Abstract
OBJECTIVE
To estimate the percentage of large-for-gestational age (LGA) neonates associated with maternal overweight and obesity, excessive gestational weight gain, and gestational diabetes mellitus (GDM)—both individually and in combination—by race or ethnicity.
METHODS
We analyzed 2004–2008 linked birth certificate and maternal hospital discharge data of live, singleton deliveries in Florida. We used multivariable logistic regression to assess the independent contributions of mother’s prepregnancy body mass index (BMI), gestational weight gain, and GDM status on LGA (birth weight-for-gestational age 90th percentile or greater) risk by race and ethnicity while controlling for maternal age, nativity, and parity. We then calculated the adjusted population-attributable fraction of LGA neonates to each of these exposures.
RESULTS
Large-for-gestational age prevalence was 5.7% among normal-weight women with adequate gestational weight gain and no GDM and 12.6%, 13.5% and 17.3% among women with BMIs of 25 or higher, excess gestational weight gain, and GDM, respectively. A reduction ranging between 46.8% in Asian and Pacific Islanders and 61.0% in non-Hispanic black women in LGA prevalence might result if women had none of the three exposures. For all race or ethnic groups, GDM contributed the least (2.0–8.0%), whereas excessive gestational weight gain contributed the most (33.3–37.7%) to LGA.
CONCLUSION
Overweight and obesity, excessive gestational weight gain, and GDM all are associated with LGA; however, preventing excessive gestational weight gain has the greatest potential to reduce LGA risk.
Large for gestational age (LGA) describes a neonate who, at birth, weighs at or above the 90th percentile for his or her gestational age. In the United States, approximately 9% of neonates are born LGA annually.1 For the mother, delivering an LGA neonate increases the risk of prolonged labor, cesarean delivery, shoulder dystocia, and birth trauma. An LGA neonate is more likely to have fetal hypoxia and intrauterine death and to develop diabetes, obesity, metabolic syndrome, asthma, and cancer later in life.2
The individual effects of pregravid maternal body mass index (BMI, calculated as weight (kg)/[height (m)]2), gestational weight gain, and diabetes during pregnancy on fetal growth are well documented. Maternal overweight and obesity, excessive gestational weight gain, and diabetes are all independent risk factors for delivering an LGA neonate.3–5 Although studies suggest the relative risks associated with each of these risk factors are similar, the prevalence of these conditions varies with notable disparities across race and ethnicity. For example, the prevalence of pregravid obesity is 29% in non-Hispanic black women compared with 7% among Asian and Pacific Islanders6; the prevalence of gestational diabetes mellitus (GDM) is nearly 10% among Asian and Pacific Islanders compared with 4% among non-Hispanic black women.7 Additionally, there are complex interactions between these risk factors so it is unclear what proportion of LGA neonates is attributable to each exposure either individually or in combination.
Each of these risk factors may be amenable to intervention. However, the timing and complexity of interventions differ and few data are available that describe the potential effect on LGA if one or more of these risks is removed. The purpose of this analysis was to estimate the percentage of LGA neonates attributable to maternal overweight and obesity, excessive gestational weight gain, and GDM—both individually and in combination—across different race or ethnic groups.
MATERIALS AND METHODS
We analyzed live, singleton deliveries occurring from March 2004 through December 2008 in Florida. We used the state’s revised birth certificate, which incorporates parts of the 2003 U.S. Standard Certificate of Live Birth and is linked to the state’s Hospital Inpatient Discharge Database. The process describing the linkage of the two sources has been previously described elsewhere.7,8 The Florida State Health Department transferred deidentified data to the Centers for Disease Control and Prevention for analysis, and this analysis was deemed by the Centers for Disease Control and Prevention to be institutional review board-exempt.
We used birth certificate data to obtain information on maternal characteristics such as age, educational attainment, marital status, race or ethnicity, insurance status, parity, smoking status, birth country, prepregnancy weight and height, maternal weight at delivery, diabetes in pregnancy, and enrollment in the Special Supplemental Nutrition Program for Women, Infants, and Children. Self-reported maternal race categories on Florida’s birth certificate have been previously described.7,8 For our analysis, we grouped maternal race or ethnicity into four categories: non-Hispanic white, non-Hispanic black, Asian and Pacific Islander, and Hispanic. Haitian women were classified into one of these four race or ethnic categories based on what race they indicated for themselves.
