Abstract
Objectives
Bipolar disorder (BD) is a severe mental illness with high healthcare costs and poor outcomes. Increasing numbers of youths are diagnosed with BD, and many adults with BD report their symptoms started in childhood, suggesting BD can be a developmental disorder. Studies advancing our understanding of BD have shown alterations in facial emotion recognition in both children and adults with BD compared to healthy comparison (HC) participants, but none have evaluated the development of these deficits. To address this, we examined the effect of age on facial emotion recognition in a sample that included children and adults with confirmed childhood-onset type-I BD, with the adults having been diagnosed and followed since childhood by the Course and Outcome in Bipolar Youth study.
Methods
Using the Diagnostic Analysis of Non-Verbal Accuracy, we compared facial emotion recognition errors among participants with BD (n = 66; ages 7–26 years) and HC participants (n = 87; ages 7–25 years). Complementary analyses investigated errors for child and adult faces.
Results
A significant diagnosis-by-age interaction indicated that younger BD participants performed worse than expected relative to HC participants their own age. The deficits occurred for both child and adult faces and were particularly strong for angry child faces, which were most often mistaken as sad. Our results were not influenced by medications, comorbidities/substance use, or mood state/global functioning.
Conclusions
Younger individuals with BD are worse than their peers at this important social skill. This deficit may be an important developmentally salient treatment target, i.e., for cognitive remediation to improve BD youths’ emotion recognition abilities.
Keywords: adolescents, bipolar disorder, child psychiatry, development, emotion, face
Bipolar disorder (BD) is a severe mental illness that often has debilitating emotional, psychosocial, and occupational consequences for patients and their families (1–6), including a high risk for suicide and psychiatric hospitalization (4–10). In adults, BD is among the more common psychiatric disorders, with an estimated prevalence of 1–4% (11, 12), and was twice as expensive as major depressive disorder during the 1990s and 2000s in terms of health care costs and missed workdays (5, 6). BD diagnoses are also on the rise among children and adolescents, with increasing rates of youths discharged from inpatient hospitalization and outpatient visits since the mid-1990s in the United States and abroad (13–15). Studies suggest that the psychological and economic costs of BD in youths are comparable to those of BD in adults (1–4, 7–9). Childhood-onset BD often continues into adulthood, leading to additional poor outcomes and reduced quality of life (1–3, 16, 17). Moreover, up to 40% of adults report their BD started as children or adolescents, rather than as adults (18, 19).
In concert, these data support two positions. First, BD is often a developmental disorder, which can start in childhood/adolescence and continue into adulthood (1–3, 16, 17). Second, we need greater understanding of the developmental underpinnings of cognitive and emotional dysfunction occurring in BD throughout the transition from childhood to adulthood (20). Such knowledge is vital to increasing diagnostic specificity, whereby biological markers and assays can augment clinical history. This knowledge is also a key to identifying new biologically informed treatment targets for BD, including both pharmacological agents (21–23) and cognitive remediation (24, 25).
Towards this end, studying facial emotion recognition in youths and adults with BD may begin to advance our knowledge of the developmental neurobiology of BD. According to several models of BD, alterations in emotional face processing may represent a key pathophysiological change related to the emotional disturbances present in the illness (26–28). For example, a consensus model based on neuroimaging findings suggests that BD influences a network of brain regions that modulate responses to external emotional signals such as affective faces (26). Emotional facial expressions serve as critical social signals, with humans hard-wired to have a neural response to faces from birth (29–31). Across numerous studies, adults with BD show deficits in facial emotion recognition compared to healthy comparison (HC) adults (32–43). Moreover, these deficits correlate with mood symptom severity, impairments in social functioning, and reduced quality of life (35–40), suggesting that altered emotional face processing may be related to the functional impairments involved in BD. Functional magnetic resonance (fMRI) studies of adults with BD during facial emotion processing tasks have shown alterations in several brain regions, including the amygdala, prefrontal cortex, and striatum (26, 44). Youths with BD show similar behavioral deficits in facial emotion recognition (45–54) and alterations in prefrontal, limbic, and striatal functioning (26, 44, 55–59). Youths may be particularly vulnerable to the negative effects of BD on the brain networks underlying the conscious regulation of affective experience, including a response to emotional facial expressions, because the brain regions involved in the ability to apply cognitive control over automatic behavioral responses have not yet fully matured (28, 60, 61).
Advancing our knowledge of developmental alterations in facial emotion recognition in BD requires more studies incorporating both youths and adults with BD (20, 41). To our knowledge, no behavioral studies of facial emotion recognition have yet done so, but several pioneering fMRI studies have (62–64). In these, youths with BD had greater amygdala hyperactivation than adults with BD in response to emotional faces (62, 63) but adults with BD showed greater recruitment of the sub-genual cingulate cortex in response to increasing facial anger intensity than youths with BD (64). Nonetheless, both youths and adults with BD showed similar amygdala hyperactivation to fearful faces (62), similar frontal, striatal, and limbic dysfunction during passive viewing and explicit rating of facial emotions (63), and similar failures to recruit increasing amygdala and cuneus activity in response to increasing angry and happy face intensity (64).
These studies highlight the need to examine the role of development in these facial emotion recognition alterations in childhood-onset BD across a continuum of participant ages. Unfortunately, past studies were limited by the traditional division of participants into child and adult samples, both due to research regulation and also training of investigators, but this approach does not account for important longitudinal imaging data showing the continuity of brain development from childhood into adulthood (65–67). A longitudinal study could potentially address this continuum of development by testing facial emotion recognition in individuals with childhood-onset BD as they age from childhood into adulthood, but the inherent costs—including time and money—could be substantial. Another approach that we, along with others, have taken recently is a meta-analysis comparing studies of youths with BD to studies of adults with BD (32, 44). Nevertheless, few original datasets have prospectively established the childhood-onset of BD in their BD adults and could directly address developmental effects in childhood-onset BD (44). A third way to examine facial emotion recognition developmentally would be a cross-sectional study incorporating data from children with type-I BD and also from BD adults whose type-I BD was prospectively followed since childhood, thus confirming the childhood-onset of their illness. This cross-sectional strategy could examine potential developmental alterations in facial emotion processing in individuals with childhood-onset BD, while avoiding the recall bias inherent in enrolling a new sample of adults with BD whose illness onset could only be ascertained retrospectively (18, 19).
Therefore, we used this third approach to evaluate facial emotion recognition in childhood-onset type-I BD across a developmental range from late childhood to young adulthood. We used the Diagnostic Analysis of Nonverbal Accuracy (DANVA) as our facial emotion recognition measure because of its construct validity and reliability across a wide age range (first graders to young adults) (68–70) and its prior use in both youths and adults with BD (33, 45–49). In our present cross-sectional study, we used the DANVA to assess the facial emotion recognition skills in youths with type-I BD and also in adults with BD whose childhood-onset type-I BD was established and prospectively followed since childhood by the ongoing Course and Outcome of Bipolar Youth (COBY) study (2). In ongoing work, the COBY study has demonstrated that these participants with BD unfortunately remain symptomatic and impaired years after their childhood-onset BD symptoms (1, 2, 17), so this group can serve as a good cross-sectional comparison for our newly diagnosed and symptomatic sample of youths with type-I BD. Moreover, to account for normal development in facial emotion recognition occurring from childhood to adulthood, we also enrolled age-matched HC youths and adults (61, 68, 69, 71).
The only prior studies that directly compared youths and adults with BD suggested that youths with BD show more pronounced neural alterations during facial emotion recognition than adults with BD (62–64). Our own recent developmental fMRI meta-analysis revealed more consistent neural alterations during emotional face processing in youths with BD than adults with BD (44). Based on this prior work, we hypothesized that, among participants who all had childhood-onset type-I BD, younger participants would show a greater deficit in facial emotion recognition than older participants.
