Abstract
Objectives
We examined health outcomes among 34,494 workers employed at a microelectronics and business machine facility 1969–2001.
Methods
Standardized mortality ratio (SMR) and standardized incidence ratios were used to evaluate health outcomes in the cohort and Cox regression modeling to evaluate relations between scores for occupational exposures and outcomes of a priori interest.
Results
Just over 17% of the cohort (5,966 people) had died through 2009. All cause, all cancer, and many cause-specific SMRs showed statistically significant deficits. In hourly males, SMRs were significantly elevated for non-Hodgkin’s lymphoma and rectal cancer. Salaried males had excess testicular cancer incidence. Pleural cancer and mesothelioma excesses were observed in workers hired before 1969, but no available records substantiate use of asbestos in manufacturing processes. A positive, statistically significant relation was observed between exposure scores for tetrachloroethylene and nervous system diseases.
Conclusions
Few significant exposure–outcome relations were observed, but risks from occupational exposures cannot be ruled out due to data limitations and the relative youth of the cohort.
Keywords: occupation, cancer, chemicals
BACKGROUND
Concerns about releases of trichloroethylene (TCE), perchlorethylene (PCE), and other industrial chemicals through groundwater and air emissions from several industrial facilities in a town in upstate New York led to studies of area residents by the New York State Department of Health (NYSDOH) and the Agency for Toxic Substances and Disease Registry (ATSDR) [Broome County Health Department, 1986; ATSDR, 1999, 2006a, b, 2007]. The studies found a statistically significant increase in kidney cancer incidence and a more modest increase approaching statistical significance for lung cancer among town residents. In addition, increased testicular cancer was observed among White male residents of an area where PCE contamination was reported.
These concerns led NYSDOH and Congressional representatives from New York State to request that the National Institute for Occupational Safety and Health (NIOSH) conduct a study of current and former workers of a local microelectronics and business machine facility. In response, NIOSH examined health outcomes among 34,494 former workers employed at the facility for at least 91 days between 1969 and 2001, the last complete calendar year the facility was fully owned by the founding company.
Highlights of the facility’s production and exposure history are given in Figure 1; a more detailed summary is found in Fleming et al. [2013]. The facility had two main types of manufacturing workers: machining workers, with exposures such as dust, noise, solvents, and metals; and “wet” process workers, who worked with chemical solutions used in processes used in manufacturing circuit boards and their substrates and had potential for exposure to chlorinated solvents and other industrial chemicals. The workforce also included numerous employees in non-production roles ranging from sales and office support to computer programming.
FIGURE 1.
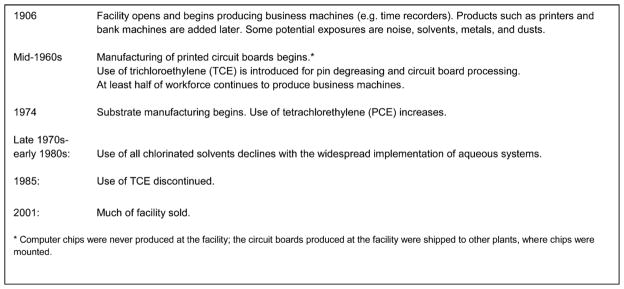
Highlights of the business machine and microelectronics production facility history, including major changes in chemical use.
Little information exists about potential health effects of work in circuit board production [LaDou and Bailar, 2007]. Studies of workers at several of the company’s other facilities with some similar operations found all cause and all cancer mortality [Beall et al., 2005], as well as cancer incidence [Bender et al., 2007], well below expectation. However, elevations in some causes of death were associated with specific locations and types of work. In a study using information from corporate mortality files for the company’s North American facilities [Clapp, 2006], increased proportionate mortality ratios (PMRs) were observed in males for all cancers combined, diseases of the nervous system (specifically multiple sclerosis, Parkinson’s disease, and amyotrophic lateral sclerosis), melanoma, and several cancer sites: brain, central nervous system (CNS), kidney, pancreas, and lymphohematopoietic system. While many of that study’s workers performed manufacturing processes not used at the facility under study here, a separate proportionate cancer mortality ratio (PCMR) study limited to the current facility observed PCMRs greater than 1.5 in males for melanoma, lymphomas, and brain and kidney cancer [Clapp and Hoffman, 2008].
The two chemicals of concern to the community, TCE and PCE, were recently reviewed by the International Agency for Research on Cancer (IARC) [Guha et al., 2012]. For TCE, the IARC group found sufficient evidence for carcinogenicity in humans, based largely on positive results for kidney cancer, with some evidence for an increased risk for NHL as well. The group-rated evidence for the carcinogenicity of PCE in humans as more limited, with the most convincing evidence for bladder cancer and some evidence for NHL and for cancers of the esophagus, kidney, and cervix.
METHODS
The current cohort study assessed health outcomes among former facility workers using standardized mortality and incidence ratios (SMR/SIR) and regression analyses. Evaluations of relations between selected health outcomes and estimated cumulative extent of potential chemical exposures comprised the primary analyses of this study. Because data were not sufficient to include all chemicals ever used at the facility in the exposure assessment, additional analyses examined the effects of duration worked in major manufacturing buildings. The study was approved by the NIOSH Human Subjects Review Board.
Health outcomes associated with TCE and PCE [Guha et al., 2012], as well as outcomes for which results were elevated in previous studies of this facility [Clapp and Hoffman, 2008] or community studies of the town in which the facility is located [Broome County Health Department, 1986; ATSDR, 1999, 2006a, 2007], were considered of a priori interest: mortality from chronic non-malignant renal disease, diseases of the nervous system, lymphohematopoietic malignancies, malignant melanoma, and malignancies of the bladder, brain, kidneys, lung, liver, and testes. Chronic obstructive pulmonary disease (COPD) mortality was of a priori interest because acids were used in a number of processes at the facility. For testicular cancer, which is a relatively rare outcome with a low fatality rate, both mortality and cancer incidence were examined.
Cohort Definition
The cohort comprised 34,494 workers employed at the facility at least 91 days between January 1, 1969 and December 31, 2001. January 1, 1969 was selected as the study begin date because electronic personnel records, while available 1965 forward, provided better information about employee locations beginning in 1969. Only direct employees of the company were included in the study, as comprehensive records on contract workers were not available. A 91-day employment minimum was imposed to exclude very short-term workers and those whose employment duration was short but not accurately quantifiable because of personnel records limitations. Foreign nationals and any worker lacking a valid US Social Security Number were excluded (n = 1,486) because of difficulties ascertaining vital status in these workers.
Vital Status and Outcome Ascertainment
Vital status was determined through December 31, 2009 by searches of the Social Security Administration’s (SSA’s) death master file, the National Death Index (NDI), and Internal Revenue Service (IRS). Death certificates were obtained from state vital statistics’ offices when cause of death information was not provided by NDI (primarily for deaths prior to 1979). For each certificate retrieved, causes of death were coded by a certified nosologist according to the International Classification of Diseases (ICD). For all vital status sources, the ICD revision in effect at time of death was used for comparability with national rates. Workers not known to be deceased before 1979 and not found on the NDI search were assumed alive at study end, because NDI is quite comprehensive [Lash and Silliman, 2001]. Workers lost to follow-up earlier were assigned the date of last known employment by the company as date last observed.
To evaluate testicular cancer incidence, subjects were linked to the cancer registries of New York, where the facility was located, and Pennsylvania, a contiguous state from which some workers commuted to the facility. Statewide registry data are available beginning in 1976 for New York and 1985 for Pennsylvania. As 93% of testicular cancer diagnoses occur in men under 55 [Howalader et al., 2012], the incidence ascertainment effort was not expanded to include retirement states, and the catchment area was defined as New York State (during 1976–2009) and Pennsylvania (1985–2009). Male workers were required to have lived in the catchment area during these years to be included in the testicular cancer analyses. The corporate personnel databases comprised the primary source for addresses for the period 1976–2004. For 2005–2009, addresses were based on IRS data. Years with no address data were filled in by extrapolation or interpolation.