Prepregnancy BMI (maternal weight in kilograms/ height in meters2) was calculated using height and prepregnancy weight information recorded on the birth certificate. Women were classified as underweight (BMI less than 18.5), normal weight (BMI 18.5–24.9), overweight (BMI 25.0–29.9), class I obese (BMI 30.0–34.9), class II obese (BMI 35–39.9), or class III obese (BMI 40.0 or greater).9
As previously described, diabetes status in pregnancy was determined by using both the birth certificate and the hospital discharge data.7,8 On the birth certificate, diabetes is recorded as prepregnancy (diagnosis before this pregnancy), gestational (diagnosis during this pregnancy), or none. Only one selection is allowed. Diabetes is identified in the hospital discharge record by the following International Classification of Diseases, 9th Revision, Clinical Modification codes: 648.8 (abnormal glucose tolerance [gestational diabetes]); 648.0 (diabetes mellitus); or 250.0–250.9 (diabetes mellitus [excludes gestational diabetes]). We used data from a previous medical record review of a small subset of the pregnancies in our linked data set to formulate rules for assigning GDM status.7 Gestational diabetes mellitus cases were defined as deliveries in which hospital discharge data included the International Classification of Diseases, 9th Revision, Clinical Modification code for gestational diabetes (648.8), except in instances in which the birth certificate indicated preexisting diabetes. Pregnancies without diabetes were those for which both the hospital discharge record and birth certificate indicated no diabetes (neither preexisting nor gestational).
Gestational weight gain was calculated from the maternal weight at delivery and prepregnancy weight as recorded on the birth certificate. We categorized pregnancy weight gain as inadequate, adequate, and excessive based on the 2009 Institute of Medicine recommendations. Gestational weight gain ranges for adequate weight gain were defined as 28–40 pounds for those with a prepregnancy BMI of less than 18.5, 25– 35 pounds for those with a prepregnancy BMI of 18.5– 24.9, 15–25 pounds for those with a prepregnancy BMI of 25.0–29.9, and 11–20 pounds for those with a prepregnancy BMI of 30 or greater (ie, all obesity classes).
Large for gestational age was defined as birth weight 90th percentile or greater for gestational age based on the distribution of birth weights in Florida from 2004–2008 and using the information recorded on birth certificates. Gestational age was calculated using the obstetric estimate also as recorded on the birth certificate.
All full-term (37–41 weeks of gestation) singleton births were eligible for inclusion in the analysis (n = 820,943). We excluded births in which hospital discharge (n = 4,938) or birth certificate (n = 3,302) records indicated preexisting diabetes, where the birth certificate indicated some form of diabetes but hospital discharge records indicated no diabetes (n = 7,752), where hospital discharge records indicated both preexisting and gestational diabetes (n = 121), and where the diabetes status from the birth certificate was missing (n = 2,349).
We also excluded the following records from our analysis: those with missing values on birth weight, prepregnancy BMI, gestational weight gain, parity, maternal age and nativity; those with implausible or extreme maternal height (less than 4’2″ or greater than 6’5″) or weight (less than 75 pounds); and those with maternal age younger than 20 years old and implausible birth weight (less than 1,000 or greater than 7,257 g [16 pounds]). Thus, our final analytic data set included 80.4% of our eligible study population, or 660,038 births.
We examined maternal demographic and behavioral characteristics overall and by maternal race or ethnicity. Potential confounders for inclusion in the logistic models were based on a review of relevant literature and the amount by which the inclusion of the variable changed the adjusted odds ratio by more than 10%. We observed evidence of confounding by parity and nativity in some racial groups and included in our final adjusted models. Although we found little evidence of confounding by other maternal characteristics, we included age because it has been found to be independently associated with BMI, gestational weight gain, and GDM in previous studies.10 We also adjusted for the other exposures not being measured in each model (ie, if modeling GDM, we adjusted for prepregnancy BMI and gestational weight gain). To determine whether race or ethnicity modified the association between LGA and the three exposures, we tested interaction terms between the three exposures and race or ethnicity by using likelihood ratio tests and required a P<.001 for statistical significance. The tests for interaction between race or ethnicity and the three exposures (independently and overall) were all significant (P<.001), except for BMI alone (P = .01).
