Abstract
A calcium phosphate cement (CPC) was shown to have the necessary attributes for endodontic materials except adequate basicity needed for antimicrobial properties. To enhance its basicity, tricalcium silicate (Ca3SiO5), a highly alkaline compound, was added to CPC at a mass fraction of 0.25, 0.5 or 0.75. The basicity, acid neutralization and physical properties of the CPC-Ca3SiO5 composites were investigated. Mineral trioxide aggregate (MTA) was used as the control. The acid neutralizing capacity of the CPC-Ca3SiO5 composites and MTA were measured by titrating the suspensions of ground set samples with a 0.2 mol / L HCl at predetermined pH levels, i.e., 11, 9.0, and 7.4. The setting time of CPC-Ca3SiO5 composites determined by the Gilmore needle method was 40 ± 10 min. Acid neutralizing capacity of CPC depended (p < 0.05) on Ca3SiO5 content. CPC containing 75 % Ca3SiO5 could neutralize slightly less acid than MTA (p < 0.05), but it had a shorter setting time than that of MTA (> 4 h) and excellent handling properties.
Keywords: acid neutralization, basicity, calcium phosphate cement, endo-materials, mineral trioxide aggregate, setting time, tricalcium silicate
1. Introduction
Calcium hydroxide (Ca(OH)2) has been historically used as a major ingredient for pulp capping or to establish apical closure and avoid surgery in the apexification procedure [1]. The treatment requires high patient compliance, multiple appointments extending over a long period of time, and susceptibility to coronal leakage or fracture of the already thin root [2, 3]. Mineral trioxide aggregates (MTA) is currently thought to be biocompatible [4] and effective in the above procedures. MTA, however, has relatively poor handling properties, namely very long hardening times [5], little washout resistance (no coherence) and noinjectablility. Calcium phosphate cement (CPC) [6], a mixture of equimolar amounts of tetracalcium phosphate (TTCP) and dicalcium phosphate anhydrous (DCPA), has the above attributes needed for use in endodontic treatment, as well as good adaptation to the dentin walls and the ability to set without shrinkage. The mechanical strength of CPC is increased in saliva or plasma-like fluid [7]. In vitro [8, 9, 10], animal and clinical studies [11, 12] have been conducted to investigate the feasibility of using CPC for these treatments. Unmodified CPC materials do not typically have the same high alkalinity, needed for anti-microbial activity, as Ca(OH)2 or MTA. Tricalcium silicate (Ca3SiO5) is the major component of Portland cement, which has nearly the same composition as that of MTA. Tricalcium silicate is a highly alkaline compound due to its ability to release Ca(OH)2 [13]. In this study, various amounts of tricalcium silicate were added to CPC to enhance its basicity. Ca3SiO5 was selected instead of Ca(OH)2 because it has lower solubility. The basicity of an endodontic material can be expressed in terms of two different aspects: (1) the highest pH value obtainable and (2) acid neutralizing capacity. The highest obtainable pH value should represent the pH of the fluids at the surface or within the pores of the material under in vivo condition, and this value can be determined by measuring the pH of a physiological-like solution in contact with the material.
The acid neutralizing capacity represents the ability of the material to absorb acids produced by bacteria/inflammatory cells while holding the pH above a specific value. This quantity is expected to increase with decreasing pH. For example, the material should be able to neutralize a greater amount of acid if the pH is allowed to drop to pH 9 compared to pH 10. Thus, this quantity should be expressed as a function of pH rather than as a single value. At the present time, none of the acid neutralizing capacity has been investigated before. Hence, the objectives of this study were to evaluate the basicity, acid neutralization capacity and physical properties of CPC-Ca3SiO5 and MTA.
2. Materials and Methods
2.1 Materials and Instruments
CPC was made of equimolar TTCP (a mass fraction of 0.73) and DCPA (a mass fraction of 0.27). Ca3SiO5 was prepared using a sol gel method [14] by firing CaCl2 and tetra-ethyl ortho silicate (Sigma, St. Louis, MO).1 One hundred grams of Ca3SiO5 were ground in a 250 mL agate jar with five agate balls (30 mm diameter) for six min in a ball mill (Retsch PM4, Brinkman, NY) at 200 rpm. MTA was purchased from Dentsply (Johnson City, TN). The titrant was a solution containing 0.2 mol/L HCl and 150 mmol/L NaCl, the latter being used as a background electrolyte. Impulsomat 614 and Dosimat 665 Titrator (Brinkman Instruments, Westbury, NY) were used in the acid titration study, and a Universal Testing Machine (United Calibration Corp., Garden Grove, CA) was used to measure diametral tensile strength (DTS). Poly-R dye (Sigma, St. Louis, MO) was used as the indicator for testing the sealing ability of CPC cement and MTA. The phase present in the set samples was identified by powder X-ray diffraction (XRD) (DMAX 2200, Rigaku Denki Co., Ltd, Woodlands, TX).