Work History Reconstruction
Work histories were constructed from several of the company’s electronic personnel databases. The databases in use during the earlier periods of the study (prior to 1984) were severely constrained by electronic storage limitations, with overwriting of within-year personnel actions such as changes in position, department, and work location creating uncertainty about the nature and date of such changes. An additional “History” database was more complete, but excluded shorter-term workers who terminated prior to 1984. The different personnel data sources frequently had conflicting information. The general approach to developing detailed work histories for each worker was to capture the level of detail offered by the History database, while giving more weight to the most temporally proximate database to resolve conflicts between sources.
Work in major manufacturing buildings (Buildings 18, 41, 46, 47, 53, 57, and 259) was identified from individual work history records starting in 1984. For earlier time periods, yearly building–department linkages for production buildings were determined from site industrial hygiene and other records and then linked to individual work histories.
Chemical Exposure Data Sources
As described in Fleming et al. [2013], chemical use and exposure information for the facility were obtained from the company: industrial hygiene monitoring results (1980–2002); industrial hygiene department documents (1974–2002); and a subset of company environmental impact assessments (1974–1980 and 1985–2002). Discussions with employees who formerly worked at the facility in manufacturing, engineering, inspection, human resources, and emergency services provided additional historical information. Estimates of quantities of volatile organic compounds used at the plant for some years during the period 1969–1980 were obtained from an ATSDR study evaluating outdoor chemical air emissions in the community around the facility [ATSDR, 2006b].
Chemical Exposure Assessment
Sufficient data were available for an exposure assessment for a number of chemical agents (lead, methylene chloride [MC], PCE, and TCE) and classes (acids and bases [e.g. hydrochloric acid, sodium hydroxide], chlorinated hydrocarbons [e.g. MC, methyl chloroform, PCE, TCE], other hydrocarbons [e.g. isopropyl alcohol and other alcohols, epoxy resin and hardener, ethylene glycol monomethyl ether and other glycol ethers, methyl ethyl ketone, formaldehyde, oil mist]) used at the facility and of potential relevance to the health outcomes of a priori interest. Source data were reviewed by NIOSH exposure assessors and used to create an exposure database [Fleming et al., 2013]. “Department” and time periods were the only factors common to work history and chemical exposure data, so evaluations of potential for chemical use focused on department–year combinations (n = 27,807 department–years). Binary assignments of chemical class/agent usage (C, likely used/not used) for each chemical class and agent were completed for each department–year combination. The resulting department–exposure matrix was matched to the detailed work history for each worker, and the estimated duration of potential exposure to each chemical class and agent accumulated.
Cumulative Exposure Scoring
The cumulative exposure score, which was used in the regression analyses, was derived as shown in Figure 2. The score represents a relative measure of the cumulative extent of potential exposure to a chemical class or agent. For each worker, the cumulative duration metric for each chemical class/agent was modified by two parameters created by the NIOSH exposure assessors: the exposure group (G) and the position factor (FP). The exposure groups categorized departments by the extent of general chemical use based on department function (with, e.g., a sales department assigned 0 and a manufacturing department in which routine hands-on work with equipment involving chemicals is performed scored at the maximum, 2). The position factors were developed to address within-department exposure differences by separating positions for workers who carried out the processing functions of the department from positions of administrative and clerical workers likely to have minimal exposure [Fleming et al., 2013]. A small group of former employees agreed that these positions were allocated correctly.
FIGURE 2.
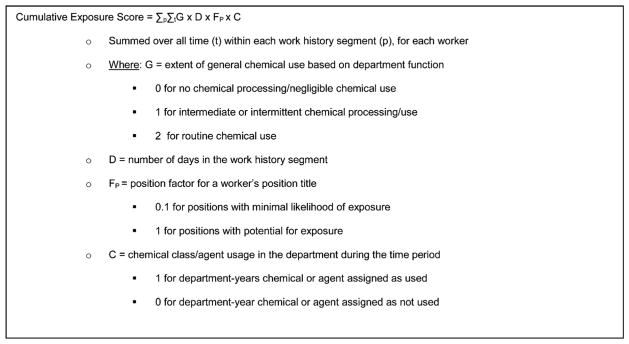
Cumulative exposure score calculation.
Covariate Data
Paycode (salaried [“exempt” in company parlance] or hourly [“non-exempt”]) was of concern because it can be associated both with occupational exposures and with lifestyle factors, like smoking, that can confound exposure–response relations for some outcomes of a priori interest [Fujishiro et al., 2012]. Anyone holding a salaried position during the first 5 years employed at the facility during the study period was coded as salaried; this relatively long period was considered because the many workers initially hired as coops, students, and supplemental staff were often classified at entry as hourly, despite, in some cases, having completed specialized higher education before hire. Birth cohort, which correlates with historical changes in smoking prevalence [Escobedo and Peddicord, 1996], was also considered in analyses to determine whether it added explanatory value to the models. Birth cohort was categorized as <1920, 1920 to <1940, 1940 to <1960, and ≥1960.
A number of cohort members (n = 10,858) were employed by the company before 1969, but potential chemical exposures could not be assessed before this time because of work history data limitations. Instead, a variable to indicate duration of employment by the company (without regard to facility, as location information was often missing) before 1969 was developed and assessed in regression models. Workers employed before 1969 worked for the company for an average of 29.8 years, with means of 12.8 of these years before 1969 and 17 years from 1969 forward.
Because of temporal changes in chemical use at the facility, a timeline was developed based on history of major process changes in manufacturing activities involving chemical use and major changes in products. Four major manufacturing eras were identified: 1969–1973, 1974–1985, 1986–1993, and 1994–2001. The effects of working during the earlier time periods were assessed in regression analysis using these cutpoints to demarcate period of hire at the facility.
Statistical Analyses
External comparisons for mortality
The NIOSH Life Table Analysis System (LTAS.net, NIOSH, Cincinnati, OH) was used to generate expected numbers for all deaths, all cancer deaths, and cause-specific deaths for each race and sex within 5-year age and 5-year calendar time periods [Schubauer-Berigan et al., 2011]. Expected and observed deaths were accumulated for each of these age and calendar time periods from April 2, 1969 through December 31, 2009. Person years at risk (PYAR) began on the later of April 2, 1969 and the date the worker had been employed at the facility for more than 90 days during the study period. PYAR ended on the earliest of date of death, date last observed, and study end date. Mortality rates from the US population, as well as New York State (excluding New York City), were used to calculate expected numbers of deaths. The expected numbers of deaths in all strata were summed to yield the total expected number for each outcome being evaluated. The SMR is the ratio of observed to expected deaths. Confidence limits were computed based on a Poisson distribution for the observed deaths [Rothman, 1986], with exact limits for outcomes with 10 or fewer deaths.
External comparisons for testicular cancer incidence
SIR and 95% confidence limits were calculated for testicular cancer. Because address data were routinely maintained for longer-term workers, we conducted two sets of analyses, one for all workers meeting inclusion criteria for the cancer incidence evaluation, and another set for longer-term workers employed at least 3 years at the facility (a cutoff selected to balance competing concerns of address reliability, which was better for longer-term workers, and statistical power, which is reduced as more short-term workers are excluded).
Many workers transferred among the company’s numerous facilities. Because testicular cancer usually affects younger men, the data were assessed to see whether restricting PYAR to the first period in the catchment area during or after eligibility criteria were met would greatly affect study power. Determination that very few cases would be affected led to the decision to proceed with this restriction.
LTAS.net was used to generate expected numbers for incident testicular cancer for White and non-White males within 5-year age and 5-year calendar time periods [Schubauer-Berigan et al., 2011] for comparison with the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) rates and, for comparison with New York State rates, within 5-year age groups and the following calendar time periods: 1976–1989, 1990–1999, and 2000–2009. Expected and observed cases were accumulated for each of these age and calendar time periods from January 1, 1976 (when the NYS Cancer Registry achieved statewide coverage) through December 31, 2009. Periods at risk were determined using both the work history and the address history. The eligibility date was defined as the date the worker had been employed at the facility for 91 days (or 3 years). If the eligibility date occurred while the worker was in the catchment area, PYAR began on that date; if the eligibility date occurred when the worker was not in the catchment area, PYAR began on the first day of the worker’s next entry into the catchment area. PYAR ended on the earliest of date of diagnosis, date last observed for the mortality study, date of death, the end of first eligible residence in the catchment area, and the study end date (December 31, 2009). The SIR and confidence limits were calculated as described above for mortality outcomes.