Using the logistic regression results, we computed relative risks and 95% confidence intervals (CIs) for BMI 25 or greater, excessive gestational weight gain, and GDM separately and for the seven mutually exclusive combinations of these three exposures by race or ethnicity.11 We then estimated the corresponding population-attributable fraction and corresponding 95% CI. The total population-attributable fraction for LGA births having any one exposure or any combination of two or more of these exposures was calculated as the sum of the population-attributable fractions for the seven mutually exclusive categories. We also calculated the population-attributable fraction of LGA among births with excessive gestational weight gain by both prepregnancy BMI and race or ethnicity. All population-attributable fraction estimates were based on adjusted logistic regressions.12 We interpreted each population-attributable fraction estimate to be the reduction in LGA prevalence that would be expected to occur if all women in the exposure categories had an LGA risk equal to that of women having normal levels of all three exposures, assuming that the risk for LGA among those with a normal exposure remained unchanged.13
RESULTS
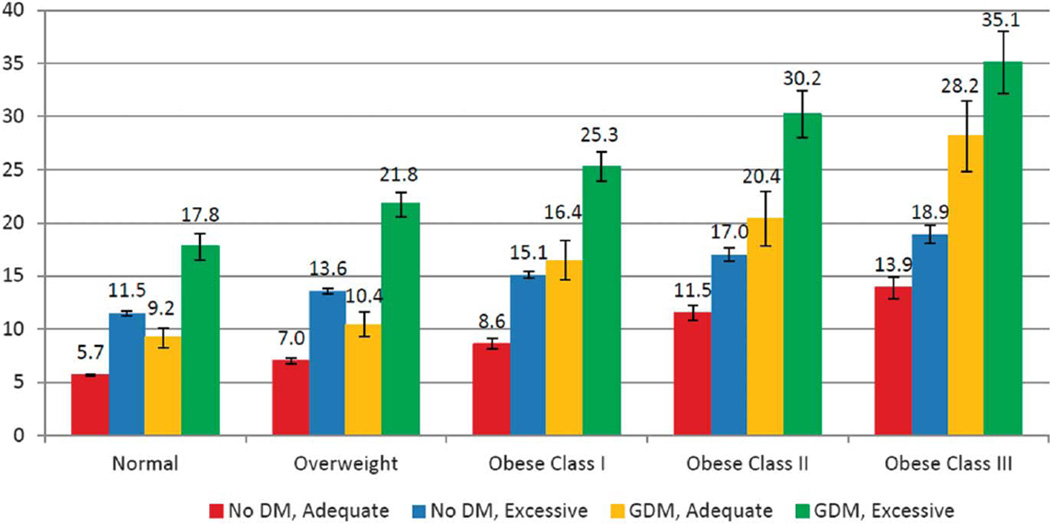
The demographic characteristics by race or ethnicity are shown in Table 1. Large-for-gestational-age prevalence was 5.7% among women who were normal weight, gained weight within recommendations, and did not have diabetes and 35.1% among women with class III obesity prepregnancy who gained excessive weight during pregnancy and had GDM (Fig. 1). Considering each factor individually, we found that the prevalence of LGA was 17.3% among women with GDM, 13.5% among women with excess gestational weight gain, and 12.6% among women who were overweight or obese (data not shown). Among women with no diabetes and adequate gestational weight gain, when examined by BMI categories, LGA prevalence was 5.7%, 7.0%, 8.6%, 11.5%, and 13.9% (Fig. 1). Large-for-gestational-age prevalence increased with increasing BMI, excessive gestational weight gain, and the presence of GDM for all women and within each racial or ethnic group (Fig. 1; Table 2). In addition, among women with excessive gestational weight gain, the prevalence of LGA was highest (38.1%) in Hispanic women with GDM and class III obesity and lowest (6.6%) in non-Hispanic black women with no diabetes and normal BMI (Table 2).
Table 1.
Maternal Characteristics
| Characteristic | White | Black | Hispanic | Asian or Pacific Islander | All Races |
|---|---|---|---|---|---|
| Total | 347,693 | 127,555 | 166,100 | 19,048 | 660,396 |
| Age (y) | |||||
| 20–29 | 57.2 | 70.8 | 58.3 | 40.7 | 59.6 |
| 30–39 | 39.7 | 26.9 | 38.8 | 55.8 | 37.4 |
| 40 or older | 3.1 | 2.3 | 2.9 | 3.5 | 2.9 |
| Education (y) | |||||
| Less than 12 | 9.0 | 17.2 | 18.1 | 7.3 | 12.8 |
| 12 | 27.4 | 40.6 | 32.0 | 19.0 | 30.9 |
| Greater than 12 | 63.5 | 42.3 | 49.9 | 73.7 | 56.3 |
| WIC status | |||||
| Yes | 28.9 | 63.7 | 48.4 | 20.9 | 40.3 |
| No | 71.1 | 36.3 | 51.6 | 79.1 | 59.7 |
| Insurance status | |||||
| Medicaid | 32.7 | 62.1 | 42.3 | 21.7 | 40.5 |
| Private | 62.3 | 31.5 | 45.1 | 67.7 | 52.2 |
| Self-pay | 3.4 | 4.9 | 10.9 | 8.6 | 5.7 |
| Other | 1.6 | 1.5 | 1.7 | 2.0 | 1.6 |
