than children without ASD in two of the large U.S. cohort studies (Gurney et al., 2006; Lyall et al., 2015; 2013). Herein, we did not observe differences in cytokine production between ASD participants with and without food allergies. However, we only had four ASD participants with reported food allergies in this study population. Thus, this likely reflects an unintentional skewing of the study towards children with ASD and no food allergies. This is certainly of interest to the autism community as the notion of food allergy/sensitivity has long been a subject of debate. However, it is also possible that the rate of true food allergy in children with ASD is not elevated. In fact, we noted a slightly higher percentage of reported food allergy in the control population in this study. Therefore, for the ASD study group, there were too few children with food allergies to make a strong statistical comparison, but there appeared to be little difference between those with a food allergy and TD children with a food allergy in terms of IL-17 and IL-13 production. Thus, while we did not note increased levels of IL-17, IL-13, and IL-4 in ASD participants with food allergies compared to TD participants, we did observe that TD participants with food allergies produced higher levels of IL-17 compared to TD controls and ASD participants without food allergies. In a recent study of typically developing children with reported food allergies, the investigators found a higher production of IL-13 and IL-4 from in vitro isolated PBMC following a stimulation with a native peanut allergy mixture (Dhuban et al., 2013). While the T cell stimulation was directed towards a specific allergen rather than a pan-T cell mitogen, the cytokine profile was similar to what was noted in the current study for the typically developing children. Furthermore, gastrointestinal symptoms, which are often associated with food sensitivities in children with autism, range in prevalence from 9% to 91%, due to differences in presentation and severity within the spectrum (de Theije et al., 2013). In addition, a larger epidemiologic study of the children in the CHARGE study, the authors observed a 38% prevalence of ASD children with a gastrointestinal diagnosis and reported food allergies compared to 16% of controls with a gastrointestinal diagnosis and reported food allergies (Lyall et al., 2015). Within our study, we had only 8.9% prevalence of reported food allergies in the ASD group. Although this study reported percentages of children with food allergies and autism to be on the lower end of what has been published, it did not appear that there was any difference in the relevant cytokines studied herein in association with those few children with reported food allergy.
In the current study, we used clinician and parent-reported data from questionnaires administered by trained personnel to evaluate the presence of asthma and allergies among children enrolled in the CHARGE study. While our criteria were fairly broad, the findings suggest that elevated IL-17 may be linked to the immune dysregulation associated with atopy, particularly in children with ASD. It is not yet clear if the increased production of PHA-stimulated IL-17 in young children with ASD is merely a secondary phenomenon or related in some way to the neurodevelopmental changes seen in autism.
Under certain pathologic conditions, IL-17 is produced by various peripheral immune cells infiltrating the central nervous system (CNS), and to a lesser extent, by CNS-resident astrocytes and microglia. IL-17 has been linked to several CNS disorders including depression and ischemic brain injury, with the greatest body of work describing its role in multiple sclerosis (MS), (reviewed in (Waisman et al., 2015)). Cells of the CNS have functional receptors for IL-17 and studies have indicated that inflammation in the periphery can influence brain function and lead to pathologic changes in the CNS (Waisman et al., 2015). Although we did not determine any potential mechanisms of IL-17 in terms of neurodevelopment or function in this study, through its direct action on endothelial cells, IL-17 could influence brain development if the blood-brain barrier (BBB) is undergoing formation, such as during gestation, or the BBB becomes permeable during infection (Waisman et al., 2015). In the CNS parenchyma, IL-17 can act either alone or in concert with other factors to directly induce neuronal damage. In addition, IL-17 signaling through receptors on astrocytes and microglia induces the expression of a host of pro-inflammatory mediators as well as factors that promote tissue repair and the resolution of inflammation. Thus, in children with autism and co-morbid asthma, chronic inflammation and elevated production of IL-17 could potentially influence changes and inflammation in the CNS over time.
In summary, we have demonstrated herein that children with asthma and/or food allergies have a differential immune profile based on neurodevelopmental outcome. Our study strongly indicates that elevated production of IL-17 is linked to a co-morbidity often reported in association with ASD, and further, is differentially expressed in the ASD children with asthma compared to TD controls with the same reported condition. Moreover, the contrast of the T cell response to mitogen stimulation between children with ASD and typically developing controls in the context of immune mediated conditions suggests that, although there is overlap in the medical phenotype, the underlying cellular function relevant to that phenotype is substantially different. Furthermore, we have highlighted the importance of considering co-morbid conditions when evaluating biologic differences between ASD and TD individuals. Finally, this report facilitates a better understanding regarding the relationship between immune dysfunction between children with one neurodevelopmental disorder and typically developing children, and how these differences may shape the behaviors as well as co-morbidities associated with such disorders.
