Abstract
Toxoplasma gondii infects a very wide range of mammals and birds, and about one-third of humans are infected with this protozoan parasite. Chronic T. gondii infection has historically been believed to be asymptomatic; however there is now evidence that links chronic infection with several psychiatric disorders. While there are drugs to treat acute toxoplasmosis, there are currently no treatments for the latent form of the parasite. Currently, T. gondii in vivo research is performed using murine models, which are limited by cost and the inability to perform high throughput assays. To develop an improved in vivo model, we adapted zebrafish to 37°C and injected them intraperitoneally with two strains of T. gondii at a concentration of 10 tissue cysts per fish, and observed them for 7 days post injection. Fish were examined by histology for the presence of T. gondii development. Intracellular parasites were observed in fish at 5 to 7 days post injection. The pattern of infection observed was similar to that found in mammalian infection, with parasites developing in the somatic muscle, heart, liver, spleen, kidney, and brain.
The cosmopolitan protozoan parasite, Toxoplasma gondii, has been observed in numerous mammalian and avian species with varying degrees of clinical disease. The only known definitive hosts of T. gondii are felids. However, other warm-blooded vertebrates, including humans, can act as an intermediate host for the parasite. In humans, it is estimated to infect one-third of the world population, making it one of the most prevalent parasitic infections in the world. Despite this wide range of hosts, T. gondii infection has never been observed in fishes (Dubey 2010). Toxoplasma gondii has been intensively studied with nearly 25 000 research articles and reviews currently indexed in the PubMed database. However, many aspects of the biology and epidemiology of T. gondii remain unknown. While there are medicines that are effective against tachyzoites of T gondii, no currently available medicines have been shown to eliminate the tissue cyst (bradyzoite) stage. Presently, potential therapeutic compounds are tested against T. gondii grown in cell culture or in a mouse model. Both models have a long history of use in drug development, but they have limitations such as cell culture is not reflective of a true in vivo infection and the murine model is expensive and not appropriate for high-throughput use. The availability of new, less expensive high-throughput in vivo models of T. gondii infection should help facilitate the development of therapeutic compounds.
Previous studies of T. gondii in fish and invertebrates have focused on the concentration and survival of the oocyst stage in prey animals as a potential vector for infection of marine mammals (Arkush et al. 2003; Lindsay & Collins 2004; Massie et al. 2010). Whereas the parasite has been shown to persist in many bivalves, some fishes, and marine water (Lindsay & Dubey 2009) for a period of time, proliferation of T. gondii has not been observed in poikilothermic aquatic organisms. In the one study in which experimental infection of a fish was attempted, the investigators were unsuccessful (Omata et al. 2005) and could not establish T. gondii infections in goldfish, Carassius auratus, that were maintained at 37 °C. Interestingly, they were able to infect and propagate in goldfish oviduct epithelial cells held at 37 °C but not 33 °C. However, fish are hosts for other several coccidian species, including Goussia, Calyptospora, etc. (Molnár 2006). Two parasites have been reported in fish that show similarities to T. gondii in that they exhibit they massive asexual proliferation in visceral organ (Ferguson & Roberts 1975; Kent, Moser & Fournie 1989). One of these has been assigned to the genus Haemogregarina (Kirmse 1978), but as no sequence data are available for these parasites, it is not possible to determine their taxonomic affinities with T. gondii or related organisms.
The zebrafish, Danio rerio, is an important laboratory model for toxicology, developmental biology, cancer and infectious disease research (Phillips & Westerfield 2014). It has a well-characterized immune system (Traver et al. 2003), and the complete genome has been sequenced with the genetic map showing an overall highly conserved synteny with the human genome (Postlethwait et al. 1998). Zebrafish have been used in numerous in vivo experiments using various bacterial and viral pathogens (reviewed in Dooley 2000; Ingham 2009; Kanther & Rawls 2010; Allen & Neely 2010). The results of these studies have shown a number of immune response pathways which are analogous to those of humans and other mammals (Meeker & Trede 2008; Meijer & Spaink 2011). The costs of housing and husbandry are considerably less than those of other animals, and thus, they are quite amenable to high-throughput methods (Zon & Peterson 2005).