Methods
Participants
Participants aged 7–30 years old were enrolled in studies approved by the Institutional Review Boards of Bradley Hospital and Brown University. After informed parental consent and child assent (youths) or informed consent (adults), participants were evaluated for psychopathology using the Child Schedule for Affective Disorders and Schizophrenia, Present and Lifetime version (K-SADS-PL) (72) administered to parents and youths separately, or the Structured Clinical Interview for DSM-IV (SCID) (73) for adult participants. All interviews were conducted by either a board-certified child/adolescent psychiatrist or a licensed clinical psychologist with high inter-rater reliability on both KSADS and SCID (κ > 0.85). To balance between sample heterogeneity and sample representativeness, participants with primary type-I BD were not excluded for comorbid conditions (e.g., oppositional defiant disorder, attention-deficit hyperactivity disorder) or substance abuse/dependence.
For participants with BD, inclusion criteria were: age 7–17 years (youths) or age 18–30 years (adults), English fluency of the participant (including one parent for youths), and meeting DSM-IV-TR criteria for type-I BD with at least one episode of mania (≥ 7 days) wherein the participant exhibited abnormally elevated/expansive and/or irritable mood and ≥ 3 DSM-IV criterion ‘B’ mania symptoms (≥ 4 if predominantly irritable mood). Thus, both groups had childhood-onset type-I BD as defined by a similar set of symptoms. Age of onset was operationally defined as the age of first manic episode. All adults with BD were originally enrolled as youths in the Brown University site of the COBY study (2), and their childhood-onset type-I BD diagnoses were confirmed by the same research interviewers who diagnosed the child participants with type-I BD. No participants were biologically related.
Exclusion criteria for participants with BD were: Wechsler Abbreviated Scale of Intelligence full-scale intelligence quotient (WASI FSIQ) ≤ 70 (74), autism or primary psychosis, color-blindness, and medical/neurological conditions potentially mimicking BD.
All participants with BD remained on their outpatient medications because this was not a treatment study. However, those taking stimulant medications were asked, but not required, to hold those medications for four drug half-lives before behavioral testing, as such medication holidays are common in standard clinical care.
For HC participants, inclusion criteria were: age 7–17 years (youths) or age 18–30 years (adults), no current or lifetime psychiatric illness or substance abuse/dependence in the participant themself, and no first-degree relatives with a history of psychiatric illness or substance abuse/dependence.
Exclusion criteria for HC participants were: WASI FSIQ ≤ 70, color-blindness, learning disorders, and serious non-psychiatric medical disorders (e.g., epilepsy).
DANVA Facial Emotion Recognition Task
Facial emotion recognition ability was assessed using the DANVA-2. The DANVA-2 has separate subtests for child and adult emotional face expressions (69). Each subtest includes 24 standardized photographs of models (12 male, 12 female) displaying one of four facial emotions (angry, sad, fearful, or happy) at two levels of intensity (high or low). Faces were presented for two seconds, and participants chose which of the four emotions listed was expressed in the photograph. Both DANVA subtests have been standardized and demonstrate construct validity, internal reliability (Cronbach’s α = 0.64–0.77), and test-retest reliability for first graders to young adults (68–70). Outcome variables for each subtest include total errors, errors per intensity level, and errors per emotion type. All participants, including the adults with BD who had been prospectively followed since childhood, were completing the DANVA for the first time.
Mood/functional measures
To characterize the BD sample with respect to mood and functional status, mania symptoms were assessed via the Young Mania Rating Scale (YMRS) (75). Depression symptoms were assessed in youths with BD with the Children’s Depression Rating Scale-Revised (CDRS-R) (76) and in adults with BD with the Hamilton Rating Scale for Depression (HAM-D) (77). Since depression symptoms were measured with different scales in youths and adults with BD, we created a sample-specific Depression z-score for each youth and adult participant with BD based on their age group mean and standard deviation (SD) [i.e., (youth participant’s CDRS-R score – mean youths’ CDRS-R score)/SD of youths’ CDRS-R scores; (adult participant’s HAM-D score – mean adults’ HAM-D score)/SD of adults’ HAM-D scores]. Overall functional impairment was assessed via the Children’s Global Assessment Scale (78) among youths with BD and the Global Assessment of Functioning (79) among adults with BD, as both use the same 1–100 scale with similar, but developmentally appropriate, prompts every 10 points (80).
Data analysis
We evaluated between-group differences in demographic variables using analysis of variance (ANOVA) for continuous data (age, FSIQ) or chi-squared tests for categorical data (sex, race). Participant’s ages were measured using the date they completed the DANVA and their date of birth.
For the DANVA data, we analyzed performance on child and adult face subtests separately, as in prior work (45–49). For our primary analyses, we focused on total errors labeling emotions for child and adult face stimuli with age as a continuous predictor variable. Secondary multivariate analyses examined the effect of facial emotion intensity (i.e., high versus low) and the effects of specific emotion types (i.e., angry, sad, fearful, or happy) on facial emotion recognition, as in prior work (46–49).
In each case, to evaluate the effect of age continuously across both BD and HC samples, we used multiple linear regression with all independent variables (i.e., diagnosis, age, diagnosis-by-age interaction, FSIQ) standardized to reduce multi-collinearity (81, p. 287). To facilitate interpretable and direct comparisons between regression analyses, we report standardized beta-weights (81, p, 80). All statistical tests were two-tailed with a 0.05 significance criterion.
For the regression analyses, we used a layered approach to avoid a potential issue with multiple comparisons, with primary analyses of total errors on child or adult faces, followed by secondary analyses testing for the effects of emotion type or intensity, and finally, with post-hoc analyses to measure the effects of potentially confounding variables. To be conservative, only analyses with a significant diagnosis-by-age interaction were decomposed via separate univariate regressions for each diagnosis group, as recommended by statistical experts (81, p. 187). Similarly, for the multivariate regression analyses, univariate regressions were only conducted if a significant diagnosis-by-age interaction was found (81, p. 617). Finally, while our primary interest was examining facial emotion recognition across age as a dimensional variable, we also examined categorical comparisons between BD and HC within separate child (< 18-years-old) versus adult (≥ 18-years-old) age groups. Specifically, we ran a 2 × 2 age group (child versus adult) by diagnosis (BD versus HC) ANCOVA with IQ as a covariate.
Results
Participant characteristics (Table 1)
Table 1.
