Abstract
Background
Despite evidence that many pain nursing home residents is poorly managed, reasons for this poor management remain unanswered.
Aims
The aim of this study was to determine if specific order sets related to pain assessment would improve pain management in nursing home (NH) residents. Outcomes included observed nurse pain assessment queries and resident reports of pain.
Design
Pre-test / post-test.
Setting
240-bed for-profit nursing home in the mid-southern region of the United States.
Subjects
43 nursing home residents capable of self-consent.
Methods
Medical chart abstraction during a two-week (14-day) period prior to the implementation of specific order sets for pain assessment (intervention) and a two-week (14-day) period following the intervention. Trained research assistants observed medication administration passes and performed subject interviews after each medication pass. One month after intervention implementation, one additional day of observations was conducted to determine data reliability.
Results
Nurses were observed to ask residents about pain more frequently, and nurses continued to ask about pain at higher rates one month after the intervention was discontinued. The proportion of residents who reported pain also significantly increased in response to increased nurse queries (e.g., “Do you have any pain right now?”), which underscores the importance of nurses directly asking residents about pain. Notably 70% of this long-stay NH population only told the nurses about their pain symptoms when asked directly.
Conclusions
Findings uncover that using specific pain order sets seems to improve the detection of pain, which should be a routine part of nursing assessment.
INTRODUCTION
Undetected and/or undertreated pain in nursing home (NH) residents is a significant public health problem. The prevalence of pain and painful conditions among NH residents has remained unchanged over the last two decades, with most studies reporting pain prevalence in nursing homes as greater than 50 percent (Leong & Nuo, 2007; Smalbrugge, Jongenelis, Pot, Beekman, & Eefsting, 2007; Weiner, Peterson, Ladd, McConnell, & Keefe, 1999; Won et al., 2004). Although documentation of pain is now required as the 5th vital sign among NH residents (Fishman, 2005), pain commonly occurring in this population remains undertreated (Monroe & Carter, 2010; Monroe, Carter, Feldt, Dietrich, & Cowan, 2013; Monroe, Herr, Mion, & Cowan, 2013; Monroe & Mion, 2012). For example, despite cancer being a cause of severe pain among NH residents (Monroe, Carter, et al., 2013), a seminal study found that 26 percent of NH residents (N=13,625) with daily cancer pain did not receive any pain medication (Bernabei et al., 1998). Similarly, another study showed that among 411 hospitalized older adults with acute hip fracture, half complained of moderate to severe pain at rest, and four out of five complained of pain during movement, yet 87 percent did not have a standing order for pain medicine (Morrison et al., 2003). Unfortunately, untreated pain can lead to longer recovery times, delayed ambulation, functional loss (Morrison et al., 2003), depression (Blixen & Kippes, 1999), and behavioral agitation in people with dementia (Husebo, Ballard, Cohen-Mansfield, Seifert, & Aarsland, 2013).
The Minimum Data Set (MDS) is a standardized assessment required in NHs that receive Medicare and/or Medicaid funding in the United Sates (US). Nursing home staff completes the MDS upon admission and quarterly thereafter, or when there is a significant change in status for each resident. The MDS is intended to be a comprehensive assessment that includes over 250 demographic, functional, cognitive, and psychosocial variables, including pain (Morris et al., 1994), and has been determined to be reliable for data collection in NH residents (Hawes et al., 1995), with acceptable reliability overall (Chu, Schnelle, Cadogan, & Simmons, 2004; Mor et al., 2003). Most NH residents, even those with mild to moderate cognitive impairment, have been shown to be able to reliably answer simple questions about their pain and whether they would prefer to take pain medication (Chu et al., 2004).
Even when pain is assessed by NH staff and reported by residents, this information does not always translate to more effective pain management. Poor pain management in the NH seems to persist regardless of the ability of residents to accurately report their own experience of pain (Cadogan et al., 2006; Chu et al., 2004; Monroe, Misra, et al., 2013). For example, a recent study showed that even though NH residents with mild to moderate dementia reported significantly more pain than comparable NH residents without a dementia diagnosis, they were prescribed significantly less opioids to treat their pain (Monroe, Misra, et al., 2013). Despite requirements for periodic pain assessments, NH residents typically have little input into their own pain management and are rarely asked directly about their pain (Cadogan et al., 2006; Chu et al., 2004; Monroe, Misra, et al., 2013). The results of one study with 255 residents in 16 NHs showed that residents with chronic pain who reported a preference for pro re nata (PRN) pain medication did not receive PRN pain medication more frequently than residents who reported a preference to not receive PRN pain medication (Cadogan, Schnelle, Yamamoto-Mitani, Cabrera, & Simmons, 2004). This finding might reflect a failure to adequately assess pain, and thus a failure to utilize the requested pain management approach. Moreover, NHs that reported a higher prevalence of pain based on the most recent MDS assessments performed significantly better on multiple care processes related to both assessment and treatment of pain relative to NHs that reported lower prevalence of pain (Cadogan et al., 2004).
The absence of adequate daily pain assessment and suboptimal pain documentation by clinicians may limit the ability to evaluate the effectiveness of pain treatment in the NH setting (Cadogan et al., 2006). Other studies have demonstrated that providing specific physician orders for the delivery of daily care processes may positively affect daily care provision in NH residents (Simmons & Patel, 2006). For example, when specific physician orders for the delivery of oral liquid nutrition supplements were used, the type (Whiteman, Ward, Simmons, Sarkisian, & Moore, 2008) and delivery time (Simmons & Patel, 2006) of oral supplements improved significantly.