Zebrafish are most often held at about 28 °C in research laboratories (Harper & Lawrence 2010), and thus, in vivo experiments with both fish and human pathogens in this model are usually conducted at this temperature (Lesley & Ramakrishnan 2008; Sullivan & Kim 2008; Vojtech et al. 2009; Sanders et al. 2010; Sanders, Peterson & Kent 2014). In its natural habitat, zebrafish generally occupy slow-moving waters with a wide range of temperatures (6–38 °C) (Spence et al. 2008). The resilience of this small fish was confirmed by the observation of zebrafish surviving in a home aquarium with a malfunctioning thermostat that resulted in heating the water to 37 °C, resulting in the death of several tropical fish species but the survival of zebrafish. Herein, we describe that successful experimental T. gondii infection occurs in zebrafish housed at 37 °C.
A total of 22 adult, 5D line zebrafish (Kent et al. 2011) were obtained from the Sinnhuber Aquatic Research Laboratory at Oregon State University and housed in 2.7-L static aquaria with gentle aeration. Fish were acclimated from 28 to 37 °C by increasing the temperature 1 °C/day. Fish were held at 37 °C and fed daily for 3 weeks prior to injection. Tissue cysts of Toxoplasma gondii were obtained and purified from the brains of experimentally infected mice following the protocol of Puvanesuaran et al. (2012). One brain from an infected mouse was homogenized by passing through a syringe with successively smaller gauge needles (18–22 gauge) several times. The homogenate was washed with PBS containing 0.05% Tween 80 twice. It was then mixed at a 1:2 ratio with a 21% dextran solution (Sigma-Aldrich) and centrifuged at 3000 g for 15 min. The pellet was washed with PBS and the cysts were counted with a haemocytometer. Two strains, representing two genotypes, ME-49 (type II) and VEG (type III) (1), were used. For each strain, the inoculum was diluted with sterile PBS to 1 cyst/μL. Fish were anesthetized with buffered tricaine methane sulphonate MS-222 (200 ppm) and injected intraperitoneally (IP) with 10 μL. Three fish from each inoculum group were killed immediately following injection as controls. The remaining fish were observed daily and held for a total of 7 days post-injection (dpi). Moribund and apparently healthy fish were killed using an overdose of MS-222 (500 ppm) and preserved in Dietrich's fixative. Preserved fish were processed for histology and paraffin-embedded, and 5-μm sections cut and stained with haematoxylin and eosin (H&E) or Giemsa. Immunohistochemical staining was performed on unstained histological sections from the infected fish for confirmation of the presence of T. gondii using a previously described protocol with polyclonal rabbit anti-T. gondii antibodies (Dubey 2010).
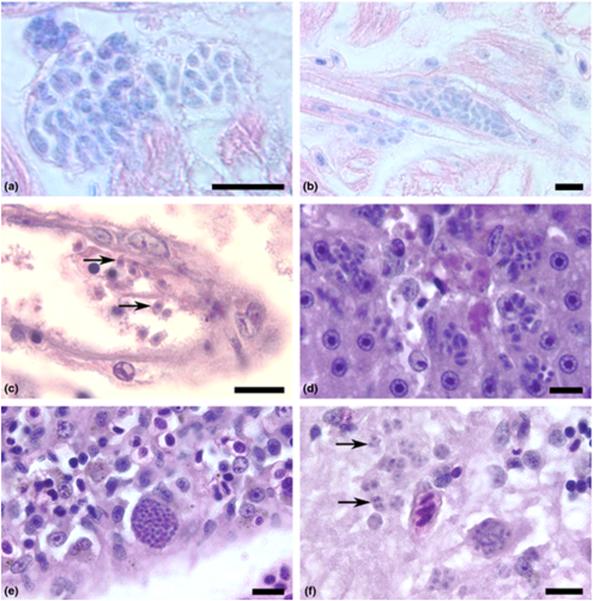
Toxoplasma gondii tachyzoites were observed in the tissues of three moribund fish at 5 (ME-49), 6 and 7 (VEG) dpi. We observed tachyzoites developing within cardiac myocytes (Fig. 1a,b), within the endothelium and free in the lumen of a small blood vessel (Fig. 1c) and in the liver (Fig. 1d), spleen (Fig. 1e), brain (Fig. 1f), ovaries, pancreas, kidney and skeletal muscles (data not shown). Corresponding slides stained with the specific antibody revealed more extensive infections in other organs, including infections in the eye and gills (Fig. 2a,b). Clinical signs present in these fish included bilateral exopthalmia, swollen abdomens, whirling swimming behaviour and generalized subdermal haemorrhaging. We were unable to determine whether these signs were caused by T. gondii infection, but lesions observed were similar with those of acute T. gondii infection in other mammals with chronic inflammation and necrosis associated with tachyzoites. Four fish died between days 3 and 7 dpi but severe post-mortem autolysis prevented histological evaluation. No parasites were observed in one moribund fish that was collected at 4 dpi.