Demographic and clinical characteristics for youths and adults with bipolar disorder and healthy comparison youths and adults
| Bipolar disorder | Healthy comparisons | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Youths (n = 39) | Adults (n = 27) | All (n = 66) | Youths (n = 45) | Adults (n = 42) | All (n = 87) | |
| Characteristics | ||||||
|
|
|
|
|
|
|
|
| Age, years, mean (SD) | 13.5 (3.0) | 21.2 (2.3) | 16.7 (4.7) | 14.0 (2.9) | 21.9 (2.2) | 17.9 (4.7) |
|
|
|
|
|
|
|
|
| WASI FSIQ, mean (SD) | 106.6 (11.2) | 106.3 (11.4) | 106.4 (11.2) | 111.4 (7.5) | 110.5 (7.6) | 110.9 (7.5) |
|
|
|
|
|
|
|
|
| Male, n (%) | 25 (64.1) | 17 (63.0) | 42 (63.6) | 23 (51.1) | 30 (71.4) | 53 (60.9) |
|
|
|
|
|
|
|
|
| Female, n (%) | 14 (35.9) | 10 (37.0) | 24 (36.4) | 22 (48.9) | 12 (28.6) | 34 (39.1) |
|
|
|
|
|
|
|
|
| White, n (%)a | 32 (84.2) | 22 (88.0) | 54 (85.7) | 35 (81.4) | 31 (75.6) | 66 (78.6) |
|
|
|
|
|
|
|
|
| Non-white, n (%)a | 6 (15.8) | 3 (12.0) | 9 (14.3) | 8 (18.6) | 10 (24.4) | 18 (21.4) |
|
|
|
|
|
|
|
|
| YMRS score, mean (SD)b | 7.2 (5.0) | 4.4 (2.8) | 6.0 (4.5) | |||
|
|
|
|
||||
| CDRS-R/HAM-D score, mean (SD)c | 29.1 (11.6) | 5.2 (4.4) | n/ad | |||
|
|
|
|
||||
| CGAS/GAF score, mean (SD)e | 62.3 (14.8) | 65.4 (11.9) | 63.6 (13.7) | |||
|
|
|
|
||||
| Onset age, years, mean (SD)f | 10.1 (3.4) | 9.1 (3.4) | 9.6 (3.4) | |||
|
|
|
|
||||
| Comorbid conditions, mean (SD) | 2.0 (1.1) | 0.7 (1.0) | 1.4 (1.2) | |||
|
|
|
|
||||
| Non-euthymic, n (%)g,h | 11 (28.2) | 4 (14.8) | 15 (22.7) | |||
|
|
|
|
||||
| Currently medicated, n (%) | 34 (87.2) | 11 (40.7) | 45 (68.2) | |||
|
|
|
|
||||
| Lithium | 11 (28.2) | 1 (3.7) | 12 (18.2) | |||
|
|
|
|
||||
| Atypical neuroleptic | 22 (56.4) | 2 (7.4) | 24 (36.4) | |||
|
|
|
|
||||
| Stimulant | 13 (33.3) | 6 (22.2) | 19 (28.8) | |||
|
|
|
|
||||
| Antidepressant | 6 (15.4) | 6 (22.2) | 12 (18.2) | |||
|
|
|
|
||||
| Antiepileptic drugs | 6 (15.4) | 5 (18.5) | 11 (16.7) | |||
|
|
|
|
||||
| Current comorbid conditions, n (%) | ||||||
|
|
||||||
| ADHD | 27 (69.2) | 9 (33.3) | 36 (54.5) | |||
|
|
|
|
||||
| Oppositional defiant disorder | 27 (69.2) | 0 (0) | 27 (40.9) | |||
|
|
|
|
||||
| Generalized anxiety disorder | 5 (12.8) | 4 (14.8) | 9 (13.6) | |||
|
|
|
|
||||
For variables with missing data, percentages are calculated relative to the number of participants with data for this variable. BD = bipolar disorder; HC = healthy comparison; WASI FSIQ = Wechsler Abbreviated Scale of Intelligence full-scale intelligence quotient; YMRS = Young Mania Rating Scale; CDRS-R = Children’s Depression Scale–Revised; HAM-D = Hamilton Rating Scale for Depression; CGAS = Children’s Global Assessment Scale; GAF = Global Assessment of Functioning; ADHD = attention-deficit hyperactivity disorder.
One BD youth, two BD adults, two HC youths, and one HCadult did not report their race.
For YMRS scores, two youths’ and one adult’s scores were not available.
For CDRS-R/HAM-D scores, two youths’ and one adult’s scores were not available.
CDRS-R and HAM-D scores are not on the same scale and thus could not be averaged between age groups.
For CGAS/GAF scores, one adult’s score was not available.
For onset age, four youths and three adults did not have a precise age recorded, although all had confirmed illness onset before 18-years-old.
For non-euthymic youths, four were hypomanic, one was in a mixed state, and two were depressed. Four participants with missing YMRS or CDRS-R scores were counted as non-euthymic.
For non-euthymic adults, none were hypomanic or in a mixed state and three were depressed. One participant with a missing YMRS score was counted as non-euthymic.
No between-group differences between the BD and HC groups in age, race, or sex were found, whether the entire diagnostic groups were considered, or whether youths and adults were considered separately (Table 2). With respect to FSIQ, the diagnosis-by-age-group interaction was not significant [F(1,149) = 0.04, p = 0.84], and youths and adults also did not differ in FSIQ [F(1,149) = 0.16, p = 0.69]. However, participants with BD, as a whole, had a significantly lower mean FSIQ (106.4 ± 11.2) than HC participants (110.9 ± 7.5), [F(1,149) = 8.52, p = 0.004]. Thus, to be conservative, all DANVA analyses co-varied for FSIQ to control for main effect of diagnosis.
Table 2.
Between group comparisons on demographic and clinical factors
| BD youths versus HC youths | BD adults versus HC adults | All BD versus all HC | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | t(82) | p-value | t(67) | p-value | t(151) | p-value |
| Age, years | 0.79 | 0.43 | 1.33 | 0.19 | 1.54 | 0.13 |
|
| ||||||
| WASI FSIQ | 2.33 | 0.02 | 1.83 | 0.07 | 2.96 | 0.004 |
|
| ||||||
| χ2 (2) | p-value | χ2 (2) | p-value | χ2 (2) | p-value | |
|
| ||||||
| Sex | 1.44 | 0.23 | 0.54 | 0.46 | 0.12 | 0.73 |
|
| ||||||
| Race | 0.11 | 0.74 | 1.51 | 0.22 | 1.23 | 0.27 |
|
| ||||||
| BD youths versus BD adults | ||||||
|
|
||||||
| ta | p-value | |||||
|
|
||||||
| YMRS score | 2.6 | 0.012 | ||||
|
|
||||||
| CDRS-R/HAM-D score | n/ab | n/ab | ||||
|
|
||||||
| CGAS/GAF score | 0.9 | 0.37 | ||||
|
|
||||||
| Onset age | 0.8 | 0.43 | ||||
|
|
||||||
| Comorbid conditions | 5.1 | < 0.001 | ||||
|
|
||||||
| χ2 (2) | p-value | |||||
|
|
||||||
| Non-euthymic | 1.63 | 0.20 | ||||
|
|
||||||
| Currently medicated | 15.86 | < 0.001 | ||||
|
|
||||||
BD = bipolar disorder; HC = healthy comparison; WASI FSIQ = Wechsler Abbreviated Scale of Intelligence full-scale intelligence quotient; YMRS = Young Mania Rating Scale; CDRS-R = Children’s Depression Scale–Revised; HAM-D = Hamilton Rating Scale for Depression; CGAS = Children’s Global Assessment Scale; GAF = Global Assessment of Functioning.
Due to missing data, degrees of freedom for these tests ranged between 57 and 63.
CDRS-R and HAM-D scores are not on the same scale and thus could not be compared between age groups.
Of note, there was no significant difference in prospectively recorded age of illness onset between youths and adults with BD, indicating that all of our participants with BD could be aggregated as childhood-onset BD for our dimensional analyses. In addition, including age of onset had no effect on any analyses. Youths with BD had significantly more comorbid conditions and higher YMRS scores, and were more likely to be currently taking psychotropic medications than adults with BD (Table 2). We examined potential influences of these and other factors in post-hoc regression analyses below which either covaried for these effects within the BD group (e.g., YMRS scores) or excluded participants (e.g., by medication).