The purpose of this quality improvement project was to determine if specific physician orders related to pain assessment and management would improve pain outcomes in the NH setting. We hypothesized that there would be a significant improvement in the assessment of pain and delivery of pain medication as a result of the intervention based on the following outcome measures:
Proportion of times residents were asked about pain by nurses during routine medication passes.
Proportion of residents who reported that the nurses asked them about pain based on a standardized interview.
Proportion of residents who reported any pain during routine medication passes.
Total number of PRN medications administered based on medication administration records.
METHODS
Subjects and Setting
Subjects for this study were recruited from a single 240-bed for-profit NH in the mid-southern region of the United States. Overall, the facility had a 92 percent occupancy rate at the time of the study, which included short-stay, long-stay and hospice beds. Total staffing (nurses + nurse aides) hours per resident per day (hprd) reported to the United States Centers for Medicare and Medicaid Services at the time of the study was 4.07 hprd, which placed this facility in a high staffing level based on national averages. The Director of Nursing reported nurse [(Registered Nurses (RN) + Licensed Practical Nurse (LPN)] ratios of 1:17 on the day shift and 1:27 on the evening shift. All study procedures were approved through a university-affiliated institutional review board.
Eligibility Criteria
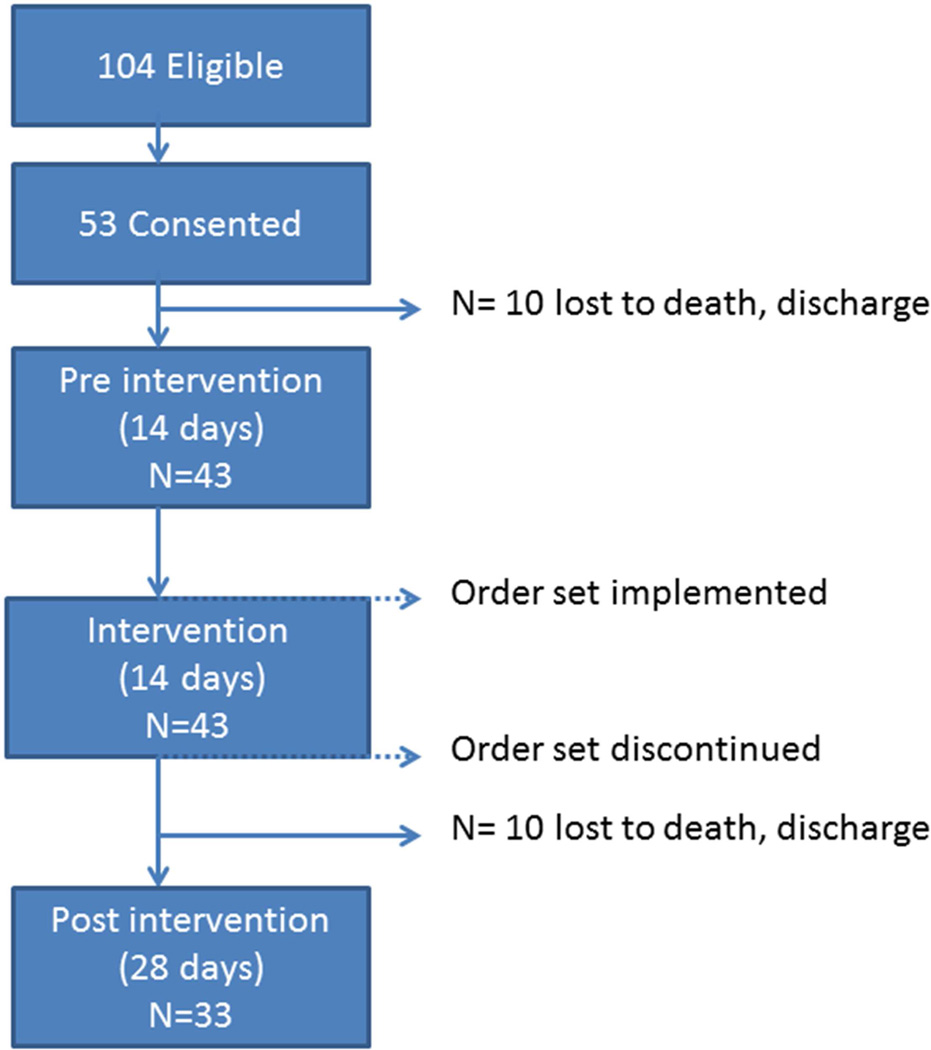
Residents were eligible for study inclusion if they were long-stay (221 total occupied beds, excludes short-stay and hospice beds), able to self-consent and had an order for any type of pain medication (scheduled or PRN). Self-consent was determined by trained research staff using a standardized evaluation form wherein structured questions were asked to determine each person’s understanding of study procedures (Monroe, Misra, et al., 2013). Of 104 eligible residents, 53 residents (51%) provided self-consent to participate in this study. Ten consented residents were subsequently lost from the study (e.g., death, discharge) leaving an analysis sample of 43 subjects for the pre-intervention to post- intervention comparisons (Figure 1).
Figure 1.