| Parity | |||||
| 0 | 41.0 | 30.1 | 37.6 | 45.1 | 38.2 |
| 1 | 35.4 | 31.1 | 36.0 | 37.4 | 34.8 |
| 2 | 15.9 | 20.7 | 17.2 | 12.3 | 17.0 |
| 3 or more | 7.7 | 18.1 | 9.2 | 5.2 | 10.0 |
| Smoking during pregnancy | |||||
| Yes | 12.3 | 3.9 | 1.8 | 1.2 | 7.7 |
| No | 87.7 | 96.1 | 98.2 | 98.8 | 92.3 |
| Nativity | |||||
| U.S. | 93.2 | 74.6 | 36.2 | 11.7 | 72.9 |
| Foreign | 6.8 | 25.4 | 63.8 | 88.3 | 27.1 |
| Height (inches) | |||||
| Mean | 64.9 | 64.8 | 63.4 | 62.7 | 64.5 |
| GDM | |||||
| GDM | 4.8 | 4.3 | 4.9 | 9.6 | 4.9 |
| No GDM | 95.2 | 95.7 | 95.1 | 90.4 | 95.1 |
| BMI (kg/m2) | |||||
| Less than 18.5 | 5.3 | 3.7 | 3.6 | 11.4 | 4.7 |
| 18.5–24.9 | 54.7 | 38.5 | 51.4 | 67.4 | 51.1 |
| 25.0–29.9 | 22.2 | 27.6 | 26.6 | 16.1 | 24.2 |
| 30.0–34.9 | 10.4 | 16.2 | 11.8 | 4.0 | 11.7 |
| 35.0–39.9 | 4.6 | 7.9 | 4.3 | 0.9 | 5.1 |
| 40 or greater | 2.8 | 6.1 | 2.3 | 0.3 | 3.2 |
| Gestational weight gain | |||||
| Inadequate | 15.3 | 22.3 | 17.5 | 22.7 | 17.4 |
| Adequate | 31.7 | 28.6 | 32.4 | 40.9 | 31.6 |
| Excessive | 53.0 | 49.1 | 50.1 | 36.4 | 51.0 |
WIC, Women, Infants, and Children; GDM, gestational diabetes mellitus; BMI, body mass index.
Data are %, excluding births with missing values of characteristic.
Number of births with missing values of characteristic, if any, is indicated.
Fig. 1.
Prevalence of large for gestational age at the 90th percentile or greater by body mass index, gestational diabetes mellitus status, and gestational weight gain for births of gestational age at 37–41 weeks. DM, diabetes mellitus; GDM, gestational diabetes mellitus.
Kim. Contributions to Large-for-Gestational-Age Births. Obstet Gynecol 2014.
Table 2.
Prevalence of Large for Gestational Age at the 90th Percentile or Greater Among Those With Excessive Gestational Weight Gain
| White (n = 347,693) |
Black (n = 127,555) |
Hispanic (n = 166,100) |
Asian or Pacific Islander (n = 19,048) |
All Races (N = 660,396) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Statistic Prevalence (%) | No DM | GDM | No DM | GDM | No DM | GDM | No DM | GDM | No DM | GDM |
| Normal BMI | 13.1 | 17.8 | 6.6 | 17.4 | 10.5 | 19.5 | 8.7 | 12.7 | 11.5 | 17.8 |
| Overweight | 16.3 | 23.2 | 7.9 | 20.3 | 12.8 | 21.0 | 10.7 | 16.2 | 13.6 | 21.8 |
| Obese class I | 18.4 | 24.3 | 8.6 | 25.2 | 15.2 | 28.3 | 15.4 | 14.9 | 15.1 | 25.3 |
| Obese class II | 21.3 | 32.8 | 9.8 | 24.9 | 17.0 | 29.5 | 21.2 | 40.0 | 17.0 | 30.2 |
| Obese class III | 24.9 | 38.0 | 11.9 | 28.0 | 18.4 | 38.1 | 29.2 | 33.3 | 18.9 | 35.1 |
DM, diabetes mellitus; GDM, gestational diabetes mellitus; BMI, body mass index.
Across the three exposures, the relative risk of an LGA neonate ranged from 1.2 (95% CI 1.16–1.25) for mothers who were overweight compared with normal weight in all race or ethnic categories to 2.9 (95% CI 1.76–4.77) for mothers who were class III obese compared with normal weight in Asian and Pacific Islander women (Table 3). The relative risk estimates for LGA among women with GDM was highest in non-Hispanic black women (2.6 [95% CI 2.5–2.8]), whereas among women with excessive gestational weight gain and maternal obesity class II and class III point estimates were highest in Asian and Pacific Islander women (2.5 [95% CI 2.2–2.8], 2.5 [95% CI 1.7–3.5], 2.9 [95% CI 1.8–4.8], respectively).
Table 3.