Figure 1.
Tachyzoites of Toxoplasma gondii developing in tissues of zebrafish raised at 37 °C. Haematoxylin and eosin stain. Bar = 10 μm. (a) Two foci of tachyzoites developing within parasitophorous vacuoles in the heart after 6 days post-injection (dpi). Giemsa stain. (b) Tachyzoites developing within a cardiac myocyte. 6 dpi, Giemsa stain. (c) Several extracellular, crescent-shaped tachyzoites (arrows) in the lumen and developing within the endothelium of a small blood vessel of an adult zebrafish after 7 dpi. Haematoxylin and eosin (HE) stain. (d) Several tachyzoites proliferating in the liver parenchyma associated with necrosis. Adult zebrafish at 6 dpi. HE stain. (e) A large pseudocyst filled with developing tachyzoites in the spleen. Adult zebrafish, 6 dpi. HE stain. (f) Numerous tachyzoites (arrows) developing in the grey matter of the midbrain of an adult zebrafish, 6 dpi. HE stain
Figure 2.
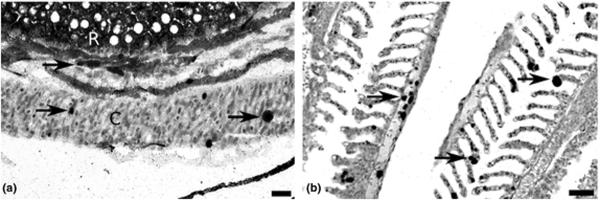
Toxoplasma gondii in the eye and gill of zebrafish raised at 37 °C at 6 days post-injection detected by immunohistochemistry. (a) Several foci of tachyzoites (arrows) developing in the choroid body (C) and the retina (R). Bar = 20 μm. (b) Tachyzoites (arrows) developing in the primary and secondary lamellae of the gills. Bar = 50 μm.
Our experiment differs from that of Omata et al. (2005) in several ways: (i) the use of zebrafish vs. goldfish, (ii) infection with bradyzoites from tissue cysts vs. tachyzoites from tissue culture, (iii) the fish were injected intraperitoneally vs. intramuscularly and (iv) the fish were examined using histological approaches. Histology is not a particularly sensitive method to detect T. gondii compared with cytology, PCR and the mouse assay (Da Silva & Langoni 2001); however, a major advantage of the zebrafish model is its small size, allowing for the examination of whole body sagittal sections that include virtually every organ on a single slide.
The zebrafish has emerged as a superb model for high-throughput screening of compounds in toxicology and infectious diseases (Carvalho et al. 2011; Bugel, Tanguay & Planchart 2014). This approach will provide a novel and robust platform for the high-throughput screening of compounds as potential therapeutics to treat the chronic (bradyzoite) form of T. gondii. In addition, the large size of the zebrafish eye relative to total body size could provide an excellent model for ocular T. gondii infections. The purpose of the present study was to determine whether an active T. gondii infection could be established in the zebrafish. The conditions described were clearly appropriate for the establishment of an acute infection, and no fish survived long enough for a chronic infection to develop. We have repeated this transmission study with cysts from mouse brains, as well as using tachyzoites from cell cultures, and have achieved the same results (unpublished data). We have also detected parasite cysts in the brain of a surviving fish 2 weeks post-infection, and we are moving forward with developing a model of chronic T. gondii infection.