Overview of Study Design
This longitudinal quality improvement project was designed as a pilot intervention with pre- and post-intervention assessments for each subject within a single NH site. Licensed nurses working in the nursing home were informed that a project about pain management was planned and received a brief explanation of the project. Trained research staff used a standardized form to abstract demographic information from each subject’s medical record (e.g., age, admission date to the NH, ethnicity, gender), medical information (diagnoses, routine and PRN medications), and their most recent MDS assessment (Version 2.0 was in use at the time of data collection). Two MDS-derived scale scores were calculated to assess cognition (Cognitive Performance Scale) and physical functioning (Activities of Daily Living). Each of these scales is described below in the ‘measures’ section. In addition, nurse documentation of both scheduled and PRN pain medication delivery was abstracted from the Medication Administration Record (MAR) and corresponding nurses notes related to pain documented in the medical record for a two-week (14-day) period prior to the intervention (pre-intervention phase) and a two-week (14-day) period following the intervention. Specific data elements included: the number of scheduled and PRN pain medications given, the type of pain medicine (i.e., opioid, non-opioid), and the frequency of pain documentation (e.g., pain as a 5th vital sign using 0–10 verbal scale; 0=no pain, 10=worse pain). Trained research staff also independently observed license nurses during their routine medication passes for two full weekdays before and after the intervention for a total of four observation days per resident subject. Finally, each subject was approached for interview by research staff on the same days as the observations using a standardized form to assess pain symptoms, preference for pain medication, and subjects’ perceptions of nurses asking them about their pain. One month after intervention implementation (post-intervention phase), research staff returned to the NH site to conduct one additional day of observations during the same medication pass periods (e.g., morning, afternoon, and evening) for a subset of the subjects (N=33; see Figure 1) during a follow-up phase. The purpose of the one-month follow-up was to determine maintenance of intervention effects. Each of these study procedures is described in more detail in the measures section.
Measures
The Minimum Data Set (2.0)
Staff in NH settings use the MDS to assess residents upon admission, quarterly, and when there is a significant change in health status (U. S. Department of Health Human Services, 2000). Specific items included in the MDS assessment are demographic, functional, psychosocial, and cognitive ability (Morris et al., 1994). The MDS has strong inter-rater item reliability (> 0.75) (Mor et al., 2003), and the MDS is reliable for data collection in NH residents on some measures (Hawes et al., 1995).
Cognitive ability
The Cognitive Performance Scale (CPS) was developed from five MDS 2.0 items relevant to cognition, i.e., comatose, short- and long-term memory, communication, and cognitive skills for decision-making (Morris et al., 1994). The CPS is scored from 0 to 6, indicating no impairment (score=0) to very severe impairment (score=6) (Morris et al., 1994). The CPS tool developers reported inter-rater reliability at 0.85 (Morris et al., 1994).
Functional ability
To examine functional ability, the Activities of Daily Living (ADL) Summary Scale was developed from seven items (each scored 0–4) on the MDS 2.0 (Morris, Fries, & Morris, 1999). The MDS-derived ADL total score has excellent reliability and strong internal consistency (alpha=0.94) with a mean score of 15.24 (SD=9.25). A total score of 0 indicates complete independence in all seven ADLs while a score of 28 demonstrates total dependence in all ADLs (Morris et al., 1999).
Observations of medication delivery by nurses
Trained research staff conducted standardized observations of routine medication passes as provided by indigenous nurses during two days (morning, afternoon, and evening, or three passes per day per subject) both before and after the intervention (total of 4 days, or 12 observations per subject). In addition, research staff conducted one day of follow-up observations for a subset (N=33, see Figure 1) of subjects approximately one month after the intervention. Research staff were as unobtrusive as possible during each observation period. Nurses within the facility were aware that a study was being conducted related to pain, but they were blinded to the specific data elements being recorded by research staff. Each observation period lasted approximately 1 ½ to 2 hours, beginning when the medication nurse first started passing medications on the unit or floor and ending when s/he approached the last consented resident targeted for observation. Using trained research assistants to observe staff interactions with NH residents has demonstrated that staff behaviors are not significantly altered by the presence of an observer (Simmons, Babineau, Garcia, & Schnelle, 2002).
During each observation period, research staff documented the following data elements: 1) total amount of time nurse spent with resident (recorded in minutes and seconds using a stop watch), 2) if the nurse asked the resident specifically about their pain (yes, no or unable to determine), 3) if the resident expressed pain either in response to a general nurse prompt (e.g., “how are you feeling today?”) or spontaneously (yes – spontaneous, yes – prompt, no – prompt, no response or unable to determine), 4) if the nurse asked the resident if they wanted medication for their pain (yes, no or unable to determine), and 5) if the resident expressed a desire for medication for their pain (yes, no or unable to determine). The most common reason for research staff documentation of “unable to determine” was related to being unable to overhear the specific content of what the nurse and/or resident said to each other during the observation period.
Structured pain interview
Trained research staff utilized a standardized 7-item interview derived from the Geriatric Pain Measure, which has been used extensively in previous studies, to assess each subject’s pain experience (Cadogan et al., 2006; Cadogan et al., 2004; Chu et al., 2004). This interview tool has been shown to be reliable and valid for the assessment of pain among NH residents with mild to moderate cognitive impairment (Cadogan et al., 2006; Cadogan et al., 2004; Chu et al., 2004; Monroe, Misra, et al., 2013). Each subject was approached for interview twice (during the morning and afternoon with at least 4 hours between interviews) on the same day(s) as the standardized observations of medication passes both before and after the intervention (to yield a maximum possible 2 interviews per person per study phase).
The following seven questions were asked at each time point (Chu et al., 2004):
Do you have any pain right now?
Do you have pain every day?
Does pain keep you from sleeping at night?
Does pain keep you from participating in activities?
Do you tell the nurse about your pain?
Does the nursing staff ask you about your pain?
Would you prefer to take medication for your pain?