Relative Risks of Large for Gestational Age at the 90th Percentile or Greater
| White (n = 347,693) | Black (n = 127,555) | |||
|---|---|---|---|---|
| Statistic | Unadjusted | Adjusted | Unadjusted | Adjusted |
| RR (95% CI)* unadjusted and adjusted† | ||||
| GDM vs no GDM | 1.43 (1.38–1.48) | 1.39 (1.35–1.44) | 2.85 (2.68–3.04) | 2.64 (2.48–2.81) |
| Excessive weight gain vs adequate weight gain | 1.81 (1.77–1.85) | 1.88 (1.84–1.93) | 1.98 (1.87–2.10) | 2.08 (1.97–2.21) |
| Overweight vs normal weight | 1.28 (1.25–1.31) | 1.25 (1.22–1.28) | 1.28 (1.21–1.36) | 1.22 (1.15–1.29) |
| Obese class I vs normal weight | 1.47 (1.43–1.51) | 1.43 (1.40–1.48) | 1.49 (1.40–1.59) | 1.43 (1.31–1.53) |
| Obese class II vs normal weight | 1.87 (1.80–1.93) | 1.83 (1.77–1.90) | 1.78 (1.65–1.93) | 1.80 (1.66–1.94) |
| Obese class III vs normal weight | 2.26 (2.18–2.35) | 2.23 (2.15–2.31) | 2.12 (1.97–2.29) | 2.24 (2.08–2.41) |
| Hispanic (n = 166,100) | Asian or Pacific Islander (n = 19,048) | All Races (N = 660,396) | |||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted |
| 1.82 (1.73–1.91) | 1.74 (1.66–1.83) | 1.47 (1.24–1.74) | 1.43 (1.21–1.69) | 1.64 (1.59–1.68) | 1.62 (1.58–1.66) |
| 1.85 (1.78–1.92) | 1.92 (1.85–1.99) | 2.35 (2.06–2.69) | 2.47 (2.16–2.82) | 1.88 (1.84–1.91) | 1.93 (1.89–1.96) |
| 1.25 (1.20–1.29) | 1.21 (1.16–1.25) | 1.30 (1.13–1.49) | 1.23 (1.07–1.42) | 1.21 (1.19–1.23) | 1.24 (1.21–1.26) |
| 1.54 (1.47–1.60) | 1.49 (1.43–1.56) | 1.68 (1.36–2.08) | 1.57 (1.27–1.95) | 1.39 (1.36–1.42) | 1.45 (1.42–1.49) |
| 1.79 (1.68–1.90) | 1.77 (1.66–1.88) | 2.50 (1.75–3.58) | 2.46 (1.71–3.52) | 1.70 (1.65–1.75) | 1.81 (1.76–1.86) |
| 2.05 (1.91–2.20) | 2.07 (1.92–2.22) | 3.08 (1.89–5.02) | 2.90 (1.76–4.77) | 2.00 (1.94–2.06) | 2.19 (2.12–2.25) |
RR, relative risk; CI, confidence interval; GDM, gestational diabetes mellitus.
All P values for unadjusted and adjusted relative risks were <.01.
Adjusted for age, parity, nativity, and the other exposure groups.
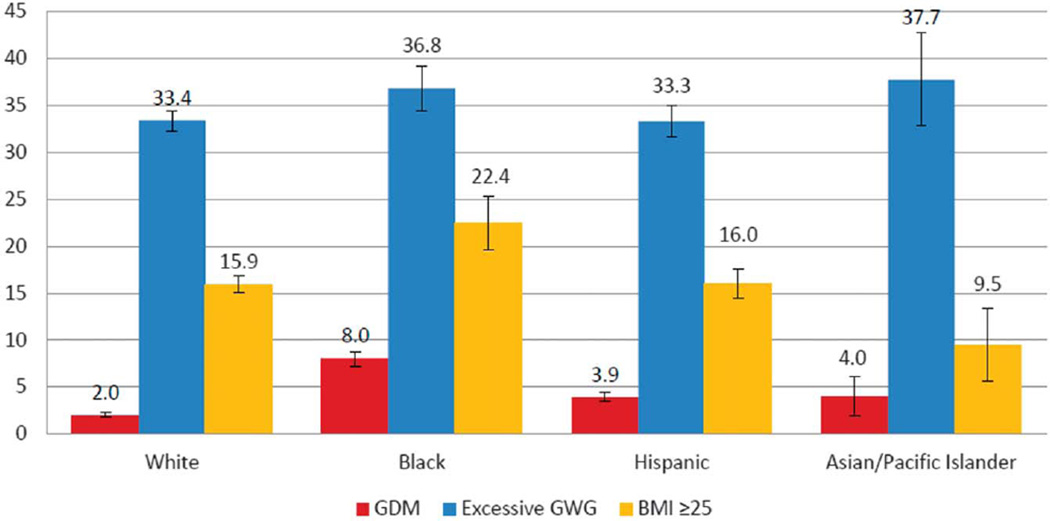
The total population-attributable fraction for having any of the three exposures ranged by race or ethnicity from 46.8% to 61.0% (Table 4). For all race or ethnic groups, GDM contributed the least to the fraction of LGA neonates ranging from 2.0% to 8.0% and excessive gestational weight gain contributed the most ranging from 33.3% to 37.7% (Fig. 2). When examining the population-attributable fractions of the mutually exclusive categories of the three exposures, we found that BMI greater than 25 in combination with excessive weight gain had the greatest contribution to LGA prevalence in the majority of the race or ethnic groups, ranging from 16.3% to 31.6% (Table 4). The exception was observed in the Asian and Pacific Islander group in which among women with normal BMI and no diabetes, excessive weight gain alone contributed 20.8% to LGA.