2.2 Sample Preparation and Acid Titration/Neutralization
The powders were prepared by combining CPC with a mass fraction of 0 (CPC), 0.25 (CPC-1), 0.5 (CPC-2) or 0.75 (CPC-3) of Ca3SiO5. The cement liquid was 0.5 mol/L Na3PO4. CPC-Ca3SiO5 samples were prepared by mixing the powder and liquid at P/L = 2.65 to produce pastes with good workability. MTA samples were prepared by following manufacturer’s instructions (P/L = 3.3) and were used as the control. After mixing, each specimen was prepared by packing the paste into a stainless steel mold (6 mm d × 3 mm h), which was subsequently covered on both the top and bottom, with two fritted glass plates (4-mm thick, 38 % porosity) and incubated at 37°C and 100 % humidity to keep the sample moist but not directly in contact with water. After 24 h, the set sample was demolded, dried at 70°C, and stored in a capped glass vial. For the basicity and acid neutralization capacity measurements, the set sample was ground by a mortar and pestle, and 0.2 g of the sample was placed in 10 mL of 150 mmol/L NaCl solution under a N2 atmosphere (to prevent atmospheric CO2 incorporation) at 37°C. A flat end combination pH electrode (Thomas Scientific, model # S450CBNC, Swedesboro, NJ, USA) was connected to a pH meter/titrator, and the pH was measured continuously to determine the highest pH obtainable. The sample suspension was under constant stirring (350 rpm) and the pH of the solution was monitored. The standard uncertainty of the pH measurement was estimated to be 0.01 pH unit.
After the maximum pH was recorded, 0.2 mol/L HCl in 150 mmol/L NaCl solution was added to reduce the pH to predetermined levels (9.0 and 7.4), and the amount of acid titrated (consumed) in order to reach each of the levels was recorded. The data would be presented as mEq of acid neutralized per unit mass of the test sample as a function of pH. The standard uncertainty of the acid neutralization capacity measurement was estimated to be 0.02 mEq acid/g of sample.
2.3 Setting Time and Mechanical Strength
The setting time of each type of material was determined using the Gilmore needle method [15]. Diametral tensile strength (DTS) was used as a measurement of mechanical strength of the materials. For DTS measurements, each set sample was prepared as described above, placed in 1 mL of distilled water and incubated at 37°C and 100 % relative humidity for 24 h. The DTS of the wet set samples (6 mm d × 3 mm h) were measured using a Universal Testing Machine, as previously described [16]. The standard uncertainty of the acid neutralization capacity measurement was estimated to be the standard deviation of the measurement.
2.4 Phase Composition Characterizations
Set samples of CPC, CPC-1, CPC-2, CPC-3, and MTA both before and after titration experiments were also characterized by powder x-ray diffraction (XRD) to determine the phase composition.
2.5 Sealing Ability Tests
Sealing abilities of wet set samples of CPC, CPC-1, CPC-2 and CPC-3, Ca3SiO5 and MTA before titration were estimated by 1 % Poly-R dye penetration test [17]. The standard uncertainty of the dye penetration measurements was estimated to be 0.1 mm based on the uncertainty of the measuring ruler.
2.6 Statistical Analysis
ANOVA and Newman-Keuls multiple comparison tests were performed to analyze the setting time, DTS, and acid neutralization capacity data.
3. Results
The setting times (mean ± s.d.; n = 3) for CPC, CPC-1, and CPC-2 were 30 ± 3 min which were not significantly different (p > 0.05) but significantly shorter (p < 0.05) than that of CPC-3 (50 ± 5 min). However, CPC-3 had a significantly shorter setting time than that of MTA (240 ± 30 min). The DTS (mean ± s.d.; n = 6) for CPC, CPC-1, CPC-2, CPC-3 were 5.38 ± 0.75, 3.74 ± 0.57, 4.79 ± 0.34, and 5.17 ± 0.56 respectively, which were not significantly different (p > 0.05) yet lower than that of MTA (13.37 ± 0.23).