The first four questions were used to determine if probable chronic pain was present, which was based on a “yes” response to question 2 (do you have pain every day?) or three or more “yes” responses to the first four questions (Cadogan et al., 2006; Cadogan et al., 2004; Chu et al., 2004; Monroe, Misra, et al., 2013). The remaining three questions were used to assess the resident’s perception of their communication about pain with nursing staff and their preference for pain medication. Response options for each question included: yes, no, don’t know, no response / unclear response, and refusal to answer.
Description of Intervention
The investigative team coordinated with the facility Medical Director and Primary Care Physician of the study subjects to document a new, specific order set for each study subject in the form of two questions that should be asked by all nurses during every medication pass to assess pain, with a related order offering pain medication: Order 1 “Do you have any pain right now?” and Order 2 “If yes, would you like some medication for it?” All new orders were documented in the middle of the month following a two-week (14-day) pre-intervention phase to be followed by a two-week (14-day) post-intervention phase such that nurse documentation related to pain assessment and treatment could be abstracted before and after the order sets were changed.
Data Analysis
Data analyses were conducted using SPSS statistical software version 22.0. Frequency distributions were used to describe the nominal variables. Due to the highly skewed nature of the continuous distributions (other than age, described by mean and SD), median and 25th-75th interquartile ranges (IQR) were used to describe those variables (length of stay, total number of pain diagnoses, MDS-derived ADL and CPS scores). To accommodate multiple and varied numbers of assessments per subject within each study phase, hierarchical or mixed-level general linear modeling was used to test for changes in the study outcome measures. Changes from pre- to post-intervention comprised the primary focus of this study and analysis. An alpha value of p < 0.05 was used for determining statistical significance. A secondary analysis also was conducted of the subset of subjects assessed during a one-month follow-up phase to determine maintenance of intervention effects. If the overall main effect of change was statistically significant, post-hoc pairwise comparisons among each of the three study phases were conducted using a Bonferroni-corrected alpha level (p < 0.017).
RESULTS
Demographic
Table 1 shows the demographic and medical characteristics of the study subjects (N=43). The subjects’ median age was 84 [min=51, max=99]. Sixty-seven percent were female and 67 percent were Caucasian. The median length of stay in the NH was 13 months. Subjects were mild to moderately cognitively impaired, as evidenced by an MDS-CPS total score average of 3 [min=0, max=4], and 37 percent with a dementia diagnosis (although all subjects were able to provide consent and communicate adequately). Subjects also were moderately physically impaired based on their MDS-ADLS total score [mean = 17; min=7, max=28].
Table 1.
Study sample characteristics
| Characteristic | Total Sample (N=43) |
|---|---|
| N (%) | |
| Gender | |
| Female | 29 (67.4) |
| Male | 14 (32.6) |
| Race / Ethnicity | |
| White | 29 (67.4) |
| Black | 14 (32.6) |
| Depression Diagnosis | |
| Absent | 19 (44.2) |
| Present | 24 (55.8) |
| Dementia Diagnosis | |
| Absent | 27 (62.8) |
| Present | 16 (37.2) |
| Pain Medication Orders | |
| Scheduled | 26 (60.5) |
| PRN | 39 (90.7) |
| Age | Mean(SD) |
| Years | 84.0 (79–90) |
| Min=51,Max=99 | |
| Length of Stay | Median(IQRa) |
| Months | 13.1 (2.6–28.1) |
| Min=<1,Max=179 | |
| Total Number of Pain-Related Diagnoses | |
| 3.0 (2–4) | |
| Min=1,Max=7 | |
| MDS-derived Activities of Daily Living Scale Total Score (MDS-ADL) | |
| 17.0 (14–20) | |
| Min=7,Max=28 | |
| MDS-derived Cognitive Performance Scale Total Score (MDS-CPS) | |
| 3.0 (2–3) | |
| Min=0,Max=4 | |
25th–75th inter-quartile range (IQR)
During the pre-intervention phase, scheduled pain medications were ordered for 61 percent, while 91 percent had PRN pain medication orders, though 100% of participants had an order for either scheduled, PRN, or both. Most subjects had multiple pain-related diagnoses, with the most common being: arthritis (49%), post-stroke pain (36%), osteoporosis (34%), and cancer (28%). In addition, 56 percent of the subjects had a diagnosis of depression. (See Table 1)
Medication Pass Observations
Of the 43 subjects, 38 (88.4%) had a total of six medication pass observations during the pre-intervention phase of the study; 37 (86.0%) had six observations during the post-intervention phase. The most common reason for fewer than six observations per subject was that no medications were scheduled during the select observation periods for individual subjects.
Prior to intervention, nurses were observed to ask subjects about their pain during approximately 21 percent of routine medication passes. (See Table 2) Following intervention, there was a statistically significant increase to 68 percent (Wald χ2(df=1)=45.77, p < 0.001). Furthermore, the rate at which subjects were observed to report pain (either in response to a nurse query or spontaneously) also increased post-intervention (Wald χ2(df=1)=5.31, p = 0.021; see Table 2). Specifically, this rate was approximately 13 percent at pre-intervention and increased to 23 percent post-intervention. During both time periods, approximately 70 percent of subjects’ reports of pain were in response to a nurse query (not spontaneous).
Table 2.