Table 4.
Population-Attributable Fractions of Large for Gestational Age at the 90th Percentile or Greater
| Statistic | No. of All Races |
White | Black | Hispanic | Asian or Pacific Islander |
|---|---|---|---|---|---|
| n | 660,396 | 347,693 | 127,555 | 166,100 | 19,048 |
| Mutually exclusive combinations of exposures* | |||||
| Unexposed (0 of the 3 exposures) | 206,895 | Reference category | Reference category | Reference category | Reference category |
| BMI (kg/m2) 25 or greater, no diabetes, adequate weight gain | 99,522 | 4.6 (4.3–4.9) | 7.5 (6.4–8.7) | 4.6 (4.0–5.3) | 1.7 (0.3–3.2) |
| BMI less than 25, GDM, adequate weight gain | 7,180 | 0.2 (0.1–0.3) | 0.2 (0.1–0.4) | 0.5 (0.3–0.6) | 1.1 (0.0–2.2) |
| BMI less than 25, no diabetes, excessive weight gain | 150,834 | 15.1 (14.5–15.7) | 10.7 (9.7–11.7) | 12.7 (11.8–13.5) | 20.8 (17.3–24.2) |
| BMI 25 or greater, GDM, adequate weight gain | 9,749 | 1.2 (1.1–1.3) | 3.2 (2.7–3.6) | 1.4 (1.2–1.7) | 1.8 (0.8–2.7) |
| BMI 25 or greater, no diabetes, excessive weight gain | 171,014 | 23.8 (23.2–24.4) | 31.6 (29.9–33.3) | 25.2 (24.1–26.3) | 16.3 (13.7–19.0) |
| BMI less than 25, GDM, excessive weight gain | 3,658 | 0.6 (0.5–0.7) | 1.0 (0.7–1.2) | 0.8 (0.6–0.9) | 2.0 (1.1–2.9) |
| BMI 25 or greater, GDM, excessive weight gain | 11,544 | 2.8 (2.6–3.0) | 6.8 (6.2–7.4) | 4.1 (3.7–4.4) | 3.0 (1.9–4.2) |
| Total of any of the 3 exposures | 453,501 | 48.3 (47.1–49.5) | 61.0 (58.1–63.9) | 49.3 (47.3–51.2) | 46.8 (41.3–52.2) |
BMI, body mass index; GDM, gestational diabetes mellitus.
Data are percent (95% confidence interval) unless otherwise specified.
The seven combinations of exposures are mutually exclusive and each population attributable fraction is the reduction in large-for-gestational-age prevalence that would result if the patients in that category had none of the three exposures. “Total of any of the three exposures” is the population-attributable fraction for large-for-gestational-age births attributable to any of the seven mutually exclusive exposures in the mother; the population-attributable fraction in this category is the sum of the preceding seven population-attributable fractions.
Fig. 2.
Population-attributable fractions and 95% confidence intervals (CIs) of large for gestational age at the 90th percentile or greater, stratified by race or ethnicity. Adjusted for age, parity, nativity, and the other exposure groups. GDM, gestational diabetes mellitus; GWG, gestational weight gain; BMI, body mass index.
Kim. Contributions to Large-for-Gestational-Age Births. Obstet Gynecol 2014.