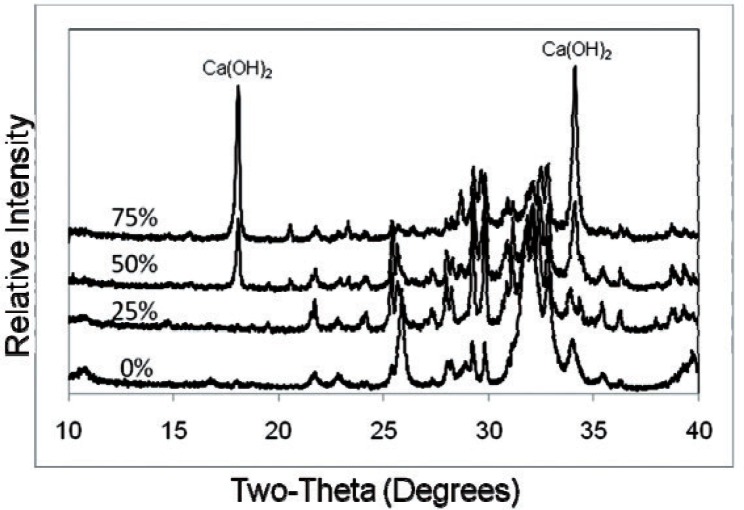
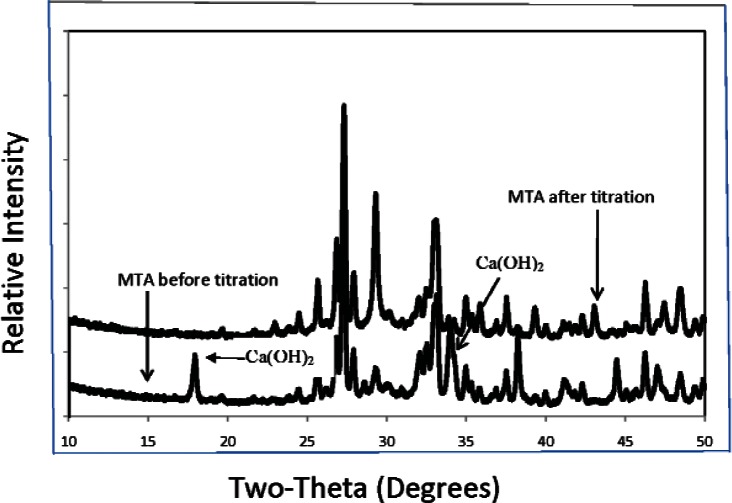
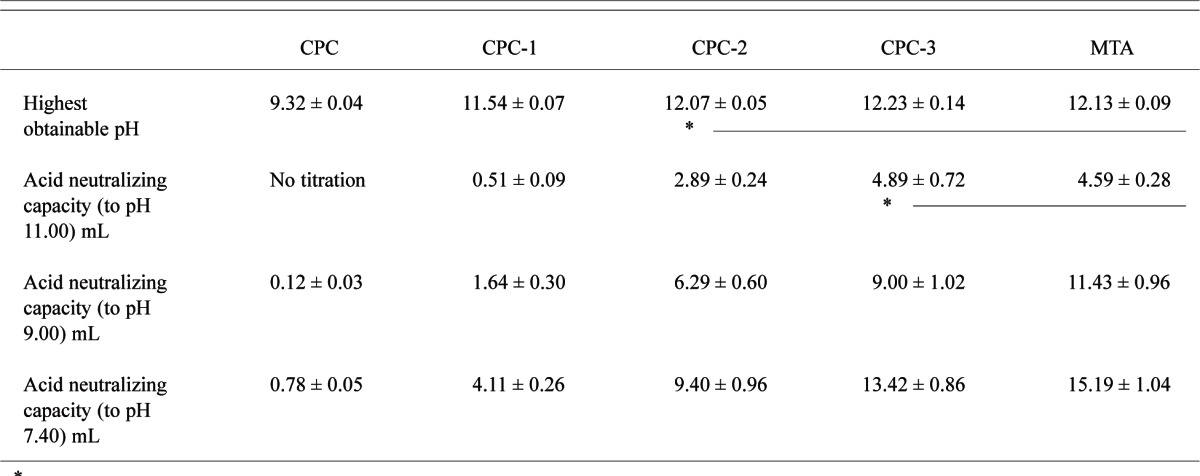
The highest obtainable pH and the amounts of HCl consumed (in mEq/g of sample) for CPC, CPC-1, CPC-2, CPC-3 and MTA (mean ± s.d.; n = 3) are shown in Table 1. The dye penetration test of CPC, CPC-1, CPC-2, CPC-3 (P/L = 2.65), Ca3SiO5 and MTA (mean ± s.d.; n = 2) indicated that the depth of penetration by measurement was completely penetrated, 0.2 ± 0.1, 0.1 ± 0.1, < 0.1, < 0.1 and < 0.1 mm respectively. Powder x-ray diffraction patterns of the CPC mixtures and MTA both before (Fig. 1 and Fig. 3) and after (Fig. 2 and Fig. 3) the titration revealed the presence of Ca(OH)2, which was largely consumed after acid neutralization.