Summary of medication pass observationsa by study phase
| N (%) | N (%) | ||
|---|---|---|---|
| Pre-Intervention (N=191) |
Post- Intervention (N=203) |
p-value | |
| Nurse Asks About Pain | < 0.001 | ||
| No | 151a (79.1) | 65a (32.0) | |
| Yes | 40a (20.9) | 138a (68.0) | |
| Baseline (N=188) |
Post (N=200) |
||
| Resident Reports Pain | 0.021 | ||
| No | 163 (86.7) | 154 (77.0) | |
| Yes | 25 (13.3) | 46 (23.0) | |
| Baseline (N=24b) |
Post (N=45b) |
||
| If Pain, Meds Offered? | 0.454 | ||
| No | 6 (25.0) | 8 (17.8) | |
| Yes | 18 (75.0) | 37 (82.2) | |
| Baseline (N=18) |
Post (N=37) |
||
|
If Pain & Meds Offered, Resident Want Meds? |
0.778 | ||
| No | 2 (11.1) | 5 (13.5) | |
| Yes | 16 (88.9) | 32 (86.5) | |
Multiple observations per participant; 38 of 43 (88.4%) had a total of 6 observations during the pre-intervention period; 37 of 43 (86.0%) had a total of 6 observations during the post-intervention period.
Missing data for 1 observation pre-intervention and 1 observation post-intervention.
For those subjects who expressed pain (spontaneously or in response to a nurse query), nurses responded by asking about their desire to take pain medication most of the time. Prior to the intervention, if a subject expressed pain during routine medication passes, nurses were observed to ask if the subject if they wanted medication for their pain during 75 percent of the observations. This proportion remained comparable at the post-intervention period (82%, Wald χ2(df=1)=0.561, p = 0.454). At both pre-intervention and post-intervention, subjects expressed a desire to take medication for their pain most of the time when asked directly by the nurses (pre-intervention: 89%; post-intervention: 87%; Wald χ2(df=1)=0.08, p = 0.778).
Resident Interviews related to Pain
Of the 43 subjects who completed pre-intervention and intervention phases (N=43), 40 also had complete interview data within both phases. The most common reason for missing interview data was "no response or refusal to answer".
Approximately 58 percent of subjects’ interview responses during the pre-intervention and intervention phases indicated probable chronic pain. There were also no statistically significant changes in any of the pain symptoms endorsed via interview from pre-intervention to intervention (Wald χ2 test, p > 0.05; see Table 3).
Table 3.
Summary of participant interview responsesa by study phase
| N (%) | N (%) | ||
|---|---|---|---|
| Interview Question | Pre-Intervention (N=80) |
Post-Intervention (N=77) |
p-value |
| Pain Right Now | 0.866 | ||
| No | 46 (57.5) | 45 (58.4) | |
| Yes | 34 (42.5) | 32 (41.6) | |
| Pain Every Day | 0.764 | ||
| No | 37 (46.2) | 34 (44.2) | |
| Yes | 43 (53.8) | 43 (55.8) | |
| Pain Prevent Sleep | 0.971 | ||
| No | 48 (60.0) | 46 (59.7) | |
| Yes | 32 (40.0) | 31 (40.3) | |
| Pain Prevent Enjoymenta | 0.925 | ||
| No | 37 (48.7) | 38 (49.4) | |
| Yes | 39 (51.3) | 39 (50.6) | |
|
Indication of Chronic Painc |
0.914 | ||
| No | 33 (42.3) | 32 (41.6) | |
| Yes | 45 (57.7) | 45 (58.4) | |
| Nurses Askc | 0.474 | ||
| No | 28 (35.9) | 23 (29.9) | |
| Yes | 50 (64.1) | 54 (70.1) | |
| Tell Nursing Staffd | 0.593 | ||
| No | 19 (24.1) | 21 (27.3) | |
| Yes | 60 (75.9) | 56 (72.7) | |
| Prefer Take Medse | 0.837 | ||
| No | 19 (24.4) | 17 (23.0) | |
| Yes | 59 (75.6) | 57 (77.0) | |
40 of the 43 participants had interview data for both baseline and post-intervention study periods; each participant had 2 interviews during baseline period for a total of 80 interviews; 3 interviews were missing in the post-interview period due to non-response or refusal.
Baseline: N=76;
Baseline: N=78;
Baseline: N=79;
Baseline: N=78, Post-Interview: N=74
Approximately 64 percent of subject responses during the pre-intervention phase indicated that nursing staff asked about their pain and 76 percent of the subjects indicated that the subjects told the nurse about their pain (see Table 3). These rates remained comparable after intervention (Nurse ask: Wald χ2(df=1)=0.51, p = 0.474; Tell nurse: Wald χ2(df=1)=0.29, p = 0.593, see Table 3). From the pre-intervention to intervention phases, subjects expressed a desire to take medication for their pain most of the time when asked directly by the nurses (76% vs77%, respectively; Wald χ2(df=1)=0.04, p = 0.837).
Chart Abstraction
Pre-intervention and intervention administrations of scheduled and PRN medications are summarized in Table 4. Prior to intervention, approximately 49 percent of the subjects (N=41) had no documented PRN pain medication administrations. This rate remained comparable during the post-intervention phase (46%). Only approximately 10% had two to three administrations per day at pre-intervention and, while not statistically significant, there was a trend toward an increasing number of PRN administrations during the post-intervention period (~20%, Wald χ2(df=1)=3.02, p = 0.082). The number of scheduled pain medications administered remained comparable from pre-intervention to intervention (Wald χ2(df=1)=1.93, p = 0.165; see Table 4).
Table 4.