Primary analyses: child face total errors
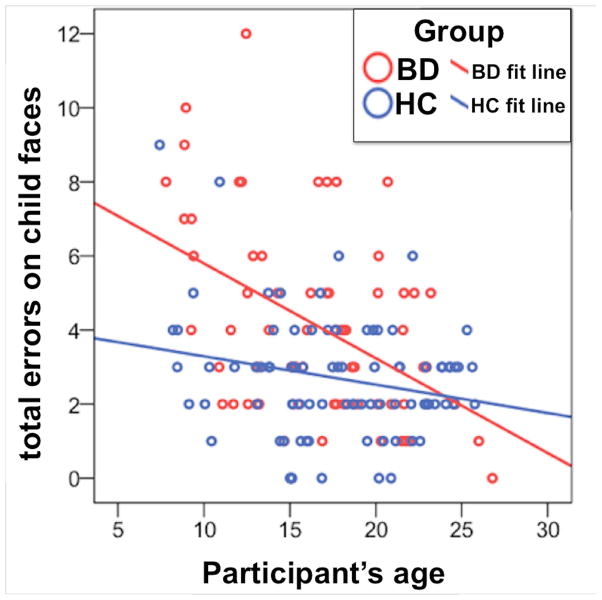
Our regression analysis of DANVA total child face errors using age as a continuous predictor revealed that participants with BD made significantly more errors than HC and younger participants made significantly more errors than older participants. However, a significant diagnosis-by-age interaction indicated that this effect of participant age on child face recognition errors was greater for participants with BD than for HC (Fig. 1) (Table 3). Confirming these findings, follow up regressions for each diagnosis revealed a significant effect of age in participants with BD and a much weaker, albeit statistically significant, effect of age in HC participants (Table 3). FSIQ exhibited no significant effects in any analysis.
Fig. 1.
Total child facial emotion recognition errors for individuals with bipolar disorder or healthy comparison participants. BD = bipolar disorder; HC = healthy comparison.
Table 3.
Regression analyses of total facial emotion errors for individuals with bipolar disorder or healthy comparison participants
| Child faces | Adult faces | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| BD versus HC | ||||||
|
|
|
|||||
| Effect | β | t(148) | p-value | β | t(148) | p-value |
|
|
|
|||||
| Diagnosis | 0.25 | 3.43 | <0.001 | 0.22 | 2.95 | 0.004 |
|
|
|
|||||
| Age | −0.34 | −4.71 | <0.001 | −0.31 | −4.22 | <0.001 |
|
|
|
|||||
| Diagnosis-by-age interaction | −0.19 | −2.61 | 0.01 | −0.18 | −2.42 | 0.02 |
|
|
|
|||||
| FSIQ | −0.07 | −1.01 | 0.31 | −0.03 | −0.39 | 0.70 |
|
|
|
|||||
| BD participants only | ||||||
| Effect | β | t(63) | p-value | β | t(63) | p-value |
|
|
|
|||||
| Age | −0.46 | −4.21 | <0.001 | −0.47 | −4.24 | <0.001 |
|
|
|
|||||
| FSIQ | −0.12 | −1.08 | 0.28 | −0.11 | −0.96 | 0.34 |
|
|
||||||
| HC participants only | ||||||
| Effect | β | t(84) | p-value | β | t(84) | p-value |
|
|
|
|||||
| Age | −0.23 | −2.10 | 0.039 | −0.17 | −1.56 | 0.123 |
|
|
|
|||||
| FSIQ | 0.003 | 0.03 | 0.98 | 0.08 | 0.76 | 0.45 |
|
|
|
|||||
Diagnosis was coded as healthy comparison (HC) = 0 and bipolar disorder (BD) = 1 for all regression analyses, so positive beta weights for diagnosis indicate greater effects in individuals with BD. FSIQ = full-scale intelligence quotient.
Primary analyses: adult face total errors
Our regression analysis of DANVA total adult face errors using age as a continuous predictor also revealed that participants with BD made significantly more errors than HC participants, and younger participants made significantly more errors than older participants. This analysis also showed a significant interaction, indicating that the effect of participant age on adult face recognition errors was greater for participants with BD than for HC participants (Fig. 2 and Table 3). Follow-up regressions revealed that the participants with BD showed a significant effect of age on performance, but the effect of age in HC participants was not significant (Table 3).
Fig. 2.
Total adult facial emotion recognition errors for individuals with bipolar disorder or healthy comparison participants. BD = bipolar disorder; HC = healthy comparison.
Secondary analyses: child face intensity and emotion effects
When evaluating child face errors by intensity level using multivariate regression, a significant interaction indicated that the effect of age differed between participants with BD and HC participants. In addition, participants with BD made significantly more errors than HC participants, and younger participants made significantly more errors than older participants (Table 4). In separate follow-up analyses for each intensity level, a significant diagnosis-by-age interaction occurred for high, but not low, intensity child faces (Table 5). Follow-up analyses for high intensity child faces split by diagnosis revealed that participants with BD showed a significant effect of age, and that HC participants showed a weaker, albeit significant, effect of age (Table 5).
Table 4.
Multivariate regressions for child and adult face errors separated by intensity or emotion type
| By intensity | By emotion type | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Wilks’ Λ | F(2,147) | p-value | Wilks’ Λ | F(4,145) | p-value | |
|
|
|
|||||
| Multivariate analysis of child faces | ||||||
|
|
|
|||||
| Effect | ||||||
|
|
|
|||||
| Diagnosis | 0.92 | 6.31 | 0.002 | 0.91 | 3.56 | 0.008 |
|
|
|
|||||
| Age | 0.86 | 12.40 | <0.001 | 0.84 | 6.78 | <0.001 |
|
|
|
|||||
| Diagnosis-by-age interaction | 0.94 | 4.34 | 0.02 | 0.93 | 2.56 | 0.04 |
|
|
|
|||||
| FSIQ | 0.99 | 0.52 | 0.60 | 0.98 | 0.69 | 0.60 |
|
|
|
|||||
| Multivariate analysis of adult faces | ||||||
| Effect | ||||||
|
|
|
|||||
| Diagnosis | 0.95 | 4.30 | 0.02 | 0.94 | 2.18 | 0.07 |
|
|
|
|||||
| Age | 0.89 | 8.90 | <0.001 | 0.84 | 6.73 | <0.001 |
|
|
|
|||||
| Diagnosis-by-age interaction | 0.96 | 3.39 | 0.04 | 0.94 | 2.30 | 0.06 |
|
|
|
|||||
| FSIQ | 1.00 | 0.08 | 0.93 | 0.99 | 0.45 | 0.77 |
|
|
|
|||||
Diagnosis was coded as healthy comparison = 0 and bipolar disorder = 1 for all regression analyses, so positive beta weights for diagnosis indicate greater effects in individuals with bipolar disorder. FSIQ = full-scale intelligence quotient.
Table 5.
Univariate regression analyses for child and adult faces separated by intensity
| Univariate high and low intensity results | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Effect | Child faces | Adult faces | ||||||||||
|
|
|
|||||||||||
| High Intensity | Low Intensity | High Intensity | Low Intensity | |||||||||
|
|
|
|
|
|||||||||
| β | t(148) | p-value | β | t(148) | p-value | β | t(148) | p-value | β | t(148) | p-value | |
|
|
|
|
|
|||||||||
| Diagnosis | 0.24 | 3.15 | 0.002 | 0.21 | 2.61 | 0.01 | 0.17 | 2.22 | 0.03 | 0.19 | 2.38 | 0.02 |
|
|
|
|
|
|||||||||
| Age | −0.33 | −4.57 | <0.001 | −0.26 | −3.42 | <0.001 | −0.26 | −3.41 | <0.001 | −0.25 | −3.19 | 0.002 |
|
|
|
|
|
|||||||||
| Diagnosis-by-age interaction | −0.21 | −2.84 | 0.005 | −0.13 | −1.70 | 0.09 | −0.19 | −2.48 | 0.01 | −0.10 | −1.34 | 0.18 |
|
|
|
|
|
|||||||||
| FSIQ | −0.05 | −0.67 | 0.51 | −0.07 | −0.95 | 0.35 | −0.02 | −0.27 | 0.78 | −0.03 | −0.33 | 0.74 |
|
|
|
|
|
|||||||||
| High intensity faces split by diagnosis | ||||||||||||
|
| ||||||||||||
| Effect | Child faces | Adult faces | ||||||||||
|
|
|
|||||||||||
| BD participants | HC participants | BD participants | HC participants | |||||||||
|
|
|
|
|
|||||||||
| β | t(63) | p-value | β | t(84) | p-value | β | t(63) | p-value | β | t(84) | p-value | |
|
|
|
|
|
|||||||||
| Age | −0.45 | −4.03 | <0.001 | −0.22 | −2.00 | 0.049 | −0.42 | −3.65 | <0.001 | −0.09 | −0.86 | 0.39 |
|
|
|
|
|
|||||||||
| FSIQ | −0.09 | −0.78 | 0.44 | 0.03 | 0.266 | 0.79 | −0.12 | −1.05 | 0.30 | 0.13 | 1.195 | 0.24 |
|
|
|
|
|
|||||||||
Diagnosis was coded as healthy comparison (HC) = 0 and bipolar disorder (BD) = 1 for all regression analyses, so positive beta weights for diagnosis indicate greater effects in individuals with BD. FSIQ = full-scale intelligence quotient.