Table 1.
Acid neutralization capacity of CPC, CPC-1, CPC-2, CPC-3 and MTA
|
Values connected by a line were not significantly different (p > 0.05).
Fig. 1.
XRD patterns of CPC, CPC-1, CPC-2 and CPC-3 before titration.
Fig. 3.
XRD patterns of MTA before and after titration with 0.2 mol / L HCl.
Fig. 2.
XRD patterns of CPC-1, CPC-2, CPC-3 after titration with 0.2 mol / L HCl.
4. Discussion
In this study, Ca3SiO5 was used as an alkaline additive instead of Ca(OH)2 because it dissolves more slowly and can improve the sealing ability of CPC as a filler. MTA contains bases (Ca2SiO4, Ca3Al2O6) other than Ca3SiO5 that may contribute to the higher acid neutralizing capacity at the levels of pH 9 and 7.4 (p > 0.05). Acid neutralizing capacity of the CPC-Ca3SiO5 composites depended strongly (p < 0.05) on the Ca3SiO5 content. CPC-3 (75 % Ca3SiO5 content) also had a relatively high neutralization capacity (p > 0.05), although MTA could neutralize more acid.
Basicity has been related to the antimicrobial property of endodontic materials. The capacity of acid neutralization by the material at different pHs should be an important property, but it has not been adequately addressed at the present time. There is neither a pre-set highest obtainable pH value nor a minimum acid neutralization capacity at a particular pH that has been established as the requirement for the antimicrobial activity. However, by combining different amounts of Ca3SiO5 with CPC, we can design CPC at different alkaline pHs if it is required.
The CPC-Ca3SiO5 samples had lower DTS (4.79 MPa) than MTA (13.3 MPa), likely due to the lower P/L ratio. However, in the area of endodontic application, strength is not as critical as that of restorative materials.
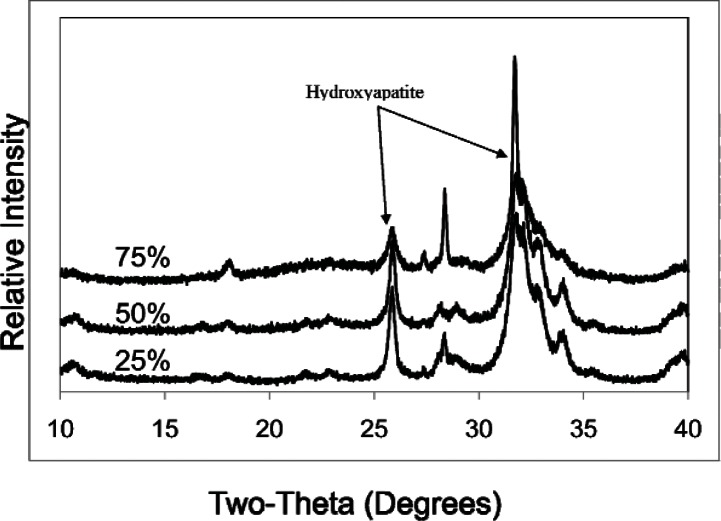
Powder XRD patterns of the CPC mixtures indicated the formation of hydroxyapatite which was found to adapt to the contours of the dentin surfaces at a microscopic levels. XRD also showed the level of Ca(OH)2 in CPC-Ca3SiO5 mixtures corresponding with the mass fraction of Ca3SiO5.
MTA samples prepared by following manufacturer’s instruction had a much longer setting time (4 h) than that of CPC-Ca3SiO5 samples (30 min to 50 min).
Although the P/L ratio used for the preparation of CPC-Ca3SiO5 samples was lower than that of MTA, it still provided an equally good seal against dye penetration and had good coherency and better handling properties than MTA.
Acknowledgments
Tricalcium silicate was prepared by Stan Frukhtbeyn in the laboratory. This work has been supported by the American Dental Association Foundation, the National Institute of Standards and Technology, and the National Institute of Dental and Craniofacial Research (DE11789).
Biography
About the authors: A. Maria Cherng is a dentist/chemist, Shozo Takagi is a senior project leader and Laurence Chow is a chief research scientist with the Paffenbarger Research Center. American Dental Association Foundation at NIST. The National Institute of Standards and Technology is an agency of the U.S. Department of Commerce.