MARS documentation for dose number of pain medication delivery (N=41)a
| N (%) | N (%) | ||
|---|---|---|---|
| Pre-Intervention | Post-Intervention | p-value | |
|
Total PRN Pain Meds Given Per Person/Day (Avg/Day) |
0.082 | ||
| 0 | 20 (48.7) | 19 (46.3) | |
| 0.5–1.0 | 17 (41.5) | 14 (34.2) | |
| ≥1.5 | 4 (9.8) | 8 (19.5) | |
|
Total Scheduled Pain Meds Given Per Person/Day (Avg/Day) |
0.165 | ||
| 0 | 19 (46.3) | 17 (41.5) | |
| 0.5–1.0 | 12 (29.3) | 14 (34.1) | |
| ≥1.5 | 10 (24.4) | 10 (24.4) | |
2 cases were missing post-intervention medication data
Follow-Up Phase
To assess whether any intervention effects would be maintained in the absence of specific orders, research staff returned to the facility one month after the new, specific order sets had been discontinued. One observation day and corresponding chart abstraction was conducted during this follow-up period. Of the original 43 study subjects, an additional 10 were lost leaving 33 remaining subjects with follow-up data. Reasons for loss included discharge, death, refusal to participate, and subjects not being available on the day of the scheduled observations or assessments (e.g., out of facility with family or medical appointment).
Observations of medication delivery by nurses
During the follow-up phase, there were a total of 69 observations for the 33 remaining study subjects. Within this subset of subjects, there was a statistically significant difference in observed rates of nurses asking about their pain during routine medication passes (Wald χ2(df=2)=47.58, p < 0.001). Post-hoc analysis revealed that, while the follow-up rate was lower than the intervention rate (42% versus 68%, respectively, p = 0.010), this rate remained significantly higher than the pre-intervention rate (21%, p = 0.010). There were no other statistically significant differences from intervention to follow-up phases for the observation-based measures of medication delivery (p > 0.05).
Structured pain interview
The pattern of interview responses for the subset of subjects with data in all three study phases (N=33) were comparable to those of the entire sample (N=43, Table 3), with no significant changes at follow-up (p > 0.05).
Chart abstraction
Within the subset of subjects with follow-up data (N=33), approximately 58 percent had no documented PRN pain medication administrations during pre-intervention followed by 39 percent during intervention and 69.7 percent in the follow-up phase (Wald χ2(df=2)=10.56, p = 0.005). Post-hoc analysis revealed that, in this subset, the increase in no documented PRN pain medication administrations during the intervention period compared to pre-intervention was statistically significant (p = 0.003). This trend was comparable to that found in the larger sample between the pre-intervention and intervention phases. As noted above, the rate for “no PRN pain medication administration” increased up to and beyond the pre-intervention rate. The number of scheduled medication administrations remained comparable throughout all study phases, including the follow-up period, for this sub-set of subjects (p > 0.05).
DISCUSSION
Finding ways to improve pain management in NH residents is imperative to both quality of care and residents’ quality of life. Although the problem of poor pain management in older adults has been documented for over a decade, studies continue to find little improvement across multiple settings, including long-term care. One suggestion to improve pain management in NH residents is to implement measures to change the way nurses approach pain assessment on a daily basis. The current quality improvement project evaluated possible beneficial effects of a simple intervention comprised of specific order sets regarding pain assessment and management that occurred during daily routine medication passes by nurses. Results confirmed hypotheses that the number of times nurses were directly observed to ask residents about their pain increased as a result of the new order set, and nurses continued to ask residents about their pain at a higher rate one month after the intervention was discontinued relative to the pre-intervention period. In addition, the proportion of residents who reported pain also significantly increased in response to the increase in specific nurse queries (e.g., “Do you have any pain right now?”), which underscores the importance of nurses asking residents directly and routinely about their pain symptoms. One notable finding was that 70 percent of this long-stay NH population only told the nurses about their pain symptoms when asked directly. Most subjects also expressed a desire for pain medication, and this proportion remained comparable before and after the intervention (89% and 87%, respectively). Similarly, when subjects expressed pain, nurses offered medication most of the time during both pre-intervention and intervention (75% and 82%, respectively).
In contrast, the proportion of residents who endorsed symptoms of probable chronic pain via a standardized interview, which was conducted outside of routine medication passes, remained comparable across study phases (58%) and consistent with the results of studies over the last several decades to assess pain prevalence in the NH populations (Ferrell, Ferrell, & Rivera, 1995; Leong & Nuo, 2007; Roy & Thomas, 1986; Shapiro, 1994; Weiner et al., 1999). Moreover, residents’ responses to interview questions related to whether or not nurses ask about their pain or if they tell the nurses about their pain also did not change as a result of the intervention. This could be because subjects responded to these interview questions based on whether or not nurses asked them at all about pain and not specific to every medication pass, per the new order set. Finally, there was a trend for the number of documented PRN pain medication administrations to increase from pre-intervention to post-intervention.
CONCLUSIONS
Several studies have demonstrated that a brief, focused pain interview, such as the one used in this study, is a reliable method for assessing pain symptoms among NH residents with mild to moderate cognitive impairment (Monroe, Misra, et al., 2013). Understanding the contributors to individual variability in pain reports in older individuals, especially those with versus without cognitive impairment (including psychophysical and neurophysiological alterations), will be critical to developing best practice assessment and management strategies in all older adults (Monroe, Gore, Chen, Mion, & Cowan, 2012), and in developing personalized pain management strategies (Bruehl et al., 2013). Interestingly, most subjects in the current quality improvement project reported a preference to take medication to manage their pain at each time point, which further supports the routine nursing practice of asking NH residents explicitly about their pain symptoms and offering their PRN pain medications accordingly.