Regression modeling
Regression models were used to evaluate the relations in this cohort between the health outcomes of a priori interest and (a) cumulative chemical exposure scores (representing a relative measure of the cumulative extent of potential exposure) or (b) duration worked in seven major manufacturing buildings. For the cumulative chemical exposure score models, the effects of chemical agents or classes for which, overall, the literature suggests associations with the outcomes of interest were assessed. In addition, associations with TCE and PCE reported in the literature or in earlier studies of the facility or surrounding community were assessed. For the building duration models, each of the manufacturing buildings was examined for each outcome of interest. Model parameter estimation used conditional logistic regression (SAS PHREG procedure, SAS Institute, Inc., Cary, NC) based on full risk sets, which is equivalent to a Cox proportional hazards analysis. Age was used as the time scale for incidence density sampling [Langholz and Goldstein, 1996]. The cases’ cumulative chemical exposure scores were compared to those of other members of the full riskset (herein referred to as “controls”) who started work at the facility at an age which was less than the case’s age at death and who survived longer than the case. Cases were allowed to serve as controls for other risk sets. For testicular cancer incidence, date of diagnosis was used in place of date of death to determine riskset eligibility and cutoff dates.
Within risk sets, cumulative exposure score data were truncated at a cutoff date defined for cases as the date of death and, for controls, as the date at which the control reached the case’s age at death, with additional truncation for lagging. A 10-year lag was used for all outcomes with one exception; as leukemia excluding chronic lymphocytic leukemia (CLL) is generally thought to have a shorter latency, a 2-year lag was used for this outcome. CLL deaths (14 of the 91 leukemia deaths) were excluded from internal analyses of leukemia because of possible differences in etiology and latency. The lag periods were applied to all exposures metrics. Either the chemical class (e.g., chlorinated hydrocarbons) or one chemical agent (e.g., TCE or PCE), but not both, was used in a given model. Model diagnostics were employed to check the assumption of proportionality of hazards for the Cox models.
The risk of many cancers varies with age and sex. Age was controlled via riskset selection methods. Univariate models included sex, paycode, and one cumulative chemical agent or class exposure score. Chemical exposure variates achieving statistical significance in the univariate models were entered into multivariable models, which were further developed using forward regression. Potential confounders were added to each base multivariable model (which included chemical agents or classes which were statistically significant in univariate models, paycode, and sex) one at a time, and confounding evaluated by comparing dose coefficients from models including and excluding the potential confounding variables. Any variable resulting in a change of 20% or more in the parameter estimate for the exposure term was considered a confounder and retained in the model. A number of temporal variates were of interest: birth cohort (potentially related to secular trends in smoking and to changes in exposure levels); time since last exposure (to provide some adjustment for the healthy worker survivor effect [Richardson et al., 2004]); hire era; and employment duration prior to 1969 (at the company, regardless of facility). Because these variates tended to be correlated, only one type of temporal variate was entered into the model at a time.
Cumulative exposure scores for many of the chemical agents and classes were highly right skewed, so log-transformed exposure scores were explored in secondary analyses. In addition, because the group and position modifying factors applied to the duration of potential chemical use to generate exposure scores were not databased, for associations at or approaching statistical significance, models with unmodified cumulative duration and logged cumulative duration were also examined to evaluate the impact of the factors on the results.
To evaluate interactions between cumulative exposure scores and manufacturing era during which the worker was hired, interaction terms were added to the model. The log-likelihoods of the model with and without the interaction terms were compared to assess the statistical significance of the interaction terms. A number of models failed to converge when interaction terms for multiple hire eras were entered in the model; in these cases, terms for the most recent eras were combined.
RESULTS
Demographics
The cohort of 34,494 workers was largely male (69.7%, n = 24,037) and White (87.2%, n = 30,078, including 5,491 workers for whom race was not known and who were assumed to be White). Of the 10,113 salaried workers, 72.0% were White males.
The cohort accrued a total of 886,851 person years of follow-up, for an average of 25.7 years. Only 5,966 workers, or 17.3%, of the cohort had died by the study end date. Workers employed less than a year at the facility (n = 8,397) comprised only 363 of these deaths. The percentage deceased was highest among hourly males, and lowest among salaried females (Table I). As there were relatively few deaths among non-White salaried workers (11 females and 206 males) and race was missing for a number of cohort members, SMR results are presented by sex and paycode, without further division by race.
TABLE I.
| Metric | Males
|
Females
|
||
|---|---|---|---|---|
| Hourly | Salaried | Hourly | Salaried | |
| Number | 15,447 | 8,590 | 8,934 | 1,523 |
| Person years at risk | 388,480 | 249,234 | 212,692 | 36,445 |
| Deceased, n (%) | 3,571 (23.1) | 1,521 (17.7) | 823 (9.2) | 51 (3.4) |
| Year of birth (mean) | 1948 | 1944 | 1954 | 1957 |
| Year of hire by company (mean) | 1974 | 1970 | 1983 | 1983 |
| Age at hire by company (mean) | 26.4 | 25.7 | 28.7 | 25.7 |
| Years of employment at facility 1969 and laterb (mean) | 8.1 | 9.8 | 5.2 | 7.2 |
| Years of follow-up (mean) | 25.1 | 29.0 | 23.8 | 23.9 |
| % Employed >90 days but <1 year | 27.7 | 3.7 | 41.9 | 6.2 |
| % Working for company before 1969 | 35.4 | 50.6 | 10.5 | 7.2 |
| % Known to have worked at other corporate facilities 1969 or later | 26.5 | 67.6 | 20.3 | 56.1 |
| % Ever potentially exposed to at least one chemical agent or class analyzed in study | 65.7 | 20.0 | 58.5 | 13.9 |
Workers employed at least 91 days at facility 1969–2001.
Salaried if ever classified as “exempt” during first 5 years employed at facility 1969 or later, otherwise hourly.
Years of employment at facility April 2, 1969 forward. Location of employment status prior to 1969 is not reliably known.
Prevalence of Exposures Within the Cohort
Proportions of workers exposed to each chemical agent or class are shown in Fleming et al. [2013]. Potential for ever exposure to a chemical agent or class analyzed in this study was much more common among hourly workers (Table I). “Other hydrocarbons” comprised the most prevalent potential exposure among hourly males, with 60.5% ever exposed. At least one-third of workers in this group had potential exposure to the following chemicals commonly used at the facility: chlorinated hydrocarbons, lead, and acids and bases. TCE and PCE were the least common potential chemical agent exposures among hourly males, with 13.9% and 15.1% exposed, respectively.
SMR and SIR Analyses
Both all cause (SMR = 0.67, 95% CI 0.65–0.69) and all cancer mortality (SMR = 0.74, 95% CI 0.71–0.77) showed a statistically significant deficit for the entire workforce. Among both hourly and salaried males, SMRs were significantly reduced for cancers of the buccal cavity and pharynx (grouped), liver, lung, and larynx (Table II). A number of outcomes, including ischemic heart disease and COPD, were in significant deficit among both salaried and hourly males, with the deficits stronger in the salaried group. In hourly males, SMRs were also significantly reduced for cancers of the stomach, but were significantly elevated for NHL and rectal cancer. Among salaried males, SMRs were also in significant deficit for cancers of the intestine and pancreas.
TABLE II.