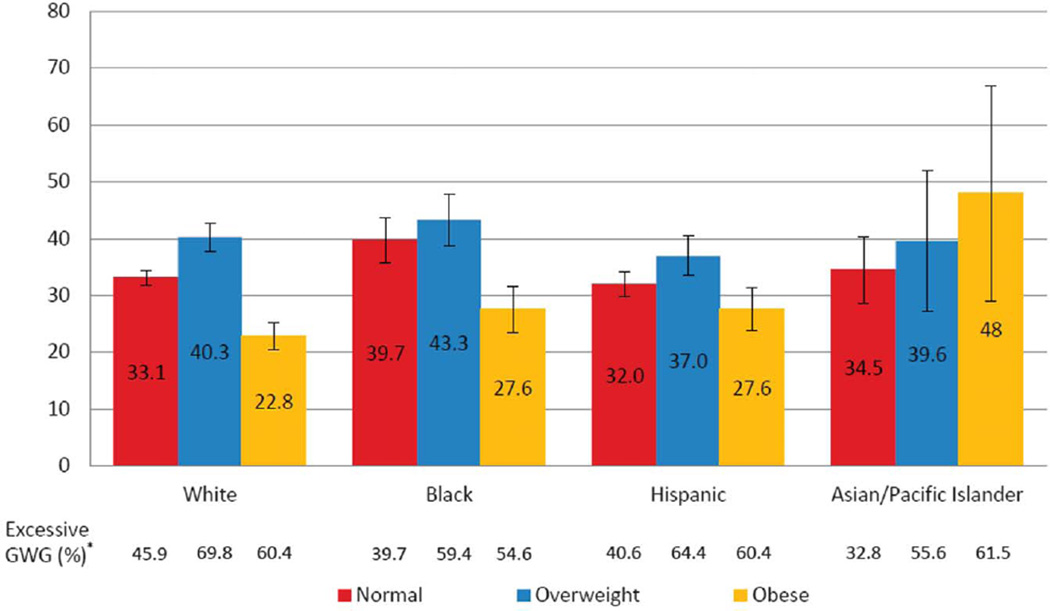
Furthermore, among births with excessive gestational weight gain, the population-attributable fractions were highest among normal weight and overweight women for all race or ethnic groups except for Asian and Pacific Islanders (Fig. 3). When further stratified by GDM, there were no consistent patterns or trends (data not shown). The prevalence of excessive gestational weight gain was highest in overweight women and lowest in normal weight women in all race or ethnic groups, except Asian and Pacific Islanders (Fig. 3).
Fig. 3.
Population-attributable fractions and 95% confidence intervals of large for gestational age at the 90th percentile or greater associated with excessive gestational weight gain (GWG), stratified by body mass index categories and race or ethnicity. Adjusted for gestational diabetes mellitus, inadequate gestational weight gain, age, parity, and nativity. *The percentage of gestational weight gain by body mass index and race or ethnicity shown in Figure 2.
Kim. Contributions to Large-for-Gestational-Age Births. Obstet Gynecol 2014.
DISCUSSION
Depending on race or ethnic, our results suggest that a reduction in LGA prevalence ranging between 46.8% and 61.0% might result if women were not overweight or obese, did not have GDM, and did not gain an excessive amount of weight. Although each of these risk conditions may be amenable to intervention, the timing and complexity of interventions differ. Lifestyle interventions aimed at healthy eating and physical activity before pregnancy may reduce overweight and obesity. Because obesity often precedes GDM, decreasing the prevalence of overweight and obesity among women of reproductive age could reduce the prevalence of both GDM and LGA. However, to increase the percentage of women entering pregnancy at a healthy weight, outreach is needed to encourage adolescent girls and young adult women to practice healthy nutrition and physical activity well before they get pregnant.14 Furthermore, preconception care guidelines recommend that all women have their BMI calculated annually and that appropriate nutrition and weight management counseling and referrals are made by clinicians.14 Effective methods to implement these guidelines for women of reproductive age are needed.
In contrast to prevention of obesity and GDM, preventing excess gestational weight gain may be more feasible as it is monitored during pregnancy. The American College of Obstetricians and Gynecologists recommends that health care providers determine a woman’s BMI at her first prenatal visit and discuss appropriate weight gain, diet, and exercise at both the initial visit and periodically throughout the pregnancy.15 Studies indicate that the most successful interventions to prevent excessive gestational weight gain closely mirror effective lifestyle programs used in nonpregnant populations; key features of these interventions include daily diet self-monitoring, frequent weight measurement, behavioral strategies, and ongoing contact with a health care provider.16 Recently, the Institute of Medicine released tools and resources for patients and health care providers to monitor weight gain and provide guidelines (www.iom.edu/healthypregnancy). One of these tools includes a pregnancy weight tracker that allows women to track their weight gain during pregnancy and compare it with recommended ranges. Further studies are needed on the efficacy of interventions to help women in all BMI groups gain within recommended gestational weight gain guidelines.
Our study is a large population-based study to examine the population-attributable fractions of LGA as a result of the combination of overweight and obesity, GDM, and excessive gestational weight gain stratified by race or ethnicity. However, the analysis has limitations. Prepregnancy weight and height were obtained from birth certificates; this information may have been obtained in clinical settings or self-reported. Estimates of obesity prevalence based on self-reported height and weight tend to be lower than those based on measured height and weight, although a previous study found minimal differences when comparing prepregnancy weight from birth certificates and clinical measurements from the first trimester.17 Therefore, if we underestimated the rate of obesity, we have underestimated the relative risks and population-attributable fraction of obesity for LGA, which would result in an underestimation of relative risk and population-attributable fraction. Second, gestational weight gain is calculated using prepregnancy weight and weight at delivery from the birth certificate. Because self-reported prepregnancy weight may be underreported and weight at delivery is more likely to have been objectively measured, we may have overestimated the rate of excessive gestational weight gain. Third, we may have underestimated the prevalence of GDM. However, because the American College of Obstetricians and Gynecologists recommends universal GDM screening for all pregnant women, we have no reason to believe that there is substantial bias in GDM diagnosis in the state of Florida. Fourth, Florida is the fourth most populous U.S. state and is diverse racially and ethnically; however, our data may not be generalizable to women outside of Florida. Finally, our study is an observational study and does not provide causal evidence for reducing LGA. As stated in the “Methods,” each population-attributable fraction is estimated to be the reduction in LGA prevalence that would occur if all women in the exposure categories had an LGA risk equal to that of women having normal levels of all three exposures.
