Abstract
Autonomic dysfunction represents a loss of normal autonomic control of the cardiovascular system associated with both sympathetic nervous system overdrive and reduced efficacy of the parasympathetic nervous system. Autonomic dysfunction is a strong predictor of future coronary heart disease, vascular disease and sudden cardiac death. In the current review, we will discuss the clinical importance of autonomic dysfunction as a cardiovascular risk marker among breast cancer patients. We will review the effects of antineoplastic therapy on autonomic function, as well as discuss secondary exposures, such as psychological stress, sleep disturbances, weight gain/metabolic derangements, and loss of cardiorespiratory fitness which may negatively impact autonomic function in breast cancer patients. Lastly, we review potential strategies to improve autonomic function in this population. The perspective can help guide new therapeutic interventions to promote longevity and cardiovascular health among breast cancer survivors.
Keywords: autonomic dysfunction, cardiovascular disease, breast cancer
Due to significant improvements in early detection and adjuvant therapy, early breast cancer patients are now expected to live long enough to be at risk for competing causes of death.1 Cardiovascular disease (CVD) is rapidly becoming the predominant cause of mortality in breast cancer survivors over 60 years of age.2 The magnitude of this problem is likely to increase with the aging of the US population, improvements in breast cancer-specific survival, and the continued use of antineoplastic agents with cardiovascular toxicities. Thus, given the nearly 3 million breast cancer survivors in the US, the number of women at excess risk for CVD is likely to increase dramatically over the next two decades, requiring specific strategies to predict and mitigate these risks.
Adjuvant therapies used in the current treatment of early breast cancer are associated with unique and varying degrees of direct (e.g., cardiac dysfunction) as well as indirect (e.g., unfavorable CVD risk factors) sequential and progressive cardiovascular insults.3 In current oncology practice, the ‘cardiovascular’ impact of cytotoxic therapies is evaluated solely by changes in resting left ventricular ejection fraction (LVEF). However, LVEF is load-, rate-, and contractility-dependent and acute declines in myocardial function can be initially compensated for in order to maintain cardiac output.4 Thus, left ventricular dysfunction is a late marker, only becoming evident after significant myocardial damage has already occurred. Therefore, alternative tools are required to identify patients at high risk for adverse cardiovascular impacts before significant damage develops.
The term “autonomic dysfunction” describes a loss of normal autonomic regulation of the cardiovascular system associated with both excessive sympathetic nervous system (SNS) activation and a reduced ability of the parasympatheic nervous system (PNS) to deactivate appropriately. Autonomic dysfunction can result in increased heart rate, atrioventricular node conduction and left ventricular contractility.5 The autonomic nervous system also regulates various hormonal systems including: the hypothalamic-pituitary-adrenal (HPA) axis, the reninangiotensin-aldosterone system (RAAS), and the endocannabinoid system. Thus, the autonomic dysfunction may also promote oxidative stress, reduce vasodilation, increase chronic inflammation, and accelerate atherosclerosis progression leading to CVD.6, 7 Clinically, the onset and progression of autonomic dysfunction can manifest through chronically elevated heart rates and a loss of normal heart rate variability (HRV), which becomes both a marker of increased risk and, through decreased cardiac resilience, a mediator of adverse cardiovascular consequences.8, 9
The current review will highlight the emerging data on autonomic dysfunction as a cardiovascular risk marker among breast cancer patients. We will review current methods for assessing cardiac autonomic function, the effects of anti-neoplastic therapy on autonomic function. We will also discuss secondary exposures, such as: psychological stress, sleep disturbances, weight gain/metabolic derangements, and loss of cardiorespiratory fitness that occur in breast cancer patients and may adversely impact autonomic function. Finally, potential strategies to prevent and/or mitigate autonomic dysfunction will be discussed. Ultimately, this review sets the stage for future studies to unravel potential interventions via the autonomic pathway to prevent competing risk of CVD among breast cancer patients.
Measures of Cardiac Autonomic Function
Healthy autonomic function is the capacity of the autonomic nervous system to deliver appropriate stimulatory and inhibitory signals through sympathetic and parasympathetic pathways. The interplay between sympathetic and parasympathetic inputs is vital for the regulation of cardiac output via changes in heart rate, electrical conduction, left ventricular contractility, vascular tone and blood pressure.10
Changes in cardiac autonomic function can be tracked by several techniques (Table 1). The simplest measure of cardiac autonomic status is resting heart rate. Greater autonomic dysfunction is associated with increasing resting heart rates over time.11 A more robust measure of autonomic function is heart rate variability (HRV), measured using continuous heart rate monitoring. HRV is a set of parameters which reflects interval fluctuations between sequential beats of the heart.12 Measures derived from interval differences between successive beats reflect parasympathetically-modulated changes in heart rate. Other HRV measures reflect the combined signaling of the two arms of the autonomic nervous system and reflect both intrinsic (e.g., baroreflex, renin-angiotensin, sleep cycles, circadian) and extrinsic (activity, rest) rhythms.13 In general, decreased or decreasing HRV would be a signal for worse cardiac autonomic dysfunction. However, a higher, but more disorganized, HRV pattern, detectable by certain “non-linear” HRV measures also reflects greater cardiac autonomic dysfunction.14 Ideally, HRV is measured using 24-hour ambulatory monitoring which can capture both daytime heart rate patterns and heart rate patterns during sleep, providing insights into circadian rhythm, sleep quality and possible sleep-disordered breathing or periodic limb movements,15 all of which affect cardiac autonomic functioning. However, significant clinical information can be obtained from shorter recordings performed, perhaps, at the time of clinical visits and in association with standard “bedside autonomic tests”.16
Table 1.