Our pre-intervention observational data indicated that nurses did not routinely ask residents about pain even though these residents had pain-related diagnoses and routine or PRN orders for pain medication. Nurses anecdotally reported, as part of this study, that they did not see a need to routinely ask residents about pain because they believed they already knew which residents had pain and who preferred pain medication, although our study results indicated that this was not the case. Nurses also informally acknowledged that they typically relied on patients to tell them when they had pain and/or wanted pain medication, but our findings strongly suggest that many residents won’t express pain complaints unless asked directly. Regardless, given the number of shift changes (typically day, evening, and night) and the high rate of nurse turnover in NHs, the need for a standardized routine assessment of pain is warranted in this frail, vulnerable population, many of whom have dementia.
A recent review article reported that 37 to 77 percent of NH residents have some level of cognitive impairment (Cahill, Diaz-Ponce, Coen, & Walsh, 2010). An underlying, often erroneous, assumption among many NH staff is that residents with cognitive impairment cannot reliably report their pain or their preferences for pain medication, which may influence their willingness to ask (Ng, Brammer, Creedy, & Klainin-Yobas, 2014). In a separate analysis from this dataset comparing those with and without a diagnosis of dementia, results showed that NH residents with mild and moderate levels of dementia were less likely to receive pain medication and verbally reported more intense pain relative to those without dementia (Monroe, Misra, et al., 2013).
Limitations in the current quality improvement project include a small sample of subjects in only one community NH facility in one geographic region with predominately white, female residents. Data collection procedures to examine effects of a specific pain assessment order set were also only short-term in duration. Medication pass observations were not conducted on the night shift or weekend days. Finally, subjects were not asked about their preferences for non-pharmacological treatments for pain.
Implications of the results of this quality improvement project are that using specific pain order sets may improve overall recognition of facility-level pain prevalence, which is one of many NH quality indicators. Physicians and advanced practice nurses can increase the accuracy of this information by explicitly requiring nurses to ask standardized questions about pain during daily, routine medication passes. Of note, the new version of the MDS (3.0) includes similar standardized questions related to pain, which could reinforce this daily care practice.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Ms. Jessica Harbison for her assistance in manuscript preparation.
Funding for this project was provided by the John A. Hartford Foundation, the Mayday Fund, and the Vanderbilt Institute for Clinical and Translational Research Career Development Award as well as a Pfizer/American Medical Directors Association Quality Improvement Project Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Todd B. Monroe, Assistant Professor of Nursing, Vanderbilt University School of Nursing.
Sumathi Misra, Section Chief, Palliative Medicine Program TVHS and Program Director, VU Palliative Medicine Fellowship Program, Vanderbilt University Medical Center.
Ralf C. Habermann, Assistant Professor of Internal Medicine, Vanderbilt University Medical Center.
Mary S. Dietrich, Professor, Vanderbilt University Schools of Medicine and Nursing.
Stephen P. Bruehl, Professor of Anesthesiology, Vanderbilt University School of Medicine.
Ronald L. Cowan, Radiology, Vanderbilt University Medical Center and Vice Chair for Education and Professor of Psychiatry, Vanderbilt University School of Medicine.
Paul A. Newhouse, Jim Turner Professor of Cognitive Disorders and Professor of Psychiatry, Pharmacology, and Medicine, Vanderbilt University School of Medicine; and Director, Vanderbilt Center for Cognitive Medicine, Vanderbilt University Medical Center.
Sandra F. Simmons, Associate Professor of Internal Medicine, Vanderbilt University School of Medicine.
REFERENCES
- Bernabei R, Gambassi G, Lapane K, Landi F, Gatsonis C, Dunlop R, Mor V. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279(23):1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- Blixen CE, Kippes C. Depression, social support, and quality of life in older adults with osteoarthritis. Image - the Journal of Nursing Scholarship. 1999;31(3):221–226. doi: 10.1111/j.1547-5069.1999.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Apkarian AV, Ballantyne JC, Berger A, Borsook D, Chen WG, Lin Y. Personalized medicine and opioid analgesic prescribing for chronic pain: Opportunities and challenges. Journal of Pain. 2013;14(2):103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadogan MP, Schnelle JF, Al-Sammarrai NR, Yamamoto-Mitani N, Cabrera G, Osterweil D, Simmons SF. A standardized quality assessment system to evaluate pain detection and management in the nursing home. Journal of the American Medical Directors Association. 2006;7(3 Suppl):11–19. doi: 10.1016/j.jamda.2005.12.011. S10. [DOI] [PubMed] [Google Scholar]
- Cadogan MP, Schnelle JF, Yamamoto-Mitani N, Cabrera G, Simmons SF. A minimum data set prevalence of pain quality indicator: Is it accurate and does it reflect differences in care processes? Journals of GerontologySeries A: Biological Sciences and Medical Sciences. 2004;59(3):281–285. doi: 10.1093/gerona/59.3.m281. [DOI] [PubMed] [Google Scholar]
- Cahill S, Diaz-Ponce AM, Coen RF, Walsh C. The underdetection of cognitive impairment in nursing homes in the Dublin area. The need for on-going cognitive assessment. Age and Ageing. 2010;39(1):128–131. doi: 10.1093/ageing/afp198. [DOI] [PubMed] [Google Scholar]