There are many elements involved in the restricted host range of intracellular parasites, intrinsic host factors such as specific immunological components, receptors, incompatibility of host cells or parasite-related factors such as permissive environmental conditions, for example temperature. T. gondii is a rather promiscuous intracellular pathogen that can infect both mammals and birds and in vitro can infect virtually any cell line. Our experience with zebrafish suggests that for poikilothermic species, the major limitation to infection is temperature.
ACKNOWLEDGEMENTS
The authors thank the Sinnhuber Aquatic Research Laboratory for providing zebrafish used in this study and the Oregon State University Veterinary Diagnostic Laboratory for histological slide preparation. This work was funded by the Tartar Foundation, Department of Microbiology, Oregon State University, NIH R01 U01AI082180-05 (RMc,YZ), and the Cornwell and Mann, Morel, Rooney-Alden, and Engel families and “Taking Out Toxo” (RMc, YZ).
REFERENCES
- Allen JPJ, Neely MNM. Trolling for the ideal model host: zebrafish take the bait. Future Microbiology. 2010;5:563–569. doi: 10.2217/fmb.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkush KD, Miller MA, Leutenegger CM, Gardner IA, Packham AE, Heckeroth AR, Tenter AM, Barr BC, Conrad PA. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis) International Journal for Parasitology. 2003;33:1087–1097. doi: 10.1016/s0020-7519(03)00181-4. [DOI] [PubMed] [Google Scholar]
- Bugel SM, Tanguay RL, Planchart A. Zebrafish: a marvel of high-throughput biology for 21st century toxicology. Current Environmental Health Reports. 2014;1:341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R, de Sonneville J, Stockhammer OW, Savage NDL, Veneman WJ, Ottenhoff THM, Dirks RP, Meijer AH, Spaink HP. A high-throughput screen for tuberculosis progression. PLoS ONE. 2011;6:e16779. doi: 10.1371/journal.pone.0016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley K. Zebrafish: a model system for the study of human disease. Current Opinion in Genetics & Development. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis of Animals and Humans. 2nd edn CRC Press; Boca Raton, Florida: 2010. [Google Scholar]
- Ferguson HW, Roberts RJ. Myeloid leucosis associated with sporozoan infection in cultured turbot (Scophthalmus maximus L.) Journal of Comparative Pathology. 1975;85:317–326. doi: 10.1016/0021-9975(75)90074-2. [DOI] [PubMed] [Google Scholar]
- Harper C, Lawrence C. The Laboratory Zebrafish. CRC Press; Boca Raton: 2010. [Google Scholar]
- Ingham PW. The power of the zebrafish for disease analysis. Human Molecular Genetics. 2009;18:R107–R112. doi: 10.1093/hmg/ddp091. [DOI] [PubMed] [Google Scholar]
- Kanther M, Rawls JJF. Host-microbe interactions in the developing zebrafish. Current Opinion in Immunology. 2010;22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Buchner C, Watral VGV, Sanders JL, LaDu J, Peterson TST, Tanguay RLR. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Diseases of Aquatic Organisms. 2011;95:73–79. doi: 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Moser M, Fournie JW. Coccidian parasites (Apicomplexa: Eucoccidorida) in hardy head fish, Atherinomorus capricornensis (Woodland) Journal of Fish Diseases. 1989;12:179–183. [Google Scholar]
- Kirmse PD. Haemogregarina sachai n.sp. from cultured turbot Scophthalmus maximus (L.) in Scotland. Journal of Fish Diseases. 1978;1:337–342. [Google Scholar]
- Lesley R, Ramakrishnan L. Insights into early mycobacterial pathogenesis from the zebrafish. Current Opinion in Microbiology. 2008;11:277–283. doi: 10.1016/j.mib.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D, Collins M. Survival of Toxoplasma gondii oocysts in Eastern oysters (Crassostrea virginica) Journal of Parasitology. 2004;90:1054–1057. doi: 10.1645/GE-296R. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Dubey JP. Long-term survival of Toxoplasma gondii sporulated oocysts in seawater. The Journal of parasitology. 2009;95:1019–1020. doi: 10.1645/GE-1919.1. [DOI] [PubMed] [Google Scholar]