When evaluating child face errors by emotion type using multivariate regression, a significant interaction indicated that the effect of age differed between participants with BD and HC participants. In addition, participants with BD made significantly more errors than HC participants, and younger participants made significantly more errors than older participants (Table 4).
Follow-up analyses of child face emotion errors split by emotion type revealed that, for angry child faces, there was a significant interaction between diagnosis and age and that there were significant main effects of diagnosis and age (Table 6). For sad child faces, individuals with BD showed worse performance overall than HC participants, young participants showed worse performance overall than older participants, but the effect of age did not differ across diagnoses. For fearful child faces, only a significant effect of age was found, and no significant effects were found for happy child faces (Table 6). Confirmatory analyses separated by diagnosis for angry child faces revealed that the effect of age was only significant for participants with BD and was not significant for HC participants (Table 6).
Table 6.
Univariate regression analyses for child faces separated by emotion
| Effect | Angry | Sad | Fearful | Happy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| β | t(148) | p-value | β | t(148) | p-value | β | t(148) | p-value | β | t(148) | p-value | |
|
|
|
|
|
|||||||||
| Diagnosis | 0.16 | 2.14 | 0.03 | 0.24 | 2.95 | <0.001 | 0.13 | 1.53 | 0.13 | 0.13 | 1.59 | 0.12 |
|
|
|
|
|
|||||||||
| Age | −0.34 | −4.62 | <0.001 | −0.20 | −2.53 | 0.01 | −0.16 | −2.02 | 0.045 | −0.09 | −1.12 | 0.26 |
|
|
|
|
|
|||||||||
| Diagnosis-by-age interaction | −0.20 | −2.76 | 0.006 | −0.12 | −1.50 | 0.14 | −0.04 | −0.55 | 0.58 | −0.10 | −1.17 | 0.24 |
|
|
|
|
|
|||||||||
| FSIQ | −0.12 | −1.53 | 0.13 | 0.03 | 0.32 | 0.75 | −0.06 | −0.70 | 0.48 | 0.01 | 0.10 | 0.92 |
|
|
|
|
|
|||||||||
| Effect | Angry child faces split by diagnosis | |||||||||||
|
|
||||||||||||
| BD participants | HC participants | |||||||||||
|
|
|
|||||||||||
| β | t(63) | p-value | β | t(84) | p-value | |||||||
|
|
|
|||||||||||
| Age | −0.50 | −4.69 | <0.001 | −0.18 | −1.70 | 0.09 | ||||||
|
|
|
|||||||||||
| FSIQ | −0.16 | −1.51 | 0.14 | −0.04 | −0.40 | 0.69 | ||||||
|
|
|
|||||||||||
Diagnosis was coded as HC = 0 and BD = 1 for all regression analyses, so positive beta weights for diagnosis indicate greater effects in individuals with BD. BD = bipolar disorder; HC = healthy comparison; FSIQ = full-scale intelligence quotient.
As an exploratory analysis to determine whether angry child faces were mistaken for other categories differently across groups, we conducted a repeated-measures multiple regression analyses with the type of error that participants made in response to angry child faces as the dependent variable (i.e., sad-instead-of-angry, fearful-instead-of-angry, happy-instead-of-angry). This analysis showed that angry child faces were mislabeled differently across sad, fearful, and happy categories, but also that participants with BD and HC participants, across age groups, differed in the types of errors they made (Table 7). This effect was found even while accounting for the main effects of diagnosis, age, and their interaction (Table 7). Follow up t-tests between participants with BD and HC participants, pooled across ages, for each error type showed that participants with BD mislabeled significantly more angry child faces as sad [t(151) = 3.52, p = 0.001] than HC participants, but not as fearful [t(151) = 0.78, p = 0.44] or happy [t(151) = 1.63, p = 0.11].
Table 7.
Repeated-measures regression analysis for emotion recognition errors in response to angry child faces by error-type
| Effect | Within-subjects effects | |
|---|---|---|
| F(2,296) | p-value | |
| Error-type | 6.65 | < 0.001 |
| Error-type by diagnosis | 2.03 | 0.007 |
| Error-type by age | 2.62 | 0.07 |
| Error-type by diagnosis-by-age interaction | 1.01 | 0.37 |
| Error-type by FSIQ | 0.88 | 0.41 |
| Effect | Between-subjects effects | |
| F(1,148) | p-value | |
| Diagnosis | 4.56 | 0.03 |
| Age | 21.35 | < 0.001 |
| Diagnosis-by-age interaction | 7.63 | 0.006 |
| FSIQ | 2.34 | 0.13 |
Diagnosis was coded as healthy comparison = 0 and bipolar disorder = 1 for all regression analyses, so positive beta weights for diagnosis indicate greater effects in individuals with bipolar disorder. FSIQ = full-scale intelligence quotient.
Secondary analysis: adult face intensity and emotion effects
When evaluating adult face errors by intensity level using multivariate regression, a significant interaction indicated that the effect of age differed between participants with BD and HC participants. In addition, participants with BD made significantly more errors than HC participants, and younger participants made significantly more errors than older participants (Table 4). As in the analyses of child face errors, in separate follow-up analyses of adult face errors for each intensity level, this diagnosis-by-age interaction occurred for high, but not low, intensity adult faces (Table 5). Follow-up analyses for high intensity adult faces split by diagnosis confirmed that participants with BD showed a significant effect of age whereas HC participants did not (Table 5).
When evaluating adult face errors by emotion type using multivariate regression, a significant effect of age was found, but the effects of diagnosis and the diagnosis-by-age interaction were not significant (Table 4).
Categorical analysis: comparisons between child and adult age groups
Evaluating age categorically, the ANCOVA for child face errors found significant main effects for diagnosis [F(1,148) = 11.94, p < 0.001] and age group [F(1,148) = 13.59, p < 0.001], but the diagnosis-by-age-group interaction was non-significant [F(1,149) = 3.83, p = 0.05]. Similarly, the ANCOVA for adult face errors also found significant main effects for diagnosis [F(1,148) = 9.05, p = 0.003] and age group [F(1,148) = 6.28, p = 0.013], but the diagnosis-by-age-group interaction was non-significant [F(1,148) = 3.39, p = 0.07]. Follow up comparisons between the four groups (youths with BD, HC youths, adults with BD, and HC adults) were not conducted due to the non-significant interactions.