Observed Deaths and Standardized Mortality Ratios in the Cohort*, Males, and Females, 1969–2009
| Cause | Males
|
Females
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hourlya
|
Salaried
|
Hourly
|
Salaried
|
|||||||||
| Obs. | SMR | 95% CI | Obs. | SMR | 95% CI | Obs. | SMR | 95% CI | Obs. | SMR | 95% CI | |
| All causes (119 Base) | 3,571 | 0.76 | 0.73–0.78 | 1,521 | 0.51 | 0.49–0.54 | 823 | 0.73 | 0.68–0.79 | 51 | 0.51 | 0.38–0.67 |
| All cancers | 1,005 | 0.83 | 0.78–0.88 | 455 | 0.56 | 0.51–0.61 | 291 | 0.86 | 0.76–0.96 | 20 | 0.64 | 0.39–0.99 |
| MN buccal cavity and pharynx | 14 | 0.52 | 0.28–0.87 | 3 | 0.17 | 0.03–0.49 | 5 | 1.41 | 0.46–3.29 | 0 | 0.00 | 0.00–11.7 |
| MN digestive organs and peritoneum | 250 | 0.83 | 0.73–0.94 | 130 | 0.65 | 0.54–0.77 | 50 | 0.73 | 0.54–0.96 | 2 | 0.35 | 0.04–1.26 |
| MN esophagus | 29 | 0.71 | 0.48–1.03 | 18 | 0.64 | 0.38–1.00 | 4 | 1.14 | 0.31–2.91 | 0 | 0.00 | 0.00–12.9 |
| MN stomach | 23 | 0.62 | 0.39–0.93 | 18 | 0.79 | 0.47–1.25 | 3 | 0.48 | 0.10–1.41 | 1 | 1.69 | 0.04–9.44 |
| MN intestine except rectum | 81 | 0.81 | 0.64–1.00 | 43 | 0.66 | 0.47–0.88 | 14 | 0.51 | 0.28–0.86 | 1 | 0.46 | 0.01–2.54 |
| MN rectum | 36 | 1.71 | 1.20–2.36 | 10 | 0.71 | 0.34–1.31 | 5 | 1.00 | 0.33–2.34 | 0 | 0.00 | 0.00–7.95 |
| MN biliary passages, liver, and gall bladder | 19 | 0.51 | 0.31–0.80 | 11 | 0.42 | 0.21–0.76 | 9 | 1.09 | 0.50–2.08 | 0 | 0.00 | 0.00–5.09 |
| MN pancreas | 56 | 0.91 | 0.69–1.18 | 28 | 0.66 | 0.44–0.95 | 15 | 0.87 | 0.49–1.43 | 0 | 0.00 | 0.00–2.71 |
| MN peritoneum and other and unspecified digestive organs | 6 | 1.57 | 0.58–3.43 | 2 | 0.83 | 0.10–3.00 | 0 | 0.00 | 0.00–2.67 | 0 | 0.00 | 0.00–33.2 |
| MN respiratory system | 296 | 0.69 | 0.61–0.77 | 106 | 0.36 | 0.29–0.43 | 68 | 0.81 | 0.63–1.02 | 2 | 0.29 | 0.04–1.05 |
| MN larynx | 7 | 0.48 | 0.19–0.98 | 0 | 0.00 | 0.00–0.37 | 2 | 1.71 | 0.21–6.18 | 0 | 0.00 | 0.00–36.8 |
| MN trachea, bronchus, and lung | 287 | 0.70 | 0.62–0.78 | 103 | 0.36 | 0.29–0.44 | 64 | 0.78 | 0.60–0.99 | 2 | 0.30 | 0.04–1.07 |
| MN pleura | 1 | 1.08 | 0.03–6.00 | 3 | 5.42 | 1.12–15.8 | 0 | 0.00 | 0.00–47.6 | 0 | 0.00 | 0.00–695 |
| MN breast | 0 | 0 | 0.00–2.42 | 0 | 0.00 | 0.00–3.59 | 62 | 0.97 | 0.74–1.24 | 7 | 0.98 | 0.39–2.02 |
| MN female genital organs | NA | NA | NA | NA | NA | NA | 34 | 0.89 | 0.61–1.24 | 1 | 0.25 | 0.00–4.96 |
| MN ovary | NA | NA | NA | NA | NA | NA | 24 | 1.19 | 0.77–1.78 | 1 | 0.54 | 0.01–3.20 |
| MN male genital organs | 112 | 0.93 | 0.76–1.12 | 52 | 0.76 | 0.57–1.00 | NA | NA | NA | NA | NA | NA |
| MN prostate | 110 | 0.92 | 0.76–1.11 | 52 | 0.77 | 0.58–1.01 | NA | NA | NA | NA | NA | NA |
| MN testes | 2 | 1.10 | 0.13–3.97 | 0 | 0.00 | 0.00–3.38 | NA | NA | NA | NA | NA | NA |
| MN urinary tract organs | 69 | 1.12 | 0.87–1.42 | 26 | 0.62 | 0.40–0.90 | 9 | 0.97 | 0.44–1.84 | 0 | 0.00 | 0.00–5.04 |
| MN kidney | 37 | 1.22 | 0.86–1.68 | 13 | 0.60 | 0.32–1.03 | 6 | 1.07 | 0.39–2.34 | 0 | 0.00 | 0.00–7.78 |
| MN bladder and other urinary organs | 32 | 1.02 | 0.70–1.44 | 13 | 0.63 | 0.34–1.09 | 3 | 0.81 | 0.17–2.36 | 0 | 0.00 | 0.00–14.3 |
| MN other and unspecified sites | 131 | 0.84 | 0.71–1.00 | 74 | 0.68 | 0.53–0.86 | 33 | 0.80 | 0.55–1.13 | 6 | 1.49 | 0.55–3.25 |
| Melanoma | 19 | 1.01 | 0.61–1.57 | 18 | 1.29 | 0.76–2.04 | 3 | 0.69 | 0.14–2.03 | 0 | 0.00 | 0.00–7.33 |
| MN skin (non-melanoma) | 6 | 0.96 | 0.35–2.08 | 2 | 0.45 | 0.05–1.63 | 0 | 0.00 | 0.00–5.11 | 0 | 0.00 | 0.00–65.1 |
| Mesothelioma | 7 | 2.18 | 0.88–4.50 | 1 | 0.34 | 0.01–1.92 | 0 | 0.00 | 0.00–11.4 | 0 | 0.00 | 0.00–114 |
| MN connective tissue | 4 | 0.59 | 0.16–1.52 | 3 | 0.64 | 0.13–1.86 | 5 | 1.92 | 0.62–4.47 | 1 | 3.12 | 0.08–17.4 |
| MN brain and other parts of nervous system | 24 | 0.82 | 0.52–1.22 | 24 | 1.15 | 0.73–1.71 | 6 | 0.69 | 0.25–1.51 | 1 | 1.05 | 0.03–5.86 |
| MN of lymphatic and hematopoietic tissue | 133 | 1.17 | 0.98–1.39 | 64 | 0.82 | 0.63–1.04 | 30 | 1.03 | 0.70–1.48 | 2 | 0.79 | 0.10–2.84 |
| Hodgkin’s disease | 2 | 0.45 | 0.05–1.63 | 2 | 0.70 | 0.08–2.53 | 0 | 0.00 | 0.00–3.22 | 0 | 0.00 | 0.00–23.9 |
| Non-Hodgkin’s lymphoma | 65 | 1.49 | 1.15–1.89 | 21 | 0.68 | 0.42–1.03 | 11 | 0.95 | 0.48–1.71 | 1 | 1.07 | 0.03–5.94 |
| Multiple myeloma | 21 | 0.96 | 0.59–1.46 | 12 | 0.81 | 0.42–1.41 | 2 | 0.35 | 0.04–1.26 | 1 | 2.26 | 0.06–12.6 |
| Leukemia | 45 | 1.04 | 0.76–1.39 | 29 | 0.98 | 0.65–1.40 | 17 | 1.61 | 0.94–2.57 | 0 | 0.00 | 0.00–3.65 |
| Benign and unspecified neoplasms | 13 | 0.88 | 0.47–1.51 | 6 | 0.62 | 0.23–1.36 | 1 | 0.21 | 0.01–1.18 | 0 | 0.00 | 0.00–8.38 |
| Diseases of blood and blood-forming organs | 18 | 0.97 | 0.58–1.53 | 5 | 0.41 | 0.13–0.96 | 3 | 0.55 | 0.11–1.62 | 0 | 0.00 | 0.00–6.61 |
| Diabetes mellitus | 79 | 0.70 | 0.55–0.87 | 28 | 0.36 | 0.24–0.52 | 18 | 0.48 | 0.29–0.77 | 0 | 0.00 | 0.00–1.14 |
| Mental, psychoneurotic, and personality disorders | 40 | 0.56 | 0.40–0.76 | 25 | 0.55 | 0.35–0.81 | 9 | 0.43 | 0.20–0.81 | 0 | 0.00 | 0.00–2.30 |
| Diseases of the nervous system | 87 | 0.84 | 0.67–1.04 | 58 | 0.84 | 0.64–1.09 | 32 | 0.87 | 0.59–1.23 | 3 | 1.12 | 0.23–2.38 |
| Multiple sclerosis | 4 | 0.98 | 0.27–2.51 | 2 | 0.65 | 0.08–2.35 | 3 | 0.95 | 0.20–2.77 | 0 | 0.00 | 0.00–8.38 |
| Amyotrophic lateral sclerosis | 15 | 1.45 | 0.81–2.40 | 5 | 0.64 | 0.21–1.50 | 5 | 1.61 | 0.52–3.75 | 0 | 0.00 | 0.00–14.2 |
| Parkinson’s disease | 21 | 0.87 | 0.54–1.34 | 19 | 1.21 | 0.73–1.89 | 3 | 0.73 | 0.15–2.14 | 0 | 0.00 | 0.00–21.8 |
| Heart diseases | 1,282 | 0.86 | 0.82–0.91 | 502 | 0.56 | 0.51–0.61 | 187 | 0.70 | 0.60–0.81 | 8 | 0.44 | 0.19–0.87 |
| Other diseases of the circulatory system | 262 | 0.70 | 0.62–0.79 | 136 | 0.62 | 0.52–0.73 | 85 | 0.81 | 0.65–1.00 | 7 | 0.87 | 0.35–1.79 |
| Cerebrovascular disease | 165 | 0.69 | 0.58–0.80 | 99 | 0.72 | 0.58–0.87 | 61 | 0.87 | 0.66–1.11 | 3 | 0.58 | 0.12–1.70 |
| Diseases of the respiratory system | 282 | 0.72 | 0.64–0.81 | 95 | 0.39 | 0.32–0.48 | 66 | 0.71 | 0.55–0.90 | 2 | 0.31 | 0.04–1.14 |
| Pneumonia | 70 | 0.63 | 0.49–0.80 | 16 | 0.26 | 0.15–0.43 | 11 | 0.47 | 0.23–0.84 | 1 | 0.59 | 0.02–3.30 |
| Chronic obstructive pulmonary disease | 151 | 0.74 | 0.63–0.87 | 47 | 0.35 | 0.26–0.47 | 36 | 0.72 | 0.50–1.00 | 0 | 0.00 | 0.00–1.25 |
| Other respiratory diseases | 52 | 0.86 | 0.64–1.13 | 28 | 0.71 | 0.47–1.03 | 13 | 0.88 | 0.47–1.50 | 1 | 0.86 | 0.02–4.80 |
| Diseases of the digestive system | 112 | 0.59 | 0.48–0.71 | 44 | 0.35 | 0.25–0.47 | 25 | 0.55 | 0.35–0.81 | 2 | 0.44 | 0.05–1.57 |
| Cirrhosis and other chronic liver disease | 51 | 0.54 | 0.40–0.71 | 15 | 0.23 | 0.13–0.38 | 11 | 0.62 | 0.31–1.12 | 1 | 0.45 | 0.01–2.50 |
| Diseases of skin and subcutaneous tissues | 2 | 0.37 | 0.04–1.34 | 0 | 0.00 | 0.00–1.17 | 2 | 1.03 | 0.12–3.73 | 0 | 0.00 | 0.00–20.1 |
| Diseases of musculoskeletal and connective tissues | 9 | 0.75 | 0.34–1.43 | 4 | 0.51 | 0.14–1.30 | 4 | 0.48 | 0.13–1.24 | 0 | 0.00 | 0.00–4.02 |
| Diseases of the genitourinary system | 61 | 0.73 | 0.56–0.94 | 18 | 0.35 | 0.21–0.56 | 12 | 0.50 | 0.26–0.88 | 1 | 0.53 | 0.01–2.97 |
| Acute glomerulonephritis, nephrotic syndrome, and acute renal failure | 6 | 0.67 | 0.24–1.45 | 2 | 0.35 | 0.04–1.27 | 1 | 0.42 | 0.01–2.36 | 0 | 0.00 | 0.00–22.1 |
| Chronic and unspecified nephritis and renal failure and other renal sclerosis | 38 | 0.80 | 0.56–1.09 | 10 | 0.32 | 0.16–0.60 | 8 | 0.61 | 0.26–1.20 | 0 | 0.00 | 0.00–3.33 |
| Symptoms and ill-defined conditions | 17 | 0.33 | 0.19–0.52 | 7 | 0.22 | 0.09–0.46 | 5 | 0.39 | 0.13–0.92 | 0 | 0.00 | 0.00–2.40 |
| Transportation injuries | 52 | 0.43 | 0.32–0.56 | 26 | 0.36 | 0.23–0.52 | 17 | 0.72 | 0.42–1.16 | 3 | 0.79 | 0.16–2.32 |
| Falls | 13 | 0.42 | 0.23–0.73 | 10 | 0.51 | 0.25–0.95 | 4 | 0.69 | 0.19–1.77 | 0 | 0.00 | 0.00–8.65 |
| Other injury | 44 | 0.41 | 0.30–0.55 | 17 | 0.26 | 0.15–0.42 | 15 | 0.70 | 0.39–1.15 | 0 | 0.00 | 0.00–1.02 |
| Violence | 85 | 0.58 | 0.46–0.71 | 20 | 0.21 | 0.13–0.33 | 7 | 0.32 | 0.13–0.66 | 2 | 0.49 | 0.06–1.75 |
| Other and unspecified causes | 103 | 0.88 | 0.72–1.06 | 63 | 0.80 | 0.61–1.02 | 37 | 0.93 | 0.65–1.28 | 3 | 0.67 | 0.14–1.97 |
SMR, standardized mortality ratio; CI, confidence interval; MN, malignant neoplasm; BN, benign neoplasm; NR, not reported.
Workers employed at least 91 days, 1969–2001. Results based on US population rates 1960–2007 for all outcomes except mesothelioma (based on US population rates 1999–2004) and testicular cancer. ICD rates were mapped to cause of death categories as described by Robinson et al. [2006], Schubauer-Berigan et al. [2011], and on the NISOH website (http://www.cdc.gov/niosh/ltas/rates.html).
Salaried if ever classified as “exempt” during first 5 years employed at facility 1969 or later, otherwise hourly.
The twofold elevation of mesothelioma in hourly males and the fivefold excess of pleural cancer in salaried males likely reflect the same disease across paycodes (with a difference in disease coding over time). The category comprising malignant neoplasms of the peritoneum and other and unspecified digestive organs, which might include some asbestos-related deaths, was also elevated, though not significantly so. No cases of silicosis, asbestosis, or other pneumoconioses were seen in either group (data not shown). Work histories for the 12 workers dying from these causes revealed no commonalities, except that all were hired by the company before 1969 (nine before 1960).
There were only two deaths from testicular cancer, so testicular cancer incidence was evaluated. Of the 24,037 males in the cohort, 22,144 remained for analyses with a 91-day minimum after exclusions due to lack of address (n = 379), no residential history in the catchment area during pertinent time periods (n = 1,248), death or testicular cancer diagnosis before 1976 or start of employment (n = 210), or no time in catchment area beyond 90 days employment at the facility (n = 56). A total of 13,994 men were available for the analysis with a 3-year minimum.
Of the 49 testicular cancer cases reported by New York State or Pennsylvania, 33 cases met the 91-day minimum and 20 met the 3-year minimum after diagnostic dates and time in the catchment area were considered. Incidence of testicular cancer was elevated only among salaried males, who had an elevation of borderline significance with a 91-day employment minimum (SIR = 1.82, 95% CI 1.00–3.06, n = 14) and a statistically significant doubling of risk with a 3-year employment minimum (SIR = 2.08, 95% CI 1.08–3.64) using New York State comparison rates. Among hourly males, SIRs for this outcome were near expectation with a 91-day minimum (SIR = 0.97, 95% CI 0.59–1.52, n = 19) and slightly below expectation with a 3-year minimum (SIR = 0.91, 95% CI 0.39–1.79). Findings were quite similar when SEER rates were used for comparison (data not shown).
No cause of death showed a statistically significant increase among females of either pay status. Both all cause and all cancer mortality showed statistically significant deficits which were somewhat more marked in salaried workers. Among hourly females, a number of specific causes of death were elevated, but there were few deaths and the results were not statistically significant. Of the outcomes of a priori interest with at least five cases, only leukemia and ALS showed elevations above 1.5. For both male and female workers, the SMR and SIR findings did not change markedly when New York State (excluding New York City) was used as the comparison population or when the analyses were repeated excluding workers employed less than 1 year at the facility (data not shown).
Regression Analyses
Duration worked in three of the major manufacturing buildings assessed was positively related to risk of cause-specific mortality after controlling for paycode and sex. Risk of COPD mortality was associated with time in building 47 (hazard ratio for 1 year = 1.09, 95% CI 1.02–1.16; for 5 years, a time period closer to the average tenure at the facility, hazard ratio = 1.50, 95% CI 1.08–2.10, 31 cases with time in Building 47). Building 47 performed heat treating and metal plating for the manufacture of processing machines before changing to the manufacture of advanced circuit panels in 1979. Duration in Building 53, which primarily provided support, testing and shipping for Building 47’s circuit panel manufacturing, was associated with a statistically significant increase in risk of melanoma (hazard ratio for 5 years = 7.35, 95% CI 3.41–15.9, three cases with time in Building 53). Risk of non-CLL leukemia was positively associated with duration in Building 18 (hazard ratio for 5 years = 1.59, 95% CI 1.02–2.49, 16 cases with time in Building 18), which manufactured microelectronics equipment (primarily circuit boards and substrates). No other significant or marginally significant relation between outcomes of a priori interest and duration in any of the seven major production buildings examined was observed.
For most outcomes, no candidate chemical exposure variate was significant in univariate models adjusted for sex and paycode (Table III). A positive, statistically significant relation was observed between the cumulative PCE exposure score and diseases of the nervous system. The positive relation between the cumulative TCE exposure score and non-CLL leukemia approached statistical significance and was significant for the log-transformed cumulative exposure variable (data not shown). A negative relation of borderline statistical significance was observed between the cumulative exposure score for “other hydrocarbons” and NHL. For all relations examined between chemical agents or classes and specific outcomes, the cumulative exposure score provided a somewhat better fit than duration of potential exposure (data not shown). In multivariable modeling, no other covariate proved to be a significant confounder of a relation between a cumulative exposure score and a health outcome, and there were no significant interactions with manufacturing era during which workers were hired.
TABLE III.
Cumulative Chemical Exposure Score Univariate Cox Modeling Results
| Outcome (number of cases)a | Chemical or chemical classb | Parameter estimate (per modified exposure yeara,c) | Hazard ratio at 5 modified exposure years | 95% Confidence interval (5 modified exposure-years) |
|---|---|---|---|---|
| MN bladder and other urinary organs (48) | Chlorinated hydrocarbons | −0.0539 | 0.76 | 0.52–1.12 |
| PCE | −0.0237 | 0.89 | 0.37–2.13 | |
| TCE | −0.632 | 0.04 | 0.00–5.71 | |
| MN brain and other parts of nervous system (55) | Chlorinated hydrocarbons | −0.0864 | 0.65 | 0.36–1.17 |
| MC | −0.535 | 0.07 | 0.00–3.83 | |
| PCE | −0.116 | 0.56 | 0.12–2.65 | |
| TCE | −0.933 | 0.01 | 0.00–24.6 | |
| COPD (234) | Acids and bases | 0.0177 | 1.09 | 0.98–1.22 |
| MN kidney (56) | Chlorinated hydrocarbons | 0.0000713 | 1.00 | 0.78–1.28 |
| PCE | −0.383 | 0.15 | 0.01–4.04 | |
| TCE | 0.0427 | 1.24 | 0.87–1.77 | |
| MN liver, biliary passages, and gallbladder (39) | Chlorinated hydrocarbons | −0.03672 | 0.83 | 0.55–1.26 |
| Other hydrocarbons | −0.00810 | 0.96 | 0.73–1.26 | |
| PCE | −0.04602 | 0.79 | 0.27–2.30 | |
| TCE | −0.00300 | 0.99 | 0.50–1.95 | |
| MN lung, trachea, and bronchus (456) | Chlorinated hydrocarbons | 0.00278 | 1.01 | 0.93–1.10 |
| MC | −0.0136 | 0.93 | 0.80–1.09 | |
| TCE | 0.00571 | 1.03 | 0.87–1.22 | |
| MN testes (32) | Chlorinated hydrocarbons | 0.0306 | 1.17 | 0.69–1.96 |
| Other hydrocarbons | 0.00201 | 1.01 | 0.61–1.68 | |
| PCE | 0.0193 | 1.10 | 0.38–3.17 | |
| TCE | 0.0938 | 1.60 | 0.71–3.59 | |
| Multiple myeloma (36) | Chlorinated hydrocarbons | −0.0138 | 0.93 | 0.64–1.35 |
| MC | −0.0587 | 0.75 | 0.32–1.75 | |
| Other hydrocarbons | −0.0531 | 0.77 | 0.51–1.16 | |
| PCE | −0.6473 | 0.04 | 0.00–59.7 | |
| TCE | 0.0331 | 1.18 | 0.70–1.99 | |
| Non-CLL leukemia (77) | Chlorinated hydrocarbons | 0.0191 | 1.10 | 0.92–1.32 |
| MC | −0.00786 | 0.96 | 0.67–1.38 | |
| Other hydrocarbons | 0.00942 | 1.05 | 0.90–1.22 | |
| PCE | 0.00971 | 1.05 | 0.66–1.66 | |
| TCE | 0.0541 | 1.31 | 0.98–1.75 | |
| Non-Hodgkin’s lymphoma (98) | Chlorinated hydrocarbons | −0.0212 | 0.90 | 0.73–1.11 |
| MC | −0.0680 | 0.71 | 0.43–1.17 | |
| Other hydrocarbons | −0.0430 | 0.81 | 0.65–1.00 | |
| PCE | 0.0440 | 1.25 | 0.90–1.73 | |
| TCE | −0.0269 | 0.87 | 0.57–1.35 | |
| Non-malignant chronic renal disease (56) (chronic and unspecified nephritis, renal failure, and other renal sclerosis) | Chlorinated hydrocarbons | −0.0148 | 0.93 | 0.72–1.21 |
| Lead | 0.0197 | 1.10 | 0.82–1.49 | |
| PCE | −0.0129 | 0.94 | 0.47–1.86 | |
| TCE | 0.0134 | 1.07 | 0.70–1.63 | |
| Non-malignant diseases of the nervous system (171) | Chlorinated hydrocarbons | 0.00711 | 1.04 | 0.90–1.19 |
| Lead | −0.00874 | 0.96 | 0.75–1.22 | |
| MC | 0.00776 | 1.04 | 0.83–1.31 | |
| Other hydrocarbons | 0.0126 | 1.07 | 0.95–1.20 | |
| PCE | 0.0534 | 1.31 | 1.01–1.69 | |
| TCE | −0.00204 | 0.99 | 0.73–1.34 |
CLL, chronic lymphocytic leukemia; COPD, chronic obstructive pulmonary disease; MC, methylene chloride; MN, malignant neoplasms; PCE, perchlorethylene; TCE, trichloroethylene.
Mortality for all outcomes except testicular cancer incidence.
Examples of chemicals included in chemical classes: acids and bases (hydrochloric acid or ferric chloride), chlorinated hydrocarbons (MC, methyl chloroform, PCE, TCE), other hydrocarbons (isopropyl alcohol and other alcohols, epoxy resin and hardener, ethylene glycol monomethyl ether and other glycol ethers, methyl ethyl ketone, oil mist).
Each model contains one cumulative chemical exposure score variate. All models have been adjusted for paycode and sex. Paycode is salaried if ever classified as “exempt” during first 5 years employed at facility 1969 or later, otherwise hourly. The modified cumulative exposure score-year is the duration employed in departments using a chemical or chemical class, modified by the extent of general chemical use based on department factors (0 for departments with no or negligible chemical use, 1 for intermediate/intermittent chemical processing, 2 for routine chemical use), and by the position factor for the worker’s position title (0.1 for positions with minimal exposure likelihood, 1 for positions with exposure potential). Ten-year lags have been imposed for all outcomes except leukemia (2-year lag used).
DISCUSSION
Despite an average follow-up exceeding 25 years, this cohort is young, with only 17% of cohort members deceased. The cohort shows a strong healthy worker effect, with mortality from all causes and, to a lesser extent, all cancers, showing statistically significant deficits based on United States and New York State (excluding New York City) population rates.
The current study presents an opportunity for comparison of SMR results with the findings of earlier studies which were only able, due to data limitations, to report PMR and PCMR results for this and other company facilities. Clapp and Hoffman [2008] found an elevated PCMR for lymphoma at this site; the current study observed an SMR above expectation for NHL among hourly males. Relative elevations seen earlier at this facility for malignancies of the brain and CNS and for melanoma were not evident in the current SMR results, although melanoma mortality risk was positively associated with duration working in Building 53. The relative increase in mortality from diseases of the nervous system observed by Clapp [2006] in the combined site study was echoed to some extent in the workforce studied here, with non-significant elevations of ALS in hourly male and female workers and Parkinson’s disease in salaried males, but no elevation seen for multiple sclerosis. Two outcomes, lung and liver cancer, which were on the list of outcomes of a priori interest, showed no significant elevations in either the combined site study or the current study and no significant, positive associations with exposure in the current study.
Analyses using buildings showed statistically significant duration–response relations with certain outcomes. Substantial task, process, and exposure heterogeneity likely pertain to each building, particularly over time, and department–building links are based on sparse data prior to 1984, with missing and uncertain assignments of time in building to individual workers more likely in these early, potentially more highly exposed years. Further examination of these findings may be useful, but only if information emerges that would better link departments to buildings in the early years.
While the observed association between melanoma risk and duration in Building 53 was very strong, the finding was based on only three melanoma deaths in workers from that building. All three of these workers had also spent time in Building 41, although the relation between time in that building and risk of melanoma was not significant. Chance must be considered among possible explanations for the statistical significance of this result, given the number of associations considered.
A new finding in this study was that mesothelioma and pleural cancer were in excess, with all cases in workers hired before 1969. No site industrial hygiene or process information suggests that asbestos was part of manufacturing at the site. Other possible sources for asbestos exposure which cannot be evaluated from available data include non-occupational exposures, exposures at the facility prior to the study period, onsite exposures from proximity to repair or renovation work, or occupational exposures accrued at other worksites.
The community’s interest in a study of facility workers stemmed in part from concerns about environmental contamination from TCE. Approximately 14% of hourly males were judged to have been potentially exposed to TCE through work in exposed departments. This study found positive relations between cumulative TCE exposure scores and non-CLL leukemia and between cumulative PCE exposure scores and diseases of the nervous system, but the TCE–leukemia relation was of borderline statistical significance (although clearly significant when the TCE exposure score was log-transformed). These findings are consistent with the literature. A meta-analysis of seven studies assessing the relation between TCE and leukemia reported very modest (RR 1.05–1.15), non-significant increases in risk in five of them [Alexander et al., 2006], and an Institute of Medicine (IOM) report [2003] concluded that there was insufficient evidence to determine whether TCE and/or PCE exposures are associated with leukemia risk [IOM, 2003]. An association between PCE and acute CNS effects is well established; literature on associations with chronic nervous system disease is sparse, with inconsistent results [National Research Council (NRC), 2009; Goldman et al., 2012]. Testicular cancer was also of concern following results of environmental studies near the facility. In SIR results in the current study, the excess of testicular cancers in this cohort is restricted to salaried employees. The SIR findings are consistent with those of studies that have found a greater risk for testicular cancer, or certain subtypes thereof, among men of higher socioeconomic status [Swerdlow et al., 1991; Akre et al., 1996].
In regression analyses, no exposure variate showed a statistically significant relation with testicular cancer; the hazard ratio for the cumulative TCE exposure score was elevated, but confidence intervals were wide and spanned the null. A recent IARC review of the carcinogenicity of TCE and PCE found the most compelling associations between TCE and kidney cancer and between PCE and bladder cancer [Guha et al., 2012]. In the current study, the cumulative TCE exposure score was positively but not significantly associated with kidney cancer, while the relation between PCE and bladder cancer was negative, small, and not significant. No environmental data on TCE or PCE were incorporated in the current study, which was limited to estimated workplace exposures.
Although hourly workers in this cohort had lower risks of mortality from many causes of death than the general population, they exhibited higher mortality risks than their salaried counterparts for some causes of death, including both outcomes of a priori interest and other illnesses such as ischemic heart disease and diabetes. The extent to which occupational exposures versus lifestyle or environmental factors underlie these differences is not clear, and the current study lacked data to directly control for either (although paycode was used to partially adjust for lifestyle factors in regression analyses). At this facility, hourly workers were, on average, more likely to be exposed to chemicals than their salaried counterparts. However, in regression modeling, despite the large number of associations evaluated, which could well lead to chance observation of statistically significant findings, few strong exposure–response relations were seen between specific chemicals and outcomes of interest. Collectively, these results do not suggest a strong role for occupational exposures. However, risks from occupational exposures cannot be ruled out due to substantial data limitations and the relative youth of this cohort.
While the inclusion of a semi-quantitative metric for exposure to a number of chemicals widely used at the site is a study strength, some chemicals used in smaller quantities could not be addressed due to lack of data. In addition, exposures from work before 1969 or at the company’s other facilities were not accounted for. Industrial hygiene data were very sparse prior to 1974, when exposures were likely to have been highest, and professional judgment was exercised more frequently in assigning exposures for these early years. A number of departments lacked sampling data completely; this may reflect limited exposure potential in many departments (e.g., sales) or limited conduct and retention of sampling. Furthermore, the work history databases on which the exposure assignments are based have a relatively large amount of incomplete and contradictory information about work dates, facility location, department, and position; data quality is worse for the early years. The exposure score modifiers provide a somewhat better fit for most exposure–response outcomes than that obtained using duration alone and were not inconsistent with the unmodified exposure results. However, both the exposure score modifiers and the manufacturing eras were, for practical reasons, uniform across the facility, although factors such as changes in exposure over time likely differ by process. As in any work environment, exposure variability existed between workers within the same department. Because this exposure assessment method categorized workers based on department–year, it could not account for additional variability present due to differences among tasks (and associated chemical use) within the department–year, nor could it adjust for differences in exposure level and frequency of chemical exposure among departments within the assigned exposure group. Each of these factors, which could not be addressed in this study due to data limitations, could lead to exposure misclassification and to a failure to observe exposure-related health effects.
Additional uncertainties pertain to the testicular cancer incidence component of the study, namely uncertainties about place of residence and incomplete ascertainment. The impact of missing or incorrect addresses is likely reduced in the 3-year minimum analyses. A second ascertainment issue is the likelihood of missed cases in areas not included in the catchment area, particularly among salaried workers, whom records show were more likely to move out of New York and Pennsylvania. Risk estimates for hourly workers, which likely reflect more complete ascertainment, are very close to expectation.
Despite the relatively low percentage of deceased workers in this cohort, numbers of deaths from more common outcomes of interest, such as lung cancer and COPD, were sufficient for detailed analysis involving a number of exposure variables and covariates. However, for some less common outcomes, or subtypes of outcomes, numbers were not sufficient to provide statistically precise estimates or to permit systematic assessment of whether these outcomes are related to exposures at the facility. Additional follow-up in the future could facilitate more thorough analyses of these less common outcomes and could facilitate better assessment of the healthy worker survivor effect and other temporal factors in more common outcomes.
Acknowledgments
Contract grant sponsor: National Institute for Occupational Safety and Health (NIOSH).
The authors wish to acknowledge the invaluable assistance of Faith Armstrong, Chris Gersic, Misty Hein, Patricia Laber, Patricia Reinhardt, Lisa Thomas, Chih-Yu Tseng, Kathy Waters, and Susan Woskie. We gratefully acknowledge the support of the US States and the NDI in providing death certificate information for this study. The information contained herein about deaths in New York City prior to 1979 was derived from death certificates provided by the Bureau of Vital Statistics, New York City Department of Health and Mental Hygiene. Death certificates for deaths prior to 1979 in New York State (excluding New York City) were provided by the NYSDOH, in Texas by the Texas Department of State Health Services, and in Pennsylvania by the Bureau of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Testicular cancer incidence information was provided by the NYSDOH (New York State Cancer Registry) and the Pennsylvania Department of Health (Pennsylvania Cancer Registry).
Footnotes
Disclosure Statement: The authors report no conflicts of interests.
References
- Akre O, Ekbom A, Hsieh CC, Trichopoulos D, Adami HO. Testicular nonseminoma and seminoma in relation to perinatal characteristics. J Natl Cancer Inst. 1996;88:883–889. doi: 10.1093/jnci/88.13.883. [DOI] [PubMed] [Google Scholar]
- Alexander DD, Mink PJ, Mandel JH, Kelsh MA. A meta-analysis of occupational trichloroethylene exposure and multiple myeloma or leukaemia. Occup Med. 2006;56:485–493. doi: 10.1093/occmed/kql083. [DOI] [PubMed] [Google Scholar]
- ATSDR. Cancer occurrence by common drinking water source, Broome County, NY 1981–1990. New York State Department of Health under a cooperative agreement with ATSDR; Atlanta: 1999. [Google Scholar]
- ATSDR. Health consultation: Endicott Area investigation, health statistics review, cancer and birth outcome analysis, Endicott Area, Town of Union, Broome County, New York. New York State Department of Health under a cooperative agreement with ATSDR; Atlanta: 2006a. [Google Scholar]
- ATSDR. Health consultation: Final version. Historical outdoor air emissions in the Endicott Area, International Business Machines Corporation (IBM), Village of Endicott, Broome County New York. New York State Department of Health under a cooperative agreement with ATSDR; Atlanta: 2006b. [Google Scholar]
- ATSDR. Public comment draft: Public Health Consultation Endicott Area investigation health statistics review follow-up, cancer and birth outcome analysis, Endicott Area, Town of Union, Broome County, New York. New York State Department of Health under a cooperative agreement with ATSDR; Atlanta: 2007. [Google Scholar]
- Beall C, Bender TJ, Cheng H, Herrick R, Kahn A, Matthews R, Sathiakumar N, Schymura M, Stewart J, Delzell E. Mortality among semiconductor and storage device-manufacturing workers. J Occup Environ Med. 2005;47:996–1014. doi: 10.1097/01.jom.0000183094.42763.f0. [DOI] [PubMed] [Google Scholar]
- Bender TJ, Beall C, Cheng H, Herrick RF, Kahn AR, Matthews R, Sathiakumar N, Schymura MJ, Stewart JH, Delzell E. Cancer incidence among semiconductor and electronic storage device workers. J Occup Environ Med. 2007;64:30–36. doi: 10.1136/oem.2005.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome County Health Department. Cancer occurrence by common drinking water source, Broome County, NY 1976–1980. Broome County, NY: Broome County Health Department; 1986. [Google Scholar]
- Clapp RW. Mortality among US employees of a large computer manufacturing company: 1969–2001. Environ Health. 2006;5:30. doi: 10.1186/1476-069X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp RW, Hoffman K. Cancer mortality in IBM Endicott plant workers, 1969–2001: An update on a NY production plant. Environ Health. 2008;7:13. doi: 10.1186/1476-069X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: The influence of gender and education. Am J Public Health. 1996;86:231–236. doi: 10.2105/ajph.86.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming DA, Woskie S, Jones JH, Silver SR, Luo L, Bertke S. Retrospective assessment of exposure to chemicals for a microelectronics and business machine manufacturing facility. J Occup Environ Hyg. 2013 doi: 10.1080/15459624.2013.862591. Published online 13 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro K, Stukovsky KD, Roux AD, Landsbergis P, Burchfiel C. Occupational gradients in smoking behavior and exposure to workplace environmental tobacco smoke: The multi-ethnic study of atherosclerosis. J Occup Environ Med. 2012;54:136–145. doi: 10.1097/JOM.0b013e318244501e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS, Comyns K, Korell M, Chade AR, Kasten M, Priestley B, Chou KL, Fernandez HH, Cambi F, Langston JW, Tanner CM. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71:776–784. doi: 10.1002/ana.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha N, Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Baan R, Mattock H, Straif K International Agency for Research on Cancer Monograph Working G. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012;13:1192–1193. doi: 10.1016/s1470-2045(12)70485-0. [DOI] [PubMed] [Google Scholar]
- Howalader N, Noone AM, Krapcho M, editors. SEER cancer statistics review, 1975–2009 (Vintage 2009 populations) Bethesda, MD: National Cancer Institute (NCI); 2012. [Google Scholar]
- Institute of Medicine (IOM) Gulf war and health, Vol. 2, insecticides and solvents. Washington, DC: The National Academies Press; 2003. [Google Scholar]
- LaDou J, Bailar JC. Cancer and reproductive risks in the semiconductor industry. Int J Occup Environ Health. 2007;13:376–385. doi: 10.1179/oeh.2007.13.4.376. [DOI] [PubMed] [Google Scholar]
- Langholz B, Goldstein L. Risk set sampling in epidemiologic cohort studies. Statist Sci. 1996;11:35–53. [Google Scholar]
- Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12:259–261. doi: 10.1097/00001648-200103000-00021. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC) Contaminated water supplies at Camp Lejeune: Assessing potential health effects. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- Richardson D, Wing S, Steenland K, McKelvey W. Time-related aspects of the healthy worker survivor effect. Ann Epidemiol. 2004;14:633–639. doi: 10.1016/j.annepidem.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Robinson CF, Schnorr TM, Cassinelli RT, Calvert GM, Steenland NK, Gersic CM, Schubauer-Berigan MK. Tenth revision U.S. mortality rates for use with the NIOSH Life Table Analysis System. J Occup Environ Med. 2006;48:662–667. doi: 10.1097/01.jom.0000229968.74906.8f. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. Modern epidemiology. Boston, MA: Little, Brown, and Company; 1986. [Google Scholar]
- Schubauer-Berigan MK, Hein MJ, Raudabaugh WM, Ruder AM, Silver SR, Spaeth S, Steenland K, Petersen MR, Waters KM. Update of the NIOSH Life Table Analysis System: A person-years analysis program for the Windows computing environment. Am J Ind Med. 2011;54:915–924. doi: 10.1002/ajim.20999. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Douglas AJ, Huttly SR, Smith PG. Cancer of the testis, socioeconomic status, and occupation. Br J Ind Med. 1991;48:670–674. doi: 10.1136/oem.48.10.670. [DOI] [PMC free article] [PubMed] [Google Scholar]