- Massie GN, Ware MW, Villegas EN, Black MW. Uptake and transmission of Toxoplasma gondii oocysts by migratory, filter-feeding fish. Veterinary Parasitology. 2010;169:296–303. doi: 10.1016/j.vetpar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Meeker N, Trede N. Immunology and zebrafish: spawning new models of human disease. Developmental & Comparative Immunology. 2008;32:745–757. doi: 10.1016/j.dci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Meijer AH, Spaink HP. Host-pathogen interactions made transparent with the zebrafish model. Current Drug Targets. 2011;12:1000–1017. doi: 10.2174/138945011795677809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár K. Phylum Apicomplexa. In: Woo PT, editor. Fish diseases and disorders. 2nd edn Vol. 1. CAB International; Wallingford: 2006. pp. 183–204. [Google Scholar]
- Omata AY, Umeshita Y, Murao T, Kano R, Kamiya H, Kudo A, Masukata Y, Maeda R, Saito A, Murata K. Toxoplasma gondii does not persist in goldfish (Carassius auratus) The Journal of Parasitology. 2005;91:1496–1499. doi: 10.1645/GE-3503RN.1. [DOI] [PubMed] [Google Scholar]
- Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Disease Models & Mechanisms. 2014;7:739–743. doi: 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait JH, Yan YL, Gates MA, Horne S, Amores A, Brownlie A, Donovan A, Egan ES, Force A, Gong Z, Goutel C, Fritz A, Kelsh R, Knapik E, Liao E, Paw B, Ransom D, Singer A, Thomson M, Abduljabbar TS, Yelick P, Beier D, Joly JS, Larhammar D, Rosa F, Westerfield M, Zon LI, Johnson SL, Talbot WS. Vertebrate genome evolution and the zebrafish gene map. Nature Genetics. 1998;18:345–349. doi: 10.1038/ng0498-345. [DOI] [PubMed] [Google Scholar]
- Puvanesuaran VR, Ibrahim N, Noordin R, Balakrishnan V. Isolation of viable Toxoplasma gondii cysts from brain samples for oral infection. European Review for Medical and Pharmacological Sciences. 2012;16:1179–1183. [PubMed] [Google Scholar]
- Sanders JL, Lawrence C, Nichols DK, Brubaker JF, Peterson TS, Murray KN, Kent ML. Pleistophora hyphessobryconis (Microsporidia) infecting zebrafish Danio rerio in research facilities. Diseases of Aquatic Organisms. 2010;91:47–56. doi: 10.3354/dao02245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Peterson TS, Kent ML. Early development and tissue distribution of Pseudoloma neurophilia in the zebrafish, Danio rerio. The Journal of Eukaryotic Microbiology. 2014;61:238–246. doi: 10.1111/jeu.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva AV, Langoni H. The detection of Toxoplasma gondii by comparing cytology, histopathology, bioassay in mice, and the polymerase chain reaction (PCR) Veterinary Parasitology. 2001;97:193–200. doi: 10.1016/s0304-4017(01)00404-6. [DOI] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews of the Cambridge Philosophical Society. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Sullivan C, Kim C. Zebrafish as a model for infectious disease and immune function. Fish & Shellfish Immunology. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Traver D, Herbomel P, Patton EE, Murphey RD, Yoder JA, Litman GW, Catic A, Amemiya CT, Zon LI, Trede NS. The zebrafish as a model organism to study development of the immune system. Advances in Immunology. 2003;81:253–330. [PubMed] [Google Scholar]
- Vojtech LN, Sanders GE, Conway C, Ostland V, Hansen JD. Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infection and Immunity. 2009;77:914–925. doi: 10.1128/IAI.01201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nature Reviews Drug Discovery. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]