Abstract
Polypharmacy is common, and may modify mechanisms of drug-induced liver injury. We examined the effect of these drug–drug interactions on liver safety reports of four drugs highly associated with hepatotoxicity. In the WHO VigiBase™, liver event reports were examined for acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid. Then, we evaluated the liver event reporting frequency of these 4 drugs in the presence of co-reported medications. Each of the 4 primary drugs was reported as having more than 2000 liver events, and co-reported with more than 600 different medications. Overall, the effect of 2275 co-reported drugs (316 drug classes) on the reporting frequency was analyzed. Decreased liver event reporting frequency was associated with 245 drugs/122 drug classes, including anti-TNFα, opioids, and folic acid. Increased liver event reporting frequency was associated with 170 drugs/82 drug classes; in particular, halogenated hydrocarbons, carboxamides, and bile acid sequestrants. After adjusting for age, gender, and other co-reported drug classes, multiple co-reported drug classes were significantly associated with decreased/increased liver event reporting frequency in a drug-specific/unspecific manner. In conclusion, co-reported medications were associated with changes in the liver event reporting frequency of drugs commonly associated with hepatotoxicity, suggesting that comedications may modify drug hepatic safety.
Keywords: Drug-induced liver injury, Hepatotoxicity, Concomitant medications, Spontaneous adverse event reporting, system, Quantitative signal detection methods
1. Introduction
Drug-related adverse events are a critical public health problem. In the US, serious and fatal adverse drug events (ADE) increased nearly 3-fold between 1998 and 2005, with most events due to a minority of important drugs (Moore et al., 2007). In the UK, 6.5% of adult hospital admissions were due to adverse drug reactions, resulting in an estimated $700 million annual cost (Pirmohamed et al., 2004). Drug-induced liver injury (DILI) is one of the most common adverse drug reactions, and can result in drug non-approvals, withdrawals and warnings (Senior, 2007). Drug-induced liver injury is the top cause of acute liver failure resulting in transplantation in the US and is associated with significant mortality (Carey et al., 2008). In the US, the drugs most frequently associated with acute liver failure include: acetaminophen, antimicrobials, anti-epileptics, psychotropics, and antimetabolites (Reuben et al., 2010). However, most drug classes can cause drug-induced liver injury (Suzuki et al., 2010).
In parallel with rising adverse drug events, the use of prescription medications, over-the-counter products and dietary supplements have also increased (Qato et al., 2008). The average elderly outpatient consumes 4 medications or more daily (Gurwitz et al., 2003; Hauben, 2003; Argikar and Remmel, 2009; Aleo et al., 2014; Chalasani et al., 2014), and most (63%) use complementary and alternative medications (Cheung et al., 2007), which have been increasingly associated with liver injury (Navarro et al., 2014). This polypharmacy contributes to adverse drug reactions (Gurwitz et al., 2003); large population studies reveal a sixfold increased injury risk with coadministration of medications associated with hepatotoxicity (de Abajo et al., 2004). Therefore, it is helpful to understand potential drug–drug interactions, which may contribute to drug-induced liver injury.
While drug-induced liver injury is clinically important, it is relatively uncommon, with symptomatic injury affecting approximately 1 in 10,000 patients annually (Sgro et al., 2002). In those with symptomatic drug-induced liver injury followed for 6 months, 1 in 14 will progress to liver transplant or liver-related death and nearly 1 in 5 of those remaining develop evidence of chronic injury (Fontana, 2014). With increasing polypharmacy potentially increasing the frequency of drug interactions and the likelihood of drug induced liver injury, it is imperative to examine the effect of concomitant medications on drug-induced liver injury in very large datasets. Therefore, we investigated the effect of comedications on selected drug-induced liver injury events using the largest global spontaneous adverse event reporting system, with over 8 million case reports. While analysis of this dataset does not enable causality assessment, it identifies new hypotheses on the effects of comedications on drug-induced liver injury.