Maternal overweight and obesity, diabetes, and excessive gestational weight gain are associated with fetal overgrowth and LGA, which then can lead to an increased risk in the offspring for later obesity and diabetes.4,5 Prevention efforts should include all women regardless of their prepregnancy BMI because more than 30% of LGA could be prevented among women with a normal BMI. Furthermore, preventing excessive gestational weight gain will also aid in reducing postpartum weight retention, which in turn may contribute to the development of obesity while entering into the next pregnancy, especially for closely spaced pregnancies.18 Therefore, it is important for health care providers to be aware of current gestational weight gain guidelines and make efforts to implement effective strategies to prevent excess gestational weight gain.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Donahue SM, Kleinman KP, Gillman MW, Oken E. Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol. 2010;115:357–364. doi: 10.1097/AOG.0b013e3181cbd5f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh JM, McAuliffe FM. Prediction and prevention of the macrosomic fetus. Eur J Obstet Gynecol Reprod Biol. 2012;162:125–130. doi: 10.1016/j.ejogrb.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Ferraro ZM, Barrowman N, Prud’homme D, Walker M, Wen SW, Rodger M, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med. 2012;25:538–542. doi: 10.3109/14767058.2011.638953. [DOI] [PubMed] [Google Scholar]
- 4.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–212. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Hinkle SN, Sharma AJ, Swan DW, Schieve LA, Ramakrishnan U, Stein AD. Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr. 2012;142:1851–1858. doi: 10.3945/jn.112.161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy trends in 20 states, 2003–2009. Prev Med. 2013;56:372–378. doi: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SY, England L, Sappenfield W, Wilson HG, Bish CL, Salihu HM, et al. Racial/Ethnic differences in the percentage of gestational diabetes mellitus cases attributable to overweight and obesity, Florida, 2004–2007. Prev Chronic Dis. 2012;9:E88. doi: 10.5888/pcd9.110249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Sappenfield W, Sharma AJ, Wilson HG, Bish CL, Salihu HM, et al. Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by nativity, Florida, 2004–2007. Obesity (Silver Spring) 2013;21:E33–E40. doi: 10.1002/oby.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. BMI classification. [Retrieved March 2, 2011]; Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 10.Davis EM, Babineau DC, Wang X, Zyzanski S, Abrams B, Bodnar LM, et al. Short Inter-pregnancy Intervals, Parity, Excessive Pregnancy Weight Gain and Risk of Maternal Obesity. Matern Child Health J. 2013 doi: 10.1007/s10995-013-1272-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanders WD, Rhodes PH. Large sample confidence intervals for regression standardized risks, risk ratios, and risk differences. J Chronic Dis. 1987;40:697–704. doi: 10.1016/0021-9681(87)90106-8. [DOI] [PubMed] [Google Scholar]
- 12.Graubard BI, Fears TR. Standard errors for attributable risk for simple and complex sample designs. Biometrics. 2005;61:847–855. doi: 10.1111/j.1541-0420.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 13.Levine BJ. The other causality question: estimating attributable fractions for obesity as a cause of mortality. Int J Obes (Lond) 2008;32(suppl 3):S4–S7. doi: 10.1038/ijo.2008.81. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K, Posner SF, Biermann J, Cordero JF, Atrash HK, Parker CS, et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR preconception care work group and the select panel on preconception care. MMWR Recomm Rep. 2006;55:1–23. [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists. Obesity in pregnancy. Committee Opinion No. 549. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121:213–217. doi: 10.1097/01.aog.0000425667.10377.60. [DOI] [PubMed] [Google Scholar]
- 16.Phelan S, Jankovitz K, Hagobian T, Abrams B. Reducing excessive gestational weight gain: lessons from the weight control literature and avenues for future research. Womens Health (Lond Engl) 2011;7:641–661. doi: 10.2217/whe.11.70. [DOI] [PubMed] [Google Scholar]
- 17.Park S, Sappenfield WM, Bish C, Bensyl DM, Goodman D, Menges J. Reliability and validity of birth certificate prepregnancy weight and height among women enrolled in prenatal WIC program: Florida, 2005. Matern Child Health J. 2011;15:851–859. doi: 10.1007/s10995-009-0544-4. [DOI] [PubMed] [Google Scholar]
- 18.Nehring I, Schmoll S, Beyerlein A, Hauner H, Von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94:1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]