Post-hoc analyses: global functioning and mood state
To examine the potential effects of mood state or global functioning on our results, we re-ran the child and adult face analyses excluding non-euthymic participants with BD [defined as YMRS > 12 (youths/adults); and/or CDRS-R > 40 (youths) or HAM-D > 7 (adults) (82)]. Excluding non-euthymic participants did not change our findings in either child or adult face analyses (Supplementary Table S1). We ran additional analyses solely within the BD sample, which included covariates for participants’ global functioning, YMRS, or depression scores and still found significant effects of age (Supplementary Table S2). Intriguingly, BD participants’ global functioning also showed a significant effect on child face errors even after accounting for participants’ age and FSIQ (Supplementary Table S2).
Post-hoc analyses: effect of comorbid conditions and substance abuse
To determine the influence of comorbid conditions on our results, we conducted separate regression analyses for child and adult faces within the BD group with variables coding for the presence versus absence of attention-deficit hyperactivity disorder, generalized anxiety disorder, or oppositional defiant disorder (Supplementary Table S3). Even after accounting for BD participants’ comorbid conditions, the effect of age was significant for all analyses.
To determine the influence of substance abuse on our results, we re-ran our primary analyses excluding participants with BD who had any diagnosis of abuse/dependence (Supplementary Table S1). In addition, we re-ran our primary child and adult face analyses with the presence or absence of substance abuse/dependence included as a covariate (Supplementary Table S3). Our results were consistent even after accounting for BD participants’ substance abuse/dependence.
Even though none of the participants met criteria for a psychotic disorder, two adults with BD reported some specific psychosis symptoms. When we excluded these two participants, the results of our primary child and adult face analyses did not change (Supplementary Table S1).
Post-hoc analyses: effect of medications
To determine the influence of medication usage on our findings, we ran analyses excluding participants taking each medication class (i.e., sequentially excluding all participants taking lithium, atypical neuroleptics, stimulants, antidepressants, or antiepileptic drugs) and re-ran our primary analyses. For both analyses (i.e., total errors on child faces and total errors on adult faces), we found significant effects of diagnosis, age, and the diagnosis-by-age interaction when we excluded participants taking each class of medication except atypical neuroleptics (Supplementary Table S4). Specifically, when excluding participants taking atypical neuroleptics, the diagnosis-by-age interaction was not significant for adult face total errors (p = 0.18), but the same interaction was significant for child face total errors (p = 0.02) and the interaction effects were in the same direction. The lack of a significant diagnosis-by-age interaction in this case may be a Type-II error due to lack of power because over one third of the participants with BD were taking an atypical neuroleptic. As further support for the position that medication usage did not influence our results, we conducted analyses covarying for the presence vs. absence of any medication and still found significant effects of age within the BD group (Supplementary Table S2). As a whole, the results of these post-hoc analyses suggest that the effects of diagnosis and age and the diagnosis-by-age interaction are unlikely to be due to medication effects.
Regression diagnostics
We also tested the assumptions and validity of the regression model (e.g., independent observations, no collinearity of predictors, etc.) for the primary child face error and adult face error analyses (81), and both analyses passed these checks (Supplementary Table S5). We also evaluated the effect of potential outliers on our model using outlier, residual, and influence statistics (Supplementary Table S5) (81). We confirmed the results of our primary analyses of child and adult faces even after excluding outliers or any potentially overly influential cases (Supplementary Table S6). Thus, our results are very unlikely to have been caused by the presence of outlying or overly influential data points.
Discussion
Our study compared facial emotion recognition in youths and adults with childhood-onset type-I BD relative to age-matched participants without psychopathology by including the continuous variable of age in the analysis. Our primary finding was a deficit in facial emotion recognition in individuals with childhood-onset type-I BD that was especially pronounced in younger participants, even after accounting for the normative development of facial emotion recognition ability that occurs between childhood and adulthood. This interaction was not significant when participants were dichotomized by the age of 18-years-old as is commonly done in research regulation, suggesting analyses treating age as a continuous variable may be more sensitive than those treating it categorically and may reduce the likelihood of Type-II errors. This facial emotion recognition deficit appeared to be largest for angry child faces, as participants with BD were more likely than HC participants to mistake such faces as sad. Our findings suggest that youths with childhood-onset type-I BD are impaired relative to their HC peers in facial emotion recognition ability, possibly due to a delay in the normal development of this social skill. Facial emotion recognition errors for child faces also showed a significant relationship with BD participants’ global functioning, suggesting that these deficits could be relevant to their daily lives. Future studies should follow up on these cross-sectional findings by conducting a longitudinal study prospectively following individuals with childhood-onset BD into adulthood to investigate whether indeed these individuals do show delays in the development of facial emotional recognition and if so, what the consequences are of this altered developmental trajectory.
Our behavioral results align with prior developmental comparisons made in individuals with BD using fMRI. These studies found increased amygdala activation in response to facial emotions specifically in youths with BD relative to adults with BD and HC youths (62) and greater modulation of neural activity in response to increasing emotion intensity in adults with BD versus youths with BD (64). In addition, our own quantitative meta-analysis of fMRI studies in BD found that amygdala hyperactivation versus HC was more consistently found in youths with BD than in adults with BD (44). The current findings, along with the prior work, suggest that facial emotion recognition deficits are more specific to younger participants with BD and have an underlying neurobiological signature.
Our approach was inspired by pioneering longitudinal neuroimaging studies demonstrating that brain development proceeds continuously from childhood to young adulthood (65–67). Prior developmental studies investigating BD dichotomized their samples into youth and adult groups using the 18 years of age cutoff commonly used in research regulation. These studies also employed adult BD groups whose mean age was older than the mean adult age in our study, and in the 75 studies of adult BD samples in our meta-analysis (44), the participants with BD were an average of 37 years old. However, these older participants are less similar to youths with BD due to their substantially longer history of BD and potentially greater differences from youths with BD in medication use, substance abuse, and life stress. Furthermore, all of the previous studies compared youths with childhood-onset BD to adults with adult-onset BD, so these studies could have been comparing different phenotypes of BD. In contrast, our study employed youths with childhood-onset BD and adults with type-I BD whose childhood-onset was confirmed prospectively. Thus, the impaired facial emotion recognition in younger BD participants was not an artifact caused by comparing different presentations of the illness, suggesting that the impairment is a key feature of childhood-onset type-I BD.
Moreover, our behavioral assessment contained both child and adult faces, unlike the prior fMRI studies comparing youths and adults with BD, which only used adult faces. We used both child and adult faces because behavioral data from paradigms such as the DANVA indicate that youths with BD may show greater deficits in recognizing other children’s emotions (48, 49). In this context, our current findings suggest that developmental studies of facial emotion recognition in BD should examine age as a continuous, rather than a discrete, variable to avoid potential Type-II errors, should include child faces in addition to adult faces, and should compare youths with BD to adults who also have childhood-onset BD.
Previous studies of facial emotion recognition in BD have found specific effects for certain emotions (33, 37, 40, 42, 43, 48–53), but the specific emotion that participants with BD have deficits in identifying has varied and was not consistent in a meta-analysis of many such studies (32). However, a recent study by Ruocco et al. (42), which included a large number of participants with BD and their relatives, did find strong evidence of a specific recognition deficit for angry faces. We also found evidence of a specific deficit in identifying angry child faces (33, 42, 43, 48–50), which were most often misidentified by our participants with BD as sad. Individuals with BD, across ages, were also less accurate than HC participants at identifying sad child faces, and younger participants, across diagnoses, were less accurate than older participants at identifying fearful child faces. The fact that we did not find diagnosis-by-age interactions for the child face emotions other than anger (i.e., sad, happy, fearful faces) may indicate that younger participants with BD are particularly impaired at recognizing anger in other children, which could have serious psychosocial consequences. This finding may also reflect a limitation of the DANVA, namely that a relatively small number of facial emotion exemplars are used for the child and adult face subtests. In addition, the DANVA does not include some emotions (e.g., disgust, surprise), so we could not test for specific alterations in recognizing these emotions (37, 40, 53). We also found stronger effects for high-intensity faces than low-intensity faces, unlike some studies that found the opposite (46, 49). In our case, the low intensity child and adult faces were more difficult for our HC sample than the high intensity faces [t(86) = 13.44, p < 0.001; t(86) = 8.47, p < 0.001, respectively], which may have obscured the diagnosis-by-age interaction. However, the relatively limited number of exemplars at each intensity on the DANVA might cause reduced power to detect the specific diagnosis-by-age interaction that was of greatest interest to us. Of note, the overall main effects of diagnosis (BD worse than HC) and age (younger worse than older) were found for both high and low intensity child and adult faces. In addition to only labeling a limited number of exemplars, participants did not rate their subjective impressions of the intensity of each face’s emotional expression. Such information would have allowed us to determine whether participants over- or underestimated the intensity of the emotion on each face (50). Thus, further study is warranted using facial emotion recognition paradigms with greater numbers of stimuli across a variety of emotion types and intensities [including ecologically valid blends of basic emotional facial expresses such as ‘happy surprised’ and ‘fearful surprised’ (83)], thus increasing sensitivity for emotion- and intensity-specific deficits and enhancing these studies generalizability to real-world situations.