Highlights.
We examined cytokine production and co-morbid conditions in children with autism.
Increased prevalence of asthma was observed in children with autism.
Children with autism produced increased levels of IL-17.
Increased production of IL-17 and IL-13 were associated with ASD cases with asthma.
Typically developing children with food allergies produced increased levels of IL-13.
Acknowledgments
The National Institute of Environmental Health Sciences (NIEHS) pre-doctoral training program in Environmental Health Sciences (T32-ES007058-33) funded M.E.A. This study was funded by the NIEHS Center for Children’s Environmental Health and Environmental Protection Agency (EPA) grants (2P01ES011269-11, 83543201 respectively), the NIEHS-funded CHARGE study (R01ES015359), and the NICHD funded IDDRC 054 (U54HD079125). We also thank the staff of the CHARGE study, and families involved in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Al-Ayadhi LY, Mostafa GA. A lack of association between elevated serum levels of S100B protein and autoimmunity in autistic children. J Neuroinflammation. 2012;9:54. doi: 10.1186/1742-2094-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. Journal of neuroimmunology. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2010a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav. Immun. 2010b doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav. Immun. 2011a;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of neuroimmunology. 2011b;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Schauer J, Pessah IN, Van de Water J. Preliminary evidence of the in vitro effects of BDE-47 on innate immune responses in children with autism spectrum disorders. Journal of neuroimmunology. 2009;208:130–135. doi: 10.1016/j.jneuroim.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. Journal of leukocyte biology. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Bakkaloglu B, Anlar B, Anlar FY, Öktem F, Pehlivantürk B, Ünal F, Ozbesler C, Gökler B. Atopic features in early childhood autism. European Journal of Paediatric Neurology. 2008;12:476–479. doi: 10.1016/j.ejpn.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respiratory medicine. 2003 doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nature Publishing Group. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Bradding P, Walls A, Holgate S. The role of the mast cell in the pathophysiology of asthma. Journal of Allergy and Clinical Immunology. 2006;117:1277–1284. doi: 10.1016/j.jaci.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, Khurana Hershey GK. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013;132:1194–1204. doi: 10.1016/j.jaci.2013.06.048. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. Journal of Allergy and Clinical Immunology. 2002;110:899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Berry A, Hill S. The lived experience of US parents of children with autism spectrum disorders: A systematic review and meta-synthesis. Journal of Intellectual Disabilities. 2015 doi: 10.1177/1744629515577876. [DOI] [PubMed] [Google Scholar]
- Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Archives of pediatrics & adolescent medicine. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- de Theije CGM, Bavelaar BM, Lopes da Silva S, Korte SM, Olivier B, Garssen J, Kraneveld AD. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr Allergy Immunol. 2013;25:218–226. doi: 10.1111/pai.12149. [DOI] [PubMed] [Google Scholar]
- Dhuban KB, d'Hennezel E, Ben-Shoshan M, McCusker C, Clarke A, Fiset P, Mazer B, Piccirillo CA. Altered T Helper 17 Responses in Children with Food Allergy. Int Arch Allergy Immunol. 2013;162:318–322. doi: 10.1159/000354028. [DOI] [PubMed] [Google Scholar]
- Ehrenstein von OS, Aralis H, Cockburn M, Ritz B. In Utero Exposure to Toxic Air Pollutants and Risk of Childhood Autism. Epidemiology 1. 2014 doi: 10.1097/EDE.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A, Onore C, Hertz-Picciotto I, Hansen R, Croen L, Judy V, Ashwood P. Detection of IL-17 and IL-23 in plasma samples of children with autism. American J. of Biochemistry and Biotechnology. 2008;4:114. doi: 10.3844/ajbbsp.2008.114.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, Hertz-Picciotto I, Van de Water JA, Sharp FR, Ashwood P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav. Immun. 2009;23:124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Are measles infections or measles immunizations linked to autism? [editorial] J Autism Dev Disord. 1999;29:349–350. doi: 10.1023/a:1022123822135. [DOI] [PubMed] [Google Scholar]
- Fournié GJ, Cautain B, Xystrakis E, Damoiseaux J, Lagrange MMD, Bernard I, Subra JF, Pelletier L, Druet P, Saoudi A. Cellular and genetic factors involved in the difference between Brown Norway and Lewis rats to develop respectively type- 2 and type- 1 immune- mediated diseases. Immunological Reviews. 2001;184:145–160. doi: 10.1034/j.1600-065x.2001.1840114.x. [DOI] [PubMed] [Google Scholar]