Using this large global dataset, we applied quantitative signal detection methods to identify liver adverse events reported for 4 drugs commonly associated with hepatotoxicity: acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid. These four drugs were chosen to illustrate different types of hepatotoxicity: acetaminophen causes direct dose-related toxicity, as well as hepatocellular injury at therapeutic doses (Watkins et al., 2006); isoniazid exhibits hepatocellular injury due to metabolic and epigenetic factors (Murata et al., 2007) which increases with aging (Uetrecht and Naisbitt, 2013); amoxicillin/clavulanic acid is associated with an hepatocellular, mixed and cholestatic injury with immunologic manifestations (Lucena et al., 2011) and is the most frequently identified drug causing drug-induced liver injury in Western registries (Chalasani et al., 2014); and valproate acid causes mitochondrial toxicity, particularly in infants and young children (Uetrecht and Naisbitt, 2013). Furthermore, antibiotics and antiepileptics account for >60% of drug-induced liver injury in a prospective US registry (Chalasani et al., 2014). We systematically investigated the impact of comedications on liver event reporting frequency, to identify drugs and drug classes, which were associated with increased or decreased liver event reporting frequency. We then examined the identified comedications and constructed a plausible conceptual framework to explain mechanisms by which they might alter liver injury caused by the 4 primary drugs, in order to provide testable hypotheses for future empirical research and structure future investigations of human drug-induced liver injury.
2. Methods
2.1. Study design
This data-mining study used the released version of the large global VigiBase™ database, a spontaneous adverse event reporting system. We performed data-mining analyses to quantify liver event reports for 4 primary drugs, which are known human hepatotoxicants: acetaminophen, isoniazid, valproic acid, and amoxicillin/ clavulanic acid. We then explored the potential impact of concomitant medications on liver event reporting frequency using individual comedications as well as drug classes, as outlined below.
This study did not breach the confidentiality or anonymity of reported cases. The study was conducted using only coded data, without accessing identifiable private information, and therefore did not involve human subjects [45 CFR 46.102(f)].
2.2. Data source
We used the WHO global individual case safety report database (VigiBase™, the fourth quarter issue of 2010), which is broadly utilized in pharmacovigilance research (Bjornsson and Olsson, 2006; Suzuki et al., 2010). VigiBase™ is the world’s largest spontaneous adverse event reporting system, with more than 8.4 million reports from 104 countries compiled since the WHO International Drug Monitoring Programme started in 1968 (Caster et al., 2014). The majority of database reports were received from Europe and North America; both regulatory and voluntary sources are included.
2.3. Primary study drugs
We investigated four drugs commonly associated with clinical hepatotoxicity: acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid (Suzuki et al., 2010). Acetaminophen, isoniazid, and valproic acid predominantly cause hepatocellular injury (Chalasani et al., 2014). Amoxicillin/clavulanic acid causes both hepatocellular and cholestatic injury, with cholestatic injury predominant in the elderly (Lucena et al., 2006). We used a single compound as a reference drug for acetaminophen, isoniazid, and valproic acid, and combined two drugs ‘Amoxicillin and Clavulanic Acid’ and ‘Amoxicillin and Clavulanate Potassium’ as a pooled reference for amoxicillin/clavulanic acid. For these four drugs, known drug: drug interactions were searched in Drug Bank (Law et al., 2014) and the Indiana University Division of Clinical Pharmacology P450 Drug Interaction Table website (Indiana University, 2015). These known interactions were then compared to potential drug:drug interactions identified through our data mining analysis.
2.4. Drug dictionary and classification
In the individual drug analyses, we used generic/abridged drug names, which are available in a pharmacovigilance application used for the analyses (Empirica™ Signal, Oracle, Waltham, MA, USA). In the drug class analyses, we classified co-reported medications (comedications, hereafter) using the fourth category of the Anatomical Therapeutic Chemical Classification (ATC4) of the WHO Drug Dictionary, which describes chemical subgroups (Dictionary, 2014). Drug classes were excluded when indicated only for skin, eye, or ears.
2.5. Liver events
Two custom liver event terms were created for data mining, combining groups of ‘Preferred Terms’ (codes from the Medical Dictionary for Regulatory Activity, MedDRA) indicating different types of drug-induced liver injury: ‘hepatocellular injury’ and ‘cholestatic injury’. Supplemental Table 1 summarizes the lists of ‘Preferred terms’ used to define the two custom terms (26 terms for ‘hepatocellular injury’ and 16 terms for ‘cholestatic injury’).
2.6. Analytical methods