Limitations
Limitations of our study include differences in medication status and psychiatric comorbidities found between our samples of youths and adults with BD. Specifically, the youths with BD were more likely to be taking medication than the adults with BD, so the increases in BD youths’ facial recognition errors could be medication-induced. However, neither sequentially removing participants taking each class of medication nor covarying for medication usage meaningfully influenced the statistical significance of our findings. Furthermore, medication usage may normalize many symptom-level and neural-level abnormalities in individuals with BD (84), so medication usage appears unlikely to have caused these increased emotion recognition deficits found in youths with BD (51). Youths with BD also had significantly more comorbid conditions than the adults with BD, but the results remained significant when we accounted for the increased comorbidities in the younger participants. Furthermore, previous studies investigating the effects of common comorbidities in youths with BD (e.g., anxiety, attention-deficit hyperactivity disorder) suggest a relatively BD-specific deficit in facial emotion recognition (46–49). Finally, the adults with BD in our study were part of an ongoing longitudinal study of the course and outcomes of childhood-onset BD (2, 17), so these participants may have been different from community-dwelling individuals with type-I BD who were not participating in a longitudinal study. However, the ongoing COBY study has shown that adults with childhood-onset BD continue to experience poor clinical outcomes and significant impairments in psychosocial functioning (1, 2, 8, 17), suggesting that the adults in our sample were not a biased group that was less symptomatic than adults with BD in general (4–6). Furthermore, the enrollment of COBY participants with BD is also a major strength of the study, as this strategy avoids retrospective recall bias about the onset of our participants’ BD illness. A final limitation of our study is that we only tested for linear relationships between age and facial emotion recognition performance. Although using age as a continuous linear predictor of performance is better than a categorical split between children and adults, the development of brain regions involved in many behaviors, including facial emotion recognition, may also have nonlinear components (60, 61, 71). However, our study was not designed to test for nonlinear relationships between age and performance, as we were concerned both about being underpowered and over-fitting such models with our current sample size. Future work in larger, more adequately powered samples is required to explore this important question. Overall, these cross-sectional results and their caveats underscore the need for longitudinal studies tracking the same youths with childhood-onset type-I BD into adulthood in order to better understand the altered developmental trajectory of facial emotion recognition and its functional consequences.
Conclusions
Comparing youths and adults with childhood-onset, type-I BD using age as a continuous variable, we identified a developmentally salient facial emotion recognition deficit in childhood-onset BD. Our analyses treated age continuously in accordance with known brain development and found significant and robust results, suggesting this may be a productive strategy in future developmental research in BD. Our data show that younger participants with BD are significantly worse than their age-matched child/adolescent peers in the important social skill of recognizing others’ facial emotions and also that this deficit may be related to their global functioning. Further research is warranted to test facial emotion recognition as a developmentally salient treatment target, such as for cognitive remediation to improve BD youths’ recognition of others’ emotions (24, 25). Finally, imaging studies are warranted to probe the neurodevelopmental mechanisms for these deficits (21–23), which could ultimately lead to better, more personalized diagnostic and intervention strategies, facilitating earlier recognition and improved outcomes for individuals with BD, their families, and society.
Supplementary Material
Acknowledgments
We gratefully acknowledge research funding from the National Institute of Mental Health, which supported this work (K22MH074945 and R01MH087513, Principal Investigator: DPD) and supported the Course and Outcome in Bipolar Youth (COBY) study (R01MH059929), the parent study whose Brown University site initially recruited and continues to follow the young adult participants with bipolar disorder. We also would like to thank our research participants and their families for their time and effort, without which this research would not have been possible.
Footnotes
Disclosures
The authors of this paper do not have any commercial associations that might pose a conflict of interest in connection with this paper.
References
- 1.Hunt JI, Case BG, Birmaher B, et al. Irritability and elation in a large bipolar youth sample: relative symptom severity and clinical outcomes over 4 years. J Clin Psychiatry. 2013;74:e110–117. doi: 10.4088/JCP.12m07874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leverich GS, Post RM, Keck PE, Jr, et al. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 4.Dusetzina SB, Farley JF, Weinberger M, Gaynes BN, Sleath B, Hansen RA. Treatment use and costs among privately insured youths with diagnoses of bipolar disorder. Psychiatr Serv. 2012;63:1019–1025. doi: 10.1176/appi.ps.201100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. Pharmacoeconomics. 2003;21:601–622. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- 6.Keck PE, Jr, Kessler RC, Ross R. Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatr Pract. 2008;14 (Suppl 2):31–38. doi: 10.1097/01.pra.0000320124.91799.2a. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein TR, Birmaher B, Axelson D, et al. History of suicide attempts in pediatric bipolar disorder: factors associated with increased risk. Bipolar Disord. 2005;7:525–535. doi: 10.1111/j.1399-5618.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero S, Birmaher B, Axelson DA, et al. Negative life events in children and adolescents with bipolar disorder. J Clin Psychiatry. 2009;70:1452–1460. doi: 10.4088/JCP.08m04948gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser M, Galling B, Correll CU. Suicidal ideation and suicide attempts in children and adolescents with bipolar disorder: a systematic review of prevalence and incidence rates, correlates, and targeted interventions. Bipolar Disord. 2013;15:507–523. doi: 10.1111/bdi.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holma KM, Haukka J, Suominen K, et al. Differences in incidence of suicide attempts between bipolar I and II disorders and major depressive disorder. Bipolar Disord. 2014;16:652–661. doi: 10.1111/bdi.12195. [DOI] [PubMed] [Google Scholar]
- 11.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blader JC, Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 15.Holtmann M, Duketis E, Poustka L, Zepf FD, Poustka F, Bölte S. Bipolar disorder in children and adolescents in Germany: national trends in the rates of inpatients, 2000–2007. Bipolar Disord. 2010;12:155–163. doi: 10.1111/j.1399-5618.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 16.Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: Prospective continuity with adult bipolar i disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50:1001–1016. e3. doi: 10.1016/j.jaac.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005;7:111–118. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 19.Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. J Affect Disord. 2010;126:1–13. doi: 10.1016/j.jad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Wegbreit E, Pavuluri M. Mechanistic comparisons of functional domains across pediatric and adult bipolar disorder highlight similarities, as well as differences, influenced by the developing brain. Isr J Psychiatry Relat Sci. 2012;49:75–83. [PubMed] [Google Scholar]