- Gagliani N, Vesely MCA, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015 doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Avol E, Lurmann F, Kuenzli N. Childhood Asthma and Exposure to Traffic and Nitrogen Dioxid. Epidemiology. 2005 doi: 10.1097/01.ede.0000181308.51440.75. [DOI] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav. Immun. 2011a;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicol Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular autism. 2011b;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. J Autism Dev Disord. 2008;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney JG, McPheeters ML, Davis MM. Parental report of health conditions and health care use among children with and without autism: National Survey of Children's Health. Archives of pediatrics & adolescent medicine. 2006;160:825–830. doi: 10.1001/archpedi.160.8.825. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Akiyama K, Kobayashi N, Mori A. Comparison of IL-17 production by helper T cells among atopic and nonatopic asthmatics and control subjects. Int Arch Allergy Immunol. 2005;137:51–54. doi: 10.1159/000085432. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism research : official journal of the International Society for Autism Research. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CRW. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. The FASEB Journal. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional Specialization of Interleukin-17 Family Members. Immunity. 2011 doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51:77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Streck DL, Toruner GA. Immunological characterization and transcription profiling of peripheral blood (PB) monocytes in children with autism spectrum disorders (ASD) and specific polysaccharide antibody deficiency (SPAD): case study. J Neuroinflammation. 2012;9:4. doi: 10.1186/1742-2094-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Wojno EDT, Artis D. Innate lymphoid cells and allergic inflammation. Current Opinion in Immunology. 2013;25:738–744. doi: 10.1016/j.coi.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Asthma: The importance of dysregulated barrier immunity. Eur J Immunol. 2013;43:3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- Lawler CP. The “Environment” for Autism Research: Signs of Improvement? Environ Health Perspect. 2008;116:A416–A417. doi: 10.1289/ehp.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just TH2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I. Maternal Immune-Mediated Conditions, Autism Spectrum Disorders, and Developmental Delay. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Van de Water J, Ashwood P, Hertz-Picciotto I. Asthma and Allergies in Children With Autism Spectrum Disorders: Results From the CHARGE Study. Autism Research n/a–n/a. 2015 doi: 10.1002/aur.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães ES, Pinto-Mariz F, Bastos-Pinto S, Pontes AT, Prado EA, deAzevedo LC. Immune allergic response in Asperger syndrome. Journal of Neuroimmunology. 2009;216:108–112. doi: 10.1016/j.jneuroim.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Menage P, Thibault G, Martineau J, Herault J, Muh JP, Barthelemy C, Lelord G, Bardos P. An IgE mechanism in autistic hypersensitivity? Biol Psychiatry. 1992;31:210–212. doi: 10.1016/0006-3223(92)90208-h. [DOI] [PubMed] [Google Scholar]
- Money J, Bobrow NA, Clarke FC. Autism and autoimmune disease: a family study. J Autism Child Schizophr. 1971;1:146–160. doi: 10.1007/BF01537954. [DOI] [PubMed] [Google Scholar]
- Mostafa GA, El-Sherif DF, AL-Ayadhi LY. Systemic auto-antibodies in children with autism. Journal of Neuroimmunology. 2014;272:94–98. doi: 10.1016/j.jneuroim.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Okada H, Khoury SJ. Type17 T-cells in Central Nervous System Autoimmunity and Tumors. J Clin Immunol. 2012;32:802–808. doi: 10.1007/s10875-012-9686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav. Immun. 2011 doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen R, Van de Water J, Ashwood P. Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. Journal of Neuroimmunology. 2009;216:126–129. doi: 10.1016/j.jneuroim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni E, Beltrami V, Sestini P, Pompella A, Menchetti G, Zappella M. Brief report: allergological evaluation of children with autism. J Autism Dev Disord. 1995;25:327–333. doi: 10.1007/BF02179294. [DOI] [PubMed] [Google Scholar]
- Sacco R, Lenti C, Saccani M, Curatolo P, Manzi B, Bravaccio C, Persico AM. Cluster Analysis of Autistic Patients Based on Principal Pathogenetic Components. Autism research : official journal of the International Society for Autism Research. 2012;5:137–147. doi: 10.1002/aur.1226. [DOI] [PubMed] [Google Scholar]
- Singh A, Holvoet S, Weiss M, Beaumont M, Zuercher AW, Mercenier A. Increased IL-5 and IL-13 cytokine level in ex vivo stimulated whole blood cells from grass pollen allergic donors correlate with seasonal exposure. Results in Immunology. 2011;1:18–23. doi: 10.1016/j.rinim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou M, Streifel KM, Goines PE, Lein PJ. Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol Teratol. 2013;36:3–16. doi: 10.1016/j.ntt.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Yoshihara Y, Wakuda T, Takebayashi K, Takagai S, Matsumoto K, Tsuchiya KJ, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Mori N. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PloS one. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-Related Air Pollution, Particulate Matter, and Autism. JAMA Psychiatry. 2013;70:71. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129:625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]