- Chu L, Schnelle JF, Cadogan MP, Simmons SF. Using the minimum data set to select nursing home residents for interview about pain. Journal of the American Geriatrics Society. 2004;52(12):2057–2061. doi: 10.1111/j.1532-5415.2004.52565.x. [DOI] [PubMed] [Google Scholar]
- Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. Journal of Pain and Symptom Management. 1995;10(8):591–598. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Fishman SM. Pain as the fifth vital sign: How can I tell when back pain is serious? Journal of Pain and Palliative Care Pharmacotherapy. 2005;19(4):77–79. [PubMed] [Google Scholar]
- Hawes C, Morris JN, Phillips CD, Mor V, Fries BE, Nonemaker S. Reliability estimates for the Minimum Data Set for nursing home resident assessment and care screening (MDS) Gerontologist. 1995;35(2):172–178. doi: 10.1093/geront/35.2.172. [DOI] [PubMed] [Google Scholar]
- Husebo BS, Ballard C, Cohen-Mansfield J, Seifert R, Aarsland D. The response of agitated behavior to pain management in persons with dementia. American Journal of Geriatric Psychiatry. 2013 doi: 10.1016/j.jagp.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Leong IY, Nuo TH. Prevalence of pain in nursing home residents with different cognitive and communicative abilities. Clinical Journal of Pain. 2007;23(2):119–127. doi: 10.1097/01.ajp.0000210951.01503.3b. [DOI] [PubMed] [Google Scholar]
- Monroe TB, Carter MA. A retrospective pilot study of African-American and caucasian nursing home residents with dementia who died from cancer. Journal of Pain and Symptom Management. 2010;40(4):1–3. doi: 10.1016/j.jpainsymman.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe TB, Carter MA, Feldt KS, Dietrich MS, Cowan RL. Pain and hospice care in nursing home residents with dementia and terminal cancer. Geriatrics and Gerontology International. 2013;13(4):1018–1025. doi: 10.1111/ggi.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe TB, Gore JC, Chen LM, Mion LC, Cowan RL. Pain in people with Alzheimer's disease: Potential applications for psychophysical and neurophysiological research. Journal of Geriatric Psychiatry and Neurology. 2012;25(4):240–255. doi: 10.1177/0891988712466457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe TB, Herr KA, Mion LC, Cowan RL. Ethical and legal issues in pain research in cognitively impaired older adults. International Journal of Nursing Studies. 2013;50(9):1283–1287. doi: 10.1016/j.ijnurstu.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe TB, Mion LC. Patients with advanced dementia: How do we know if they are in pain? Geriatric Nursing (New York, N.Y.) 2012;33(3):226–228. doi: 10.1016/j.gerinurse.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe TB, Misra SK, Habermann RC, Dietrich MS, Cowan RL, Simmons SF. Pain reports and pain medication treatment in nursing home residents with and without dementia. Geriatrics and Gerontology International. 2013 doi: 10.1111/ggi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor V, Angelelli J, Jones R, Roy J, Moore T, Morris J. Inter-rater reliability of nursing home quality indicators in the U.S. BMC Health Services Research. 2003;3(1):20. doi: 10.1186/1472-6963-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Mehr DR, Hawes C, Phillips C, Mor V, Lipsitz LA. MDS Cognitive Performance Scale. Journal of Gerontology. 1994;49(4):174–182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54(11):546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Magaziner J, McLaughlin MA, Orosz G, Silberzweig SB, Koval KJ, Siu AL. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103(3):303–311. doi: 10.1016/S0304-3959(02)00458-X. [DOI] [PubMed] [Google Scholar]
- Ng SQ, Brammer J, Creedy DK, Klainin-Yobas P. Tool for Evaluating the Ways Nurses Assess Pain (TENAP): Psychometric properties assessment. Pain Management Nursing. 2014;15(4):807–818. doi: 10.1016/j.pmn.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Roy R, Thomas M. A survey of chronic pain in an elderly population. Canadian Family Physician. 1986;32:513–516. [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS. Liability issues in the management of pain. Journal of Pain and Symptom Management. 1994;9(3):146–152. doi: 10.1016/0885-3924(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Simmons SF, Babineau S, Garcia E, Schnelle JF. Quality assessment in nursing homes by systematic direct observation: Feeding assistance. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002;57(10):665–671. doi: 10.1093/gerona/57.10.m665. [DOI] [PubMed] [Google Scholar]
- Simmons SF, Patel AV. Nursing home staff delivery of oral liquid nutritional supplements to residents at risk for unintentional weight loss. Journal of the American Geriatrics Society. 2006;54(9):1372–1376. doi: 10.1111/j.1532-5415.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- Smalbrugge M, Jongenelis LK, Pot AM, Beekman AT, Eefsting JA. Pain among nursing home patients in the Netherlands: Prevalence, course, clinical correlates, recognition and analgesic treatment--an observational cohort study. BMC Geriatrics. 2007;7:3. doi: 10.1186/1471-2318-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Department of Health Human Services. Minimum Data Set (MDS) Version 2.0. 2000 Retrieved July 2008 from http://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MinimumDataSets20/index.html?redirect=/MinimumDataSets20/
- Weiner D, Peterson B, Ladd K, McConnell E, Keefe F. Pain in nursing home residents: an exploration of prevalence, staff perspectives, and practical aspects of measurement. Clinical Journal of Pain. 1999;15(2):92–101. doi: 10.1097/00002508-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Whiteman E, Ward K, Simmons SF, Sarkisian CA, Moore AA. Testing the effect of specific orders to provide oral liquid nutritional supplements to nursing home residents: A quality improvement project. Journal of Nutrition, Health, and Aging. 2008;12(9):622–625. doi: 10.1007/BF03008272. [DOI] [PubMed] [Google Scholar]
- Won AB, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. Journal of the American Geriatrics Society. 2004;52(6):867–874. doi: 10.1111/j.1532-5415.2004.52251.x. [DOI] [PubMed] [Google Scholar]