Footnotes
Certain commercial equipment, instruments, or materials are identified in this paper to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by the NIST or the ADAF, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
Contributor Information
A. Maria Cherng, Email: maria.cherng@nist.gov.
Shozo Takagi, Email: shozo.takagi@nist.gov.
Laurence C. Chow, Email: laurence.chow@nist.gov.
5. References
- [1].Frank A. Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Ass. 1966;72:87–93. doi: 10.14219/jada.archive.1966.0017. [DOI] [PubMed] [Google Scholar]
- [2].Weisenseel JA, Jr, Hicks ML, Pelleu GB., Jr Calcium hydroxide as an apical barrier. J Endod. 1987;13:1–5. doi: 10.1016/S0099-2399(87)80084-5. [DOI] [PubMed] [Google Scholar]
- [3].Schumacher JW, Rutledge RE. An alternative to apexification. J Endod. 1993;19:529–531. doi: 10.1016/S0099-2399(06)81497-4. [DOI] [PubMed] [Google Scholar]
- [4].Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy. A review. Part 2, root-canal filling materials. Int Endod J. 2003;36:147–160. doi: 10.1046/j.1365-2591.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz RS, Mauger M, Clement DJ, Walker WA. Mineral trioxide aggregate: A new material for endodontics. J Am Dent Assoc. 1999;130:967–975. doi: 10.14219/jada.archive.1999.0337. [DOI] [PubMed] [Google Scholar]
- [6].Brown WE, Chow LC. A new calcium phosphate cement. Cem Res Prog. 1987. 1986:352–79. [Google Scholar]
- [7].Ishikawa K, Takagi S, Chow LC, Ishikawa Y, Eanes ED, Asaoka K. Behavior of a calcium phosphate cement in simulated blood plasma in vitro. Dent Mater. 1994;10:26–32. doi: 10.1016/0109-5641(94)90018-3. [DOI] [PubMed] [Google Scholar]
- [8].Sugawara A, Chow LC, Takagi S, Chohayeb H. An in vitro evaluation of sealing ability of a calcium phosphate cement when used as a root canal sealer-filler. J Endod. 1990;16:162–165. doi: 10.1016/S0099-2399(06)81963-1. [DOI] [PubMed] [Google Scholar]
- [9].Goodell GG, Mork TO, Hutter JW, Nicoll BK. Linear dye penetration of a calcium phosphate cement apical barrier. J Endod. 1997;23:174–177. doi: 10.1016/S0099-2399(97)80270-1. [DOI] [PubMed] [Google Scholar]
- [10].Chau J, Hutter JW, Mork TO, Nicoll BK. An in vitro study of furcation perforation repair using calcium phosphate cement. J Endod. 1997;23:588–592. doi: 10.1016/S0099-2399(06)81129-5. [DOI] [PubMed] [Google Scholar]
- [11].Chohayeb AA, Chow LC, Tsaknis PJ. Evaluation of calcium phosphate as a root canal sealer-filler material. J Endod. 1987;13:384–387. doi: 10.1016/S0099-2399(87)80198-X. [DOI] [PubMed] [Google Scholar]
- [12].Hong YC, Wang JT, Hong CY, Brown W, Chow LC. The periapical tissue reaction to calcium phosphate cement in the teeth of monkeys. J Biomed Mater Res. 1991;25:485–498. doi: 10.1002/jbm.820250406. [DOI] [PubMed] [Google Scholar]
- [13].Peterson VK, Neumann DA, Livingston RA. Inelastic neutron scattering investigation of hydrating tricalcium and dicalcium silicate mixture pastes: Ca(OH)2 formation and evolution of strength. J Mater Res. 2006;21:1836–1842. [Google Scholar]
- [14].Zhao WY, Chang J. Sol-gel synthesis and in vitro bioactivity of tricalcium silicate powders. Mater Lett. 2004;58:2350–2353. [Google Scholar]
- [15].ASTM Standard test method for time of setting of hydraulic cement paste by Gilmore needles. ASTM International Standard C. 2002:266–299. [Google Scholar]
- [16].Cherng A, Takagi S, Chow LC. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J Biomed Mater Res. 1996;35:273–277. doi: 10.1002/(sici)1097-4636(19970605)35:3<273::aid-jbm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [17].Cherng A, Chow LC, Takagi S. In vitro evaluation of a calcium phosphate cement root canal filler/sealer. J Endo. 2001;27:613–615. doi: 10.1097/00004770-200110000-00003. [DOI] [PubMed] [Google Scholar]