- 21.Wegbreit E, Ellis JA, Nandam A, et al. Amygdala functional connectivity predicts pharmacotherapy outcome in pediatric bipolar disorder. Brain Connect. 2011;1:411–422. doi: 10.1089/brain.2011.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavuluri MN, Passarotti AM, Fitzgerald JM, Wegbreit E, Sweeney JA. Risperidone and divalproex differentially engage the fronto-striato-temporal circuitry in pediatric mania: A pharmacological functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2012;51:157–170. doi: 10.1016/j.jaac.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavuluri MN, Ellis JA, Wegbreit E, Passarotti AM, Stevens MC. Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania. Behav Brain Res. 2012;226:493–503. doi: 10.1016/j.bbr.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 25.Eldar S, Apter A, Lotan D, et al. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am J Psychiatry. 2012;169:213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strakowski SM, Adler CM, Almeida J, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. J Am Acad Child Adolesc Psychiatry. 2008;47:1273–1288. doi: 10.1097/CHI.0b013e318185d2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 30.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Bolten M, Nast I, Skrundz M, Stadler C, Hellhammer DH, Meinlschmidt G. Prenatal programming of emotion regulation: Neonatal reactivity as a differential susceptibility factor moderating the outcome of prenatal cortisol levels. J Psychosom Res. 2013;75:351–357. doi: 10.1016/j.jpsychores.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Pan YJ, Tseng HH, Liu SK. Affect recognition across manic and euthymic phases of bipolar disorder in Han-Chinese patients. J Affect Disord. 2013;151(2):791–4. doi: 10.1016/j.jad.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 34.Derntl B, Seidel EM, Kryspin-Exner I, Hasmann A, Dobmeier M. Facial emotion recognition in patients with bipolar I and bipolar II disorder. Br J Clin Psychol. 2009;48:363–375. doi: 10.1348/014466509X404845. [DOI] [PubMed] [Google Scholar]
- 35.Fulford D, Peckham AD, Johnson K, Johnson SL. Emotion perception and quality of life in bipolar I disorder. J Affect Disord. 2014;152–154:491–497. doi: 10.1016/j.jad.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derntl B, Seidel EM, Schneider F, Habel U. How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr Res. 2012;142:58–64. doi: 10.1016/j.schres.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoertnagl CM, Muehlbacher M, Biedermann F, et al. Facial emotion recognition and its relationship to subjective and functional outcomes in remitted patients with bipolar I disorder. Bipolar Disord. 2011;13:537–544. doi: 10.1111/j.1399-5618.2011.00947.x. [DOI] [PubMed] [Google Scholar]
- 38.Seidel EM, Habel U, Finkelmeyer A, Hasmann A, Dobmeier M, Derntl B. Risk or resilience? Empathic abilities in patients with bipolar disorders and their first-degree relatives. J Psychiatr Res. 2012;46:382–388. doi: 10.1016/j.jpsychires.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Rowland JE, Hamilton MK, Vella N, Lino BJ, Mitchell PB, Green MJ. Adaptive associations between social cognition and emotion regulation are absent in schizophrenia and bipolar disorder. Front Psychol. 2012;3:607. doi: 10.3389/fpsyg.2012.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaefer KL, Baumann J, Rich BA, Luckenbaugh DA, Zarate CA., Jr Perception of facial emotion in adults with bipolar or unipolar depression and controls. J Psychiatr Res. 2010;44:1229–1235. doi: 10.1016/j.jpsychires.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daros AR, Ruocco AC, Reilly JL, Harris MS, Sweeney JA. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr Res. 2014;153:32–37. doi: 10.1016/j.schres.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruocco AC, Reilly JL, Rubin LH, et al. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr Res. 2014;158:105–112. doi: 10.1016/j.schres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goghari VM, Sponheim SR. More pronounced deficits in facial emotion recognition for schizophrenia than bipolar disorder. Compr Psychiatry. 2013;54:388–397. doi: 10.1016/j.comppsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wegbreit E, Cushman GK, Puzia ME, et al. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71:926–935. doi: 10.1001/jamapsychiatry.2014.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 46.McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E. Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry. 2003;160:1172–1174. doi: 10.1176/appi.ajp.160.6.1172. [DOI] [PubMed] [Google Scholar]
- 47.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 48.McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 49.Seymour KE, Pescosolido MF, Reidy BL, et al. Emotional face identification in youths with primary bipolar disorder or primary attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:537–546. e3. doi: 10.1016/j.jaac.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shankman SA, Katz AC, Passarotti AM, Pavuluri MN. Deficits in emotion recognition in pediatric bipolar disorder: the mediating effects of irritability. J Affect Disord. 2013;144:134–140. doi: 10.1016/j.jad.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- 52.Brotman MA, Rich BA, Schmajuk M, et al. Attention bias to threat faces in children with bipolar disorder and comorbid lifetime anxiety disorders. Biol Psychiatry. 2007;61:819–821. doi: 10.1016/j.biopsych.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 53.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brotman MA, Skup M, Rich BA, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry. 2008;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wegbreit E, Passarotti AM, Ellis JA, et al. Where, when, how high, and how long? The hemodynamics of emotional response in psychotropic-naive patients with adolescent bipolar disorder. J Affect Disord. 2013;147:304–311. doi: 10.1016/j.jad.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Garrett AS, Reiss AL, Howe ME, et al. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:821–831. doi: 10.1016/j.jaac.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Somerville LH, Fani N, McClure-Tone EB. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36:408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim P, Thomas LA, Rosen BH, et al. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. Am J Psychiatry. 2012;169:642–649. doi: 10.1176/appi.ajp.2012.11081245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brotman MA, Tseng WL, Olsavsky AK, et al. Fronto-limbic-striatal dysfunction in pediatric and adult patients with bipolar disorder: impact of face emotion and attentional demands. Psychol Med. 2014;44:1639–1651. doi: 10.1017/S003329171300202X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deveney CM, Brotman MA, Thomas LA, et al. Neural response during explicit and implicit face processing varies developmentally in bipolar disorder. Soc Cogn Affect Neurosci. 2014;9:1984–1992. doi: 10.1093/scan/nsu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 66.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nowicki S, Jr, Carton J. The measurement of emotional intensity from facial expressions. J Soc Psychol. 1993;133:749–750. doi: 10.1080/00224545.1993.9713934. [DOI] [PubMed] [Google Scholar]
- 69.Nowicki S, Jr, Duke MP. Individual differences in the nonverbal communication of affect: the Diagnostic Analysis of Nonverbal Accuracy Scale. J Nonverbal Behav. 1994;18:9–35. [Google Scholar]
- 70.McIntire KA, Danforth MM, Schneider HG. Measuring cue perception: Assessment of reliability and validity. N Am J Psychol. 1999;1:261–266. [Google Scholar]
- 71.Hartley CA, Lee FS. Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology. 2015;40:50–60. doi: 10.1038/npp.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 73.First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 74.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 2005. [Google Scholar]
- 75.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 76.Poznanski E, Freeman LN, Mokros HB. Children’s Depression Rating Scale-Revised. Psychopharmacol Bull. 1985;21:979–984. [Google Scholar]
- 77.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 79.Hall RC. Global Assessment of Functioning. A modified scale Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 80.Schorre BE, Vandvik IH. Global assessment of psychosocial functioning in child and adolescent psychiatry. A review of three unidimensional scales (CGAS, GAF, GAPD) Eur Child Adolesc Psychiatry. 2004;13:273–286. doi: 10.1007/s00787-004-0390-2. [DOI] [PubMed] [Google Scholar]
- 81.Cohen J, Cohen P, West SG, Aiken LA. Applied multiple regression/correlations analysis for the behavioral sciences. 3. Mahwah: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 82.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150:384–388. doi: 10.1016/j.jad.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 83.Du S, Tao Y, Martinez AM. Compound facial expressions of emotion. Proc Natl Acad Sci U S A. 2014;111:E1454–1462. doi: 10.1073/pnas.1322355111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.