Clinical approaches to characterizing cardiac autonomic functioning over time
| Measures obtained | Measure of Dysfunction | |
|---|---|---|
| ECG | Heart rate (HR) | ↑ Resting HR |
| Lack of variation in length of beat-to-beat intervals | ||
| Bedside autonomic function tests | HR responses to respiratory maneuvers and lying down to standing up | ↓in HR response to tests |
| Continuous ECG (Minutes to 24-hours) | Digitized to get beat-to-beat intervals for normal heart beats and counts of ectopic beats | |
| Under controlled conditions | Usually resting. PNS predominance at rest. Limited data about time domain and frequency domain HRV. | ↑ Resting HR ↓ in short-term HRV measures |
| During normal activities (24-hour Holter recording) | Full spectrum of time domain, frequency domain and “non-linear” HRV measures. Heart rate turbulence. Accurate count of atrial and ventricular ectopic beats. Characterization of HR patterns during sleep. Requires research quality scanning for most sensitive measures. |
↑ or ↓ in daytime HR. ↑ in nighttime HR. ↓in time domain/frequency domain HRV measures reflecting loss of regulatory function at circadian, ultradian, short-term and beat-to-beat levels. ↑ or ↓ in non-linear measures reflect more disorganized rhythm. ↓ in heart rate turbulence reflects loss of regulatory function in response to a sudden drop in cardiac output. ↑ in atrial or ventricular ectopy counts and complexity. ↑ in HR characteristics of poor sleep and/or sleep-disordered breathing including increased periodic and Cheyne-Stokes respiratory patterns as seen on beat-to-beat plots of HR tachograms. |
| Exercise testing | Heart rate recovery (HRR) | ↓ in HR recovery at 1-minute post peak exercise |
| Heart rate response Chronotropic incompetence |
↓ in peak HR at peak exercise. Inability to raise HR. |
Heart rate recovery (HRR) and chronotropic competence, assessed after submaximal or maximal exercise stress testing, also reflect the health of cardiac autonomic regulation. Traditionally, HRR is measured as the difference in heart rate assessed at peak exercise and 1-minute post exercise. A reduction of less than 12 beats/min or the 10th percentile within the first minute reflects inadequate reactivation of the parasympathetic nervous system post-stress.17 Chronotropic competence describes the ability of the heart to adjust its intrinsic rate appropriately for the level of cardiovascular demand during exercise testing. Chronotropic competence is commonly determined from measurement of heart rate reserve (difference between heart rate at peak exercise compared to rest) or achievement of age-predicted maximal heart rate.
Autonomic Dysfunction is a Prognostic Marker of Short-Term and Long-Term CVD Risk
The importance of autonomic dysfunction as a marker of CVD risk was first demonstrated in a series of studies of canines subjected to transient ischemia in the post-myocardial infarction (MI) setting. In these studies, the presence of parasympathetic activity (or lack of sympathetic activation) was associated with a lower incidence of sudden cardiac death.18 A subsequent study in humans demonstrated that decreased HRV and an impaired baroreceptor reflex were prognostic of cardiac death independent of baseline LVEF among post-MI patients. After 21 months of follow-up, impaired 24-hour HRV was associated with a three-fold higher risk of cardiac mortality in men and women (HR 2.8 95% CI,1·2–6.2) compared with individuals with normal HRV measures.19 Cole et al. extended these findings by showing that HRR after exercise testing was strongly predictive of all-cause mortality after multivariable adjustment (HR, 2.0; 95 % CI, 1.5- 2.7) among individuals referred for exercise testing.20 This has been corroborated by others and found to be independent of angiographic severity and cardiac function.21 Importantly, more recent studies suggest that resting heart rate, the simplest measure of autonomic functioning, is also a powerful predictor of future CVD events and survival. Cooney et al. found that among 10,519 men and 11,334 women followed in a Finish population-based study, a 15 beat increase in resting heart rate was associated with a 24% and 32% increase in future cardiovascular death in men and women, respectively.22 Moreover, in a 2012 study of 112, 680 men and women pooled from 12 cohort studies, higher resting heart rate (≥80 beats/min compared to <65 beats/min) was associated with an increased risk of both CVD events (HR, 1.44, 95% CI: 1.29-1.60) and all-cause mortality (HR 1.54, 1.43-1.66).23
Relationships between autonomic dysfunction and cardiovascular risk have also been demonstrated in studies specifically focused on women. Mora et al. investigated the clinical value of HRR independently and in combination with exercise capacity in predicting 20-year CVD mortality.24 Women with HRR and exercise capacity values below the median had nearly a 4-fold higher risk of CVD death after adjustment for traditional CVD risk factors (HR 3.5; 95% CI: 1.6-7.9) compared with women with values above the median. More recently, Gulati et al. found that achieving one standard deviation (1-SD) below age-predicted peak heart rate was the during exercise testing was the strongest predictor of adverse outcomes (HR 3.5; 95% CI: 2.9-4.2) in asymptomatic women over 15.9 ±2.2 years.25 Taken together, these studies indicate that autonomic dysfunction is also a strong predictor of both short-term and long-term CVD mortality in women.
Antineoplastic Exposure and Autonomic Dysfunction in Breast Cancer Patients
Multiple pathways between anthracycline-based therapy and cardiotoxicity have been delineated.26 Though less well-elucidated, several lines of evidence suggest that anthracyclines act directly on adrenergic nerve tissue to alter autonomic function. In animal models of heart failure, left ventricular dysfunction often presents with enhanced sympathetic activity as evidenced by elevated circulating concentrations of norepinephrine.27 Local activation of adrenergic receptors and subsequent depletion of myocardial norepinephrine occur as heart failure ensues.28 Thus, elevated circulating levels of norepinephrine and depletion of myocardial norepinephrine are characteristic of sympathetic hyperactivity during acute injury. Previous studies among patients exposed to anthracyclines have also demonstrated high circulating levels of norepinephrine as well as reductions in cardiac autonomic function (i.e. impaired HRV from short-term recordings) prior to left ventricular dysfunction.29, 30 Studies using radiolabeled metaiodobenzylguanidine (MIBG) scintigraphy to capture myocardial norepinephrine uptake have also demonstrated decreases in norepinephrine uptake with increasing anthracycline exposure.31, 32 Importantly, alterations in circulating and cardiac neuronal norepinephrine concentrations occurred prior to left ventricular dysfunction and were dose-dependent, substantiating the hypothesis that anthracyclines impact norepinephrine turnover both locally and systemically to impact autonomic signaling prior to clinically evident heart failure. These data support the hypothesis that cardiac autonomic regulation is altered with anthracycline-based therapy and should be detectable prior to the onset of LV dysfunction.
Several small clinical studies support the hypothesis that anthracycline therapy is associated with autonomic dysfunction in women with breast cancer. In a study of 47 breast cancer patients, with an average follow-up of 3 years post-chemoendocrine adjuvant therapy, our group found that resting heart rate was significantly elevated in patients compared with age-matched controls (91 ± 15 vs. 76 ± 8 bpm, respectively p=0.004) despite normal LVEF (>50%).33 In a second study, we demonstrated that 50% of breast cancer patients previously treated with anthracycline-trastuzumab containing chemotherapy for HER2 positive operable breast cancer presented with sinus tachycardia (resting HR >110 bpm) compared with 0% age-matched controls.34 Finally, in examining resting heart rate across the breast cancer continuum, we showed that mean resting heart rate was 74 ±10 bpm in breast cancer patients prior to adjuvant therapy compared to 91 ±17, 89 ±16, and 92 ±17 bpm in patients during adjuvant therapy, after adjuvant therapy, or in those with metastatic disease, respectively.35
While studies from other groups have shown similar results,36-38 the exact time course of autonomic dysfunction following anthracycline exposure is unclear, likely because of small sample sizes, diverse measures of autonomic function, and lack of long-term follow-up. Further work is needed to determine whether anthracycline exposure alone or in combination with radiation therapy and/or other cytoxic agents has additive adverse effects on autonomic function. Importantly, recent data suggest that thoracic radiation exposure is associated with a higher risk of autonomic dysfunction and subsequent all-cause mortality among Hodgkin lymphoma survivors.39 Studies are lacking on the question of whether radiation for the treatment of breast cancer independently impacts autonomic function, although there is compelling evidence for the development of ischemic heart disease and myocardial fibrosis among women after radiotherapy.40 Lastly, cyclophosphamide and taxanes are commonly used in combination with anthracyclines as first-line therapy for breast cancer. Two small studies have demonstrated no effect of taxanes by themselves on HRV or blood pressure control.41, 42 Cyclophosphamide commonly causes nausea and vomiting, which are mediated by the autonomic nervous system and are generally associated with other symptoms of autonomic activation; however, susceptibility to nausea and fatigue during treatment has also been associated with pre-treatment differences in psychological factors.43 Further studies are required to assess whether this relationship of psychological factors and symptoms impacts objective measures of cardiac autonomic functioning and subsequent levels or autonomic dysfunction.
Additional Mechanisms Leading to Autonomic Dysfunction in Breast Cancer
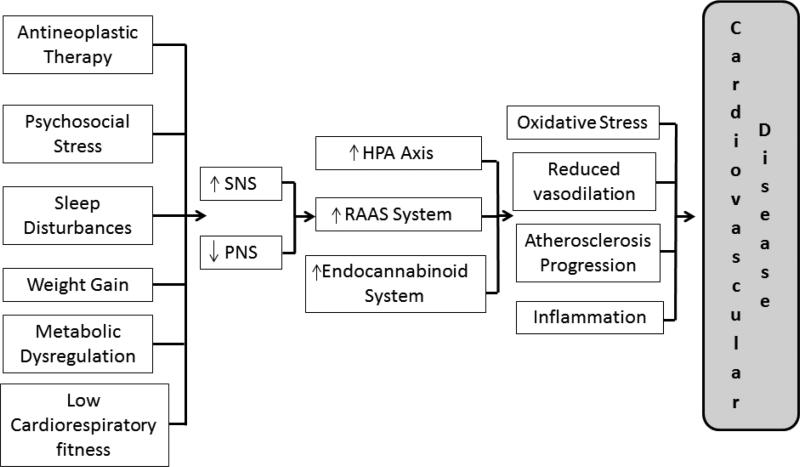
The Figure outlines the potential mechanisms associated with both autonomic dysfunction and increased CVD risk in breast cancer patients. In addition to anthracyclines and other therapies such as radiation treatment, psychosocial stress, sleep disturbances, weight gain/metabolic dysregulation, and low cardiorespiratory fitness can be present in breast cancer patients, both at the time of diagnosis and as a consequence of diagnosis and treatment. Each of these potentially modifiable risk factors can result in even greater autonomic dysfunction and potentially higher CVD risk. A discussion of each of these factors will be presented below.
Figure. Potential mechanisms associated with both autonomic dysfunction and increased cardiovascular disease (CVD) risk in breast cancer patients.
Breast cancer diagnosis is associated with therapy-induced cardiovascular injury and lifestyle perturbations leading to increased activation of the sympathetic nervous system (SNS) and decreased activation of the parasympathetic nervous system (PNS). In turn, this autonomic imbalance triggers the hypothalamic-pituitary-adrenal (HPA) axis, the renin-angiotensin-aldosterone system (RAAS), and the endocannabinoid system, leading to oxidative stress, reduced vasodilation, inflammation, and atherosclerosis progression that promotes CVD.
Psychosocial stress
Growing epidemiologic evidence supports a link between psychological stress and CVD, with autonomic dysfunction as one of the proposed pathophysiologic mechanisms.44, 45 Psychological stress results in a perception of chronic threat and an upregulation of the sympathetic-adrenal-medullary axis and release of catecholamines (norepinephrine and epinephrine) prompting chronically heightened cardiovascular responses. The downstream effects of chronic psychological stress include fatigue and depression which can exacerbate key factors involved in SNS activity. For example, in the post-adjuvant setting, breast cancer patients who report chronic fatigue have significantly higher norepinephrine levels and lower HRV compared to less fatigued counterparts (p=0.02).46 Importantly, over 30% of breast cancer survivors have cancer-related fatigue.47 Moreover, breast cancer patients with depression are more likely to exhibit autonomic dysfunction compared to their counterparts without depression.48 Depression is also highly prevalent at one year post diagnosis (50%) and remains elevated out to 5-years.49 Taken together, psychological stress leading to fatigue and depression is common in the breast cancer setting and each share common pathways implicated in the development of autonomic dysfunction.50, 51
Circadian - Sleep
Basal heart rate and HRV are, in part, regulated by central and cardiomyocyte circadian clocks.52-54 The master circadian pacemaker, located within the hypothalamic suprachiasmatic nuclei (SCN), directly regulates heart rate via neural and neuroendocrine pathways or indirectly through the sleep/wake cycle.55, 56 Circadian rhythms are integral in the sleep-wake cycle, hormonal secretions, as well as other physiological processes in coordination with peripheral clocks found tissues such as liver, adipose, and heart.57
Sympathetic and parasympathetic activities vary with sleep/wake cycles.58, 59 Heart rate is lowest during deep non-REM sleep compared to wakefulness. During REM sleep, sympathetic-nerve activity and subsequent blood pressure and heart rate are reported to be similar to waking hours. Disturbances in sleep/wake potentially influence sympathetic activity and timing, previously linked to increased cardiovascular risks.60, 61 In a study of 52,610 men and women followed for a mean 11.4 years, insomnia symptoms were associated with a graded increase in risk of acute myocardial infarction.62 For example, in adjusted models individuals with insomnia symptoms had a 2-fold greater risk of MI compared those with no symptoms (HR 2.1; 95% CI: 1.1-4.0). In addition, difficulty maintaining sleep every night (HR 1.5 95% CI: 1.2-1.9), a feeling of nonrestorative sleep more than once a week (HR 1.4; 95% CI: 1.1-1.8) was associated with significant MI risk after adjustment for potential confounders compared with those with no sleep problems.
Importantly, sleep/wake cycles are commonly disturbed in cancer patients.63, 64 More severe sleep disorders occur at a frequency 2-3 times greater than the general population.65, 66 In a recent study by Savard et al., 962 patients with non-metastatic cancer in the perioperative phase (T1) were studied and followed over 18 month period.67 At T1, 31% had insomnia symptoms, defined as a complaint of sleep difficulties and need for hypnotic medication 1-2 night per week, while 28% had more severe insomnia syndrome. In that study, breast cancer patients had the highest rates of insomnia (42-69%) and insomnia syndrome (25-36%). Over an 18 month period, rates of insomnia decreased significantly across all types of cancers, though they remained higher than in the general population. Importantly, sleep remained significantly impaired and persisted throughout treatment. These results highlight the prevalence of severe forms of insomnia prior to, during, and beyond active treatment in breast cancer which can subsequently influence sympathetic activity and autonomic timing, linked to increased cardiovascular risks.
Weight Gain / Metabolic Derangements
Previous studies support a strong correlation between weight change and autonomic functioning. For example, weight gain of 10% above baseline body weight has been shown to be associated with sympathetic activation.68 Conversely, corresponding weight loss of approximately 4 kg has been shown to be associated with enhancement in parasympathetic activity well as improvements in heart rate recovery.69, 70 Prior work has linked chronic sympathetic activation as a potential mechanism between weight gain and CVD risk.71
Importantly, breast cancer patients are more likely to gain weight, experience changes in visceral adiposity, and report other features of the metabolic syndrome compared to healthy women. 72, 73 Breast cancer patients commonly gain an average of 3-6 pounds over the course of treatment.74 Sarcopenic obesity, defined as increased fat mass with concomitant skeletal muscle loss, can occur as a result of weight gain in breast cancer patients.75 Skeletal muscle changes related to sarcopenia may contribute to an unfavorable metabolic milieu leading to greater sympathetic activation. Moreover, skeletal muscle abnormalities can cause metabolic distress during exercise and disturb normal patterns of cardiorespiratory control.76 Muscle metabolites evoked during exercise can stimulate a muscle reflex leading to elevated heart rate, blood pressure, and enhanced muscle sympathetic nerve activity which, when chronically stimulated, can adversely impact LV function.77 Delineating whether these potential mechanisms link fat distribution, autonomic control, and CVD risk in breast cancer patients will require future studies.
Cardiorespiratory Fitness
Cardiorespiratory fitness, defined as the efficiency of O2 transport and utilization, is a robust marker of CVD mortality risk.78-80 One proposed mechanism for CV protection associated with fitness is through an autonomic pathway. Indeed, multiple studies have shown a strong relationship between cardiorespiratory fitness and autonomic control,81-85 with a reported correlation coefficient of 0.53 between HRV and VO2max (mL.kg.−1min−1). Direct correlations between cardiorespiratory fitness and autonomic function are less well delineated in the breast cancer literature. We have demonstrated lower cardiorespiratory fitness levels among breast cancer patients compared to age-matched controls, with lower fitness sustained at 7 years post-treatment.86, 87 Similarly, we have shown significant differences in resting heart rate and chronotropic competence (peak heart rate- resting heart rate) as a function of the timing of adjuvant therapy and stage of disease (p<0.001).35 However, to date, no study has determined the relationship between loss of cardiorespiratory fitness and impairment in autonomic function in a breast cancer population or assessed longitudinal patterns of change. It will be important to elucidate the relative contribution of cardiorespiratory fitness to autonomic dysfunction or vice versa in order to tailor treatment therapies that might help minimize this adverse cycle.
Strategies to Improve Autonomic Function
Exercise training is an attractive intervention to improve autonomic dysfunction due to the ability of exercise to simultaneously impact cardiopulmonary and skeletal muscle O2 consumption, both important regulators of sympathetic activity.88 In a recent study of individuals in a cardiac rehabilitation program participating in aerobic training 3 times a week for 30-50 minutes, patients with abnormal autonomic function at baseline (defined as impaired HRR) who normalized HRR with exercise had a significant survival benefit.89 In addition to aerobic training, resistance training has been show to maintain lean muscle mass, improve O2 transport from circulation to muscle mitochondria, and to increase the oxidative capacity of the muscle, which may be important in preserving normal skeletal muscle sympathetic nervous system activity.90 Overall, prior work suggests that exercise training can shift the autonomic balance and modify survival among those post-MI.91
We have shown that exercise training is a safe and feasible intervention in the oncology setting.92, 93 Moreover there is emerging data that exercise training has positive effects on autonomic function among cancer patients. One recent study in cancer patients demonstrated improvement in heart rate response during submaximal exercise testing among individuals randomized to combination aerobic/resistance training compared to controls [mean change 4.3 bpm (95% CI: 2.9-5.7), p<0.001].94 In a study of cancer patients in both the acute treatment phase and post-treatment phase, Neiderer et al. found that participation in a 16-week, moderate intensity exercise intervention improved cardiac autonomic regulation, particularly heart rate variability.95 Among breast cancer patients, our group has also recently completed a study showing improvements in VO2peak as well as a mitigating effect on change in resting heart rate after endurance exercise 3 times a week for 12 weeks.96 Independent of changes in VO2peak, there is robust data on the impact of exercise training on improvement of quality of life and reduction of cancer related fatigue in this population.97
Exercise training in breast cancer patients has also been associated with improved sleep, though there is controversy on type and timing of exercise to facilitate sleep. The importance of adequate sleep is often overlooked. In a recent study, sleep quality, as measured objectively using actigraphy, was a predictor of survival in patients with advanced breast cancer.98 Untreated sleep disorders can significantly add to the downward spiral of exhaustion, weight gain and psychological distress in cancer patients; in these cases, referral to sleep medicine specialists for sleep studies is recommended. The efficacy of sleep interventions, such as cognitive behavior therapy, to alter sleep patterns have been substantiated.99, 100 Also efficacious in this context is yoga therapy which also has been shown to have a positive impact on sleep patterns in breast cancer patients.101
Beyond exercise training and sleep-related interventions, initial studies of pharmacologic therapies directed toward increasing HRV and normalizing autonomic dysfunction have been shown to mitigate the negative effects of chemotherapy on cardiac function among cancer patients. For example, beta-blockade has been shown to offset anthracycline-induced cardiomyopathy among cancer patients,102, 103 as well as reduce the incidence of heart failure.104 Though no direct relationship between beta-blockade and autonomic dysfunction has been elucidated, beta-blockers are protective in cardiac failure by preventing the deleterious link between chronically enhanced sympathetic activity and cardiac failure.105 A similar argument could be made for drugs that inhibit RAAS – a key pathway involved in autonomic function. Several prior studies have demonstrated the cardioprotective effect of ACE inhibitors and ARBs in patients undergoing chemotherapy.106-108 Moreover, spironolactone, an aldosterone antagonist, has been shown to reduce early morning increases in heart rate in patients with known heart failure109 and have a favorable response on HRV in patients with ischemic heart failure.110 In a recent study of 83 breast cancer patients, 25mg of spironolactone daily during anthracycline treatment mitigated a decrease in LVEF.111 Lastly, statins have shown benefit for the prevention of anthracycline-based cardiotoxicity in a small single center study.112 In the breast cancer literature, continuous statin use has been shown to decrease incident heart failure hospitalization among those receiving anthracyclines.113 While these data are promising, each of the proposed medically-targeted regimens require large clinical trials to support standard use of these medications in the breast cancer population to prevent autonomic dysfunction leading to cardiovascular disease.
Lastly, a stronger research focus on psychosocial stress among breast cancer patients and post-treatment survivors is needed. The diagnosis of cancer per se is a major trauma and a significant number of patients (estimated at 5-10%) still meet criteria for post-traumatic stress disorder (PTSD) 5 years after diagnosis.114 Systematic screening for psychosocial stress, particularly adverse childhood experiences,115-117 prior to a breast cancer diagnosis as well as during and after treatment118, 119 and development of appropriate therapies could mitigate development of CVD associated with chronic over-stimulation of SNS and/or loss of the restorative function of the PNS. Having a way for patients to access professional and knowledgeable support for acute exacerbations of symptoms of distress during treatment may reduce panic and promote better navigation of this difficult period both psychologically and physiologically. Moreover, the effect of expanding access to stress-reducing interventions such as yoga, mindfulness mediation, music therapy, and other integrative or complementary therapies that support the ability return to a relaxed state and a greater sense of efficacy should be studied as a way to reduce psychosocial stress, reduce symptoms and support improved cardiac autonomic functioning among breast cancer patients.120-122
In summary, emerging evidence shows that key factors in cardiac autonomic function, heart rate and heart rate variability, predict functioning and survival among patients with metastatic and recurrent breast cancer.123 There is a need to extend these findings to earlier stage breast cancers, as well as to perform studies on a larger scale. Data on whether restoration in autonomic functioning, using nonpharmacological or pharmacological interventions, reduces CVD risk breast cancer patients undergoing adjuvant chemotherapy will be of key importance. Other major gaps in the current knowledge include: 1) dose, type, and time course of chemotherapy associated with autonomic dysfunction, 2) etiology and prevalence of psychological stress and sleep disorders among breast cancer patients and their impact on cardiac function, 3) mechanistic links between sympathetic activity and metabolic derangements in breast cancer patients, and 4) the effectiveness of exercise interventions, sleep interventions and medical therapies to improve autonomic function in breast cancer patients. Taken together, more robust data in these areas will allow clinicians to pinpoint the timing of autonomic function insult as well as determine the most effective intervention to offset this impact (Table 2 – proposed studies). Given the enhanced mobile technology that allows for minute to minute capture of heart rate, physical activity, sleep and robust measures of autonomic functioning, as well as measures of mood states, the future looks promising to address this knowledge gap.124 Ultimately, this line of research will enhance understanding of autonomic dysfunction in the pathogenesis of CVD in breast cancer and provide insight into potential pathways to intervene to promote longevity and cardiovascular health.
Table 2.
Potential future directions to assess the relationship between autonomic dysfunction and CVD in the breast cancer setting.
| Underlying Mechanisms |
| • Identify baseline risk factors that contribute to autonomic dysfunction. |
| • Examine the impact of psychological stress and sleep disorders on autonomic dysfunction. |
| • Elucidate the mechanistic link between metabolic derangements and autonomic dysfunction. |
| • Evaluate underlying mechanisms of conventional and/or novel adjuvant therapies contributing to autonomic dysfunction. |
| Clinical Importance |
| • Perform large epidemiological studies in the breast cancer setting that could: |
| i) Determine the incidence of autonomic dysfunction before, during, and following therapy. |
| ii) Delineate the time course of therapy-induced autonomic dysfunction. |
| iii) Evaluate the association between autonomic dysfunction and CVD. |
| Prevention/Management |
| • Perform adequately-powered multicenter RCTs of both pharmacologic as well as non-pharmacologic interventions at each stage in disease progression that could: |
| i) Compare different medications, varying doses and combinations of pharmacologic therapies for use in preventive strategies. |
| ii) Establish most effective timing (before, during, or following therapy) to perform aerobic exercise training, and the optimal exercise intensity required to prevent/treat autonomic dysfunction. |
Acknowledgements
SGL is supported in part by the National Institute of General Medical Sciences/ National Institutes of Health (P20GM103644-01A1). LJ is supported in part by grants from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Lee Jones is a cofounder a commercial company, Exercise by Science, Inc.
Disclosure
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
References
- 1.Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, Czene K. Prognosis of patients with breast cancer: Causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 2.Patnaik JL, Byers T, Diguiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Mann DL, Bristow MR. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 5.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circulation research. 1971;29:437–445. doi: 10.1161/01.res.29.5.437. [DOI] [PubMed] [Google Scholar]
- 6.Caetano J, Delgado Alves J. Heart rate and cardiovascular protection. European journal of internal medicine. 2015;26:217–222. doi: 10.1016/j.ejim.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Kadoya M, Koyama H, Kurajoh M, Kanzaki A, Kakutani-Hatayama M, Okazaki H, Shoji T, Moriwaki Y, Yamamoto T, Emoto M, Inaba M, Namba M. Sleep, cardiac autonomic function, and carotid atherosclerosis in patients with cardiovascular risks: Hscaa study. Atherosclerosis. 2015;238:409–414. doi: 10.1016/j.atherosclerosis.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 8.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International journal of cardiology. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 9.Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: A review on the pathophysiologic mechanisms of neurocardiology. International journal of cardiology. 2013;166:30–37. doi: 10.1016/j.ijcard.2012.03.165. [DOI] [PubMed] [Google Scholar]
- 10.Levy MN. Autonomic interactions in cardiac control. Ann N Y Acad Sci. 1990;601:209–221. doi: 10.1111/j.1749-6632.1990.tb37302.x. [DOI] [PubMed] [Google Scholar]
- 11.Thayer JF, Lane RD. The role of vagal function in the risk for cardiovascular disease and mortality. Biol Psychol. 2007;74:224–242. doi: 10.1016/j.biopsycho.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Stein PK, Domitrovich PP, Hui N, Rautaharju P, Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: Results of graphical and nonlinear analyses. Journal of cardiovascular electrophysiology. 2005;16:954–959. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- 15.Stein PK PP. Graphical analysis of heart rate patterns to assess cardiac autonomic function. McGraw-Hill Professional; New York: 2011. [Google Scholar]
- 16.Ewing DJ, Neilson JM, Shapiro CM, Stewart JA, Reid W. Twenty four hour heart rate variability: Effects of posture, sleep, and time of day in healthy controls and comparison with bedside tests of autonomic function in diabetic patients. British heart journal. 1991;65:239–244. doi: 10.1136/hrt.65.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauer MS. Autonomic function and prognosis. Cleve Clin J Med. 2009;76(Suppl 2):S18–22. doi: 10.3949/ccjm.76.s2.04. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction. An experimental preparation for sudden cardiac death. Circulation. 1984;69:790–800. doi: 10.1161/01.cir.69.4.790. [DOI] [PubMed] [Google Scholar]
- 19.La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Atrami (autonomic tone and reflexes after myocardial infarction) investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 20.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe J, Thamilarasan M, Blackstone EH, Thomas JD, Lauer MS. Heart rate recovery immediately after treadmill exercise and left ventricular systolic dysfunction as predictors of mortality: The case of stress echocardiography. Circulation. 2001;104:1911–1916. [PubMed] [Google Scholar]
- 22.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619 e613. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Woodward M, Webster R, Murakami Y, Barzi F, Lam TH, Fang X, Suh I, Batty GD, Huxley R, Rodgers A. The association between resting heart rate, cardiovascular disease and mortality: Evidence from 112,680 men and women in 12 cohorts. European journal of preventive cardiology. 2012;21:719–726. doi: 10.1177/2047487312452501. [DOI] [PubMed] [Google Scholar]
- 24.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 25.Gulati M, Shaw LJ, Thisted RA, Black HR, Bairey Merz CN, Arnsdorf MF. Heart rate response to exercise stress testing in asymptomatic women: The st. James women take heart project. Circulation. 122:130–137. doi: 10.1161/CIRCULATIONAHA.110.939249. [DOI] [PubMed] [Google Scholar]
- 26.Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: Current evidence and underlying mechanisms. Circulation. 124:642–650. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin- Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (solvd). Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa T, Handa S, Suzuki M, Nagami K. Abnormalities in sympathoneuronal regulation are localized to failing myocardium in rabbit heart. J Am Coll Cardiol. 1994;24:210–215. doi: 10.1016/0735-1097(94)90565-7. [DOI] [PubMed] [Google Scholar]
- 29.Nousiainen T, Vanninen E, Jantunen E, Remes J, Ritanen E, Vuolteenaho O, Hartikainen J. Neuroendocrine changes during the evolution of doxorubicin-induced left ventricular dysfunction in adult lymphoma patients. Clin Sci (Lond) 2001;101:601–607. [PubMed] [Google Scholar]
- 30.Hrushesky WJ, Fader DJ, Berestka JS, Sommer M, Hayes J, Cope FO. Diminishment of respiratory sinus arrhythmia foreshadows doxorubicin-induced cardiomyopathy. Circulation. 1991;84:697–707. doi: 10.1161/01.cir.84.2.697. [DOI] [PubMed] [Google Scholar]
- 31.Wakasugi S, Wada A, Hasegawa Y, Nakano S, Shibata N. Detection of abnormal cardiac adrenergic neuron activity in adriamycin-induced cardiomyopathy with iodine-125- metaiodobenzylguanidine. J Nucl Med. 1992;33:208–214. [PubMed] [Google Scholar]
- 32.Wakasugi S, Fischman AJ, Babich JW, Aretz HT, Callahan RJ, Nakaki M, Wilkinson R, Strauss HW. Metaiodobenzylguanidine: Evaluation of its potential as a tracer for monitoring doxorubicin cardiomyopathy. J Nucl Med. 1993;34:1283–1286. [PubMed] [Google Scholar]
- 33.Jones LW, Haykowsky M, Pituskin EN, Jendzjowsky NG, Tomczak CR, Haennel RG, Mackey JR. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor--positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 34.Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuk LM, Courneya KS, Slamon DJ, Mackey JR. Cardiovascular risk profile of patients with her2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 35.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, 2nd, Douglas PS, Haykowsky M. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjeerdsma G, Meinardi MT, van Der Graaf WT, van Den Berg MP, Mulder NH, Crijns HJ, de Vries EG, van Veldhuisen DJ. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: Autonomic versus echocardiographic variables. Heart. 1999;81:419–423. doi: 10.1136/hrt.81.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagy AC, Cserep Z, Tolnay E, Nagykalnai T, Forster T. Early diagnosis of chemotherapy-induced cardiomyopathy: A prospective tissue doppler imaging study. Pathology oncology research : POR. 2008;14:69–77. doi: 10.1007/s12253-008-9013-4. [DOI] [PubMed] [Google Scholar]
- 38.Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, Volkers C, Haaksma J, de Vries EG, Sleijfer DT, van der Graaf WT. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19:2746–2753. doi: 10.1200/JCO.2001.19.10.2746. [DOI] [PubMed] [Google Scholar]
- 39.Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, Forman DE, Di Carli MF, Nohria A. Abnormal exercise response in long-term survivors of hodgkin lymphoma treated with thoracic irradiation: Evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 41.Ekholm E, Rantanen V, Syvanen K, Jalonen J, Antila K, Salminen E. Docetaxel does not impair cardiac autonomic function in breast cancer patients previously treated with anthracyclines. Anti-cancer drugs. 2002;13:425–429. doi: 10.1097/00001813-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Ekholm E, Rantanen V, Antila K, Salminen E. Paclitaxel changes sympathetic control of blood pressure. European journal of cancer. 1997;33:1419–1424. doi: 10.1016/s0959-8049(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 43.Zachariae R, Paulsen K, Mehlsen M, Jensen AB, Johansson A, von der Maase H. Chemotherapy-induced nausea, vomiting, and fatigue--the role of individual differences related to sensory perception and autonomic reactivity. Psychotherapy and psychosomatics. 2007;76:376–384. doi: 10.1159/000107566. [DOI] [PubMed] [Google Scholar]
- 44.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–1246. doi: 10.1016/j.jacc.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fagundes CP, Murray DM, Hwang BS, Gouin JP, Thayer JF, Sollers JJ, 3rd, Shapiro CL, Malarkey WB, Kiecolt-Glaser JK. Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–1147. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 48.Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M, Neri E, Taylor CB, Kraemer HC, Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosomatic medicine. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 49.Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. Bmj. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koschke M, Boettger MK, Schulz S, Berger S, Terhaar J, Voss A, Yeragani VK, Bar KJ. Autonomy of autonomic dysfunction in major depression. Psychosomatic medicine. 2009;71:852–860. doi: 10.1097/PSY.0b013e3181b8bb7a. [DOI] [PubMed] [Google Scholar]
- 51.Newton JL, Okonkwo O, Sutcliffe K, Seth A, Shin J, Jones DE. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM : monthly journal of the Association of Physicians. 2007;100:519–526. doi: 10.1093/qjmed/hcm057. [DOI] [PubMed] [Google Scholar]
- 52.Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G, Cerutti S, Lombardi F, Pagani M, Malliani A. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and rr variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 53.Massin MM, Maeyns K, Withofs N, Ravet F, Gerard P. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child. 2000;83:179–182. doi: 10.1136/adc.83.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- 55.Froy O. Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda) 26:225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- 56.Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- 57.Antoch MP, Kondratov RV, Takahashi JS. Circadian clock genes as modulators of sensitivity to genotoxic stress. Cell Cycle. 2005;4:901–907. doi: 10.4161/cc.4.7.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 59.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 61.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 62.Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: A population study. Circulation. 124:2073–2081. doi: 10.1161/CIRCULATIONAHA.111.025858. [DOI] [PubMed] [Google Scholar]
- 63.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 64.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 65.Morin CM, Daley M, Ouellet MC. Insomnia in adults. Curr Treat Options Neurol. 2001;3:9–18. doi: 10.1007/s11940-001-0020-y. [DOI] [PubMed] [Google Scholar]
- 66.Ohayon MM, Lemoine P, Arnaud-Briant V, Dreyfus M. Prevalence and consequences of sleep disorders in a shift worker population. J Psychosom Res. 2002;53:577–583. doi: 10.1016/s0022-3999(02)00438-5. [DOI] [PubMed] [Google Scholar]
- 67.Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 29:3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 68.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995;269:R222–225. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 69.Rissanen P, Franssila-Kallunki A, Rissanen A. Cardiac parasympathetic activity is increased by weight loss in healthy obese women. Obes Res. 2001;9:637–643. doi: 10.1038/oby.2001.84. [DOI] [PubMed] [Google Scholar]
- 70.Brinkworth GD, Noakes M, Buckley JD, Clifton PM. Weight loss improves heart rate recovery in overweight and obese men with features of the metabolic syndrome. Am Heart J. 2006;152:693 e691–696. doi: 10.1016/j.ahj.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 71.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–2640. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 72.Straznicky NE, Grima MT, Sari CI, Eikelis N, Lambert EA, Nestel PJ, Esler MD, Dixon JB, Chopra R, Tilbrook AJ, Schlaich MP, Lambert GW. Neuroadrenergic dysfunction along the diabetes continuum: A comparative study in obese metabolic syndrome subjects. Diabetes. 2012;61:2506–2516. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capasso I, Esposito E, Pentimalli F, Crispo A, Montella M, Grimaldi M, De Marco M, Cavalcanti E, D'Aiuto M, Fucito A, Frasci G, Maurea N, Esposito G, Pedicini T, Vecchione A, D'Aiuto G, Giordano A. Metabolic syndrome affects breast cancer risk in postmenopausal women: National cancer institute of naples experience. Cancer biology & therapy. 2010;10:1240–1243. doi: 10.4161/cbt.10.12.13473. [DOI] [PubMed] [Google Scholar]
- 74.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 75.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: Definition, cause and consequences. Current opinion in clinical nutrition and metabolic care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piepoli MF, Kaczmarek A, Francis DP, Davies LC, Rauchhaus M, Jankowska EA, Anker SD, Capucci A, Banasiak W, Ponikowski P. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006;114:126–134. doi: 10.1161/CIRCULATIONAHA.105.605980. [DOI] [PubMed] [Google Scholar]
- 77.Murphy MN, Mizuno M, Mitchell JH, Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol. doi: 10.1152/ajpheart.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 79.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr., Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 80.Berry JD, Willis B, Gupta S, Barlow CE, Lakoski SG, Khera A, Rohatgi A, de Lemos JA, Haskell W, Lloyd-Jones DM. Lifetime risks for cardiovascular disease mortality by cardiorespiratory fitness levels measured at ages 45, 55, and 65 years in men. The cooper center longitudinal study. J Am Coll Cardiol. 57:1604–1610. doi: 10.1016/j.jacc.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 82.Carter JB, Banister EW, Blaber AP. Effect of endurance exercise on autonomic control of heart rate. Sports Med. 2003;33:33–46. doi: 10.2165/00007256-200333010-00003. [DOI] [PubMed] [Google Scholar]
- 83.Carnethon MR, Jacobs DR, Jr., Sidney S, Sternfeld B, Gidding SS, Shoushtari C, Liu K. A longitudinal study of physical activity and heart rate recovery: Cardia, 1987-1993. Med Sci Sports Exerc. 2005;37:606–612. doi: 10.1249/01.mss.0000158190.56061.32. [DOI] [PubMed] [Google Scholar]
- 84.De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J. 1993;125:726–731. doi: 10.1016/0002-8703(93)90164-5. [DOI] [PubMed] [Google Scholar]
- 85.Shin K, Minamitani H, Onishi S, Yamazaki H, Lee M. Autonomic differences between athletes and nonathletes: Spectral analysis approach. Med Sci Sports Exerc. 1997;29:1482–1490. doi: 10.1097/00005768-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 86.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: A call for normative values. Journal of the American Heart Association. 2014;3:e000432. doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lakoski SG, Barlow CE, Koelwyn GJ, Hornsby WE, Hernandez J, Defina LF, Radford NB, Thomas SM, Herndon JE, 2nd, Peppercorn J, Douglas PS, Jones LW. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: The cooper center longitudinal study. Breast cancer research and treatment. 2013;138:909–916. doi: 10.1007/s10549-013-2478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scott JM, Jones LW, Hornsby WE, Koelwyn GJ, Khouri MG, Joy AA, Douglas PS, Lakoski SG. Cancer therapy-induced autonomic dysfunction in early breast cancer: Implications for aerobic exercise training. International journal of cardiology. 2014;171:e50–51. doi: 10.1016/j.ijcard.2013.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jolly MA, Brennan DM, Cho L. Impact of exercise on heart rate recovery. Circulation. 124:1520–1526. doi: 10.1161/CIRCULATIONAHA.110.005009. [DOI] [PubMed] [Google Scholar]
- 90.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: Effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 91.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 92.Jones LW, Eves ND, Peterson BL, Garst J, Crawford J, West MJ, Mabe S, Harpole D, Kraus WE, Douglas PS. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: A pilot study. Cancer. 2008;113:3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- 93.Jones LW, Eves ND, Haykowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 94.Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT, Watanabe H. Circadian variation of cardiac k+ channel gene expression. Circulation. 2003;107:1917–1922. doi: 10.1161/01.CIR.0000058752.79734.F0. [DOI] [PubMed] [Google Scholar]
- 95.Niederer D, Vogt L, Thiel C, Schmidt K, Bernhorster M, Lungwitz A, Jager E, Banzer W. Exercise effects on hrv in cancer patients. International journal of sports medicine. 2013;34:68–73. doi: 10.1055/s-0032-1314816. [DOI] [PubMed] [Google Scholar]
- 96.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE, 2nd, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: A phase ii randomized trial. Acta oncologica. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 97.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta- analysis. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837–842. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20:95–101. doi: 10.1093/sleep/20.2.95. [DOI] [PubMed] [Google Scholar]
- 100.Shapiro CM, Warren PM, Trinder J, Paxton SJ, Oswald I, Flenley DC, Catterall JR. Fitness facilitates sleep. Eur J Appl Physiol Occup Physiol. 1984;53:1–4. doi: 10.1007/BF00964680. [DOI] [PubMed] [Google Scholar]
- 101.Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Palesh OG, Chandwani K, Reddy PS, Melnik MK, Heckler C, Morrow GR. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233–3241. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 103.Kaya MG, Ozkan M, Gunebakmaz O, Akkaya H, Kaya EG, Akpek M, Kalay N, Dikilitas M, Yarlioglues M, Karaca H, Berk V, Ardic I, Ergin A, Lam YY. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: A randomized control study. International journal of cardiology. 2013;167:2306–2310. doi: 10.1016/j.ijcard.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 104.Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH. Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: Follow-up study of heart failure. Circulation. Heart failure. 2013;6:420–426. doi: 10.1161/CIRCHEARTFAILURE.112.000055. [DOI] [PubMed] [Google Scholar]
- 105.Kishi T. Heart failure as an autonomic nervous system dysfunction. Journal of cardiology. 2012;59:117–122. doi: 10.1016/j.jjcc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 107.Cadeddu C, Piras A, Mantovani G, Deidda M, Dessi M, Madeddu C, Massa E, Mercuro G. Protective effects of the angiotensin ii receptor blocker telmisartan on epirubicin- induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J. 2010;160:487 e481–487. doi: 10.1016/j.ahj.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 108.Dessi M, Piras A, Madeddu C, Cadeddu C, Deidda M, Massa E, Antoni G, Mantovani G, Mercuro G. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction. Experimental and therapeutic medicine. 2011;2:1003–1009. doi: 10.3892/etm.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovascular research. 1997;35:30–34. doi: 10.1016/s0008-6363(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 110.Korkmaz ME, Muderrisoglu H, Ulucam M, Ozin B. Effects of spironolactone on heart rate variability and left ventricular systolic function in severe ischemic heart failure. The American journal of cardiology. 2000;86:649–653. doi: 10.1016/s0002-9149(00)01046-8. [DOI] [PubMed] [Google Scholar]
- 111.Akpek M, Ozdogru I, Sahin O, Inanc M, Dogan A, Yazici C, Berk V, Karaca H, Kalay N, Oguzhan A, Ergin A. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. European journal of heart failure. 2015;17:81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 112.Acar Z, Kale A, Turgut M, Demircan S, Durna K, Demir S, Meric M, Agac MT. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 58:988–989. doi: 10.1016/j.jacc.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 113.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 114.Andrykowski MA, Cordova MJ, Studts JL, Miller TW. Posttraumatic stress disorder after treatment for breast cancer: Prevalence of diagnosis and use of the ptsd checklist-civilian version (pcl-c) as a screening instrument. Journal of consulting and clinical psychology. 1998;66:586–590. doi: 10.1037//0022-006x.66.3.586. [DOI] [PubMed] [Google Scholar]
- 115.Brown MJ, Thacker LR, Cohen SA. Association between adverse childhood experiences and diagnosis of cancer. PloS one. 2013;8:e65524. doi: 10.1371/journal.pone.0065524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kelly-Irving M, Mabile L, Grosclaude P, Lang T, Delpierre C. The embodiment of adverse childhood experiences and cancer development: Potential biological mechanisms and pathways across the life course. International journal of public health. 2013;58:3–11. doi: 10.1007/s00038-012-0370-0. [DOI] [PubMed] [Google Scholar]
- 117.Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: A systematic review. Journal of the American Association of Nurse Practitioners. 2015 doi: 10.1002/2327-6924.12215. [DOI] [PubMed] [Google Scholar]
- 118.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: Quality of life and psychosocial adjustment in the 15 months after diagnosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 119.Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: Reproductive and late health effects of treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4184–4193. doi: 10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 120.Bower JE, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Greendale G. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2012;118:3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bower JE, Crosswell AD, Stanton AL, Crespi CM, Winston D, Arevalo J, Ma J, Cole SW, Ganz PA. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2014 doi: 10.1002/cncr.29194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Greenlee H, Balneaves LG, Carlson LE, Cohen M, Deng G, Hershman D, Mumber M, Perlmutter J, Seely D, Sen A, Zick SM, Tripathy D, Society for Integrative Oncology Guidelines Working G. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. Journal of the National Cancer Institute. Monographs. 2014;2014:346–358. doi: 10.1093/jncimonographs/lgu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Giese-Davis J, Wilhelm FH, Tamagawa R, Palesh O, Neri E, Taylor CB, Kraemer HC, Spiegel D. Higher vagal activity as related to survival in patients with advanced breast cancer: An analysis of autonomic dysregulation. Psychosomatic medicine. 2015;77:346–355. doi: 10.1097/PSY.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Walsh JA, 3rd, Topol EJ, Steinhubl SR. Novel wireless devices for cardiac monitoring. Circulation. 2014;130:573–581. doi: 10.1161/CIRCULATIONAHA.114.009024. [DOI] [PMC free article] [PubMed] [Google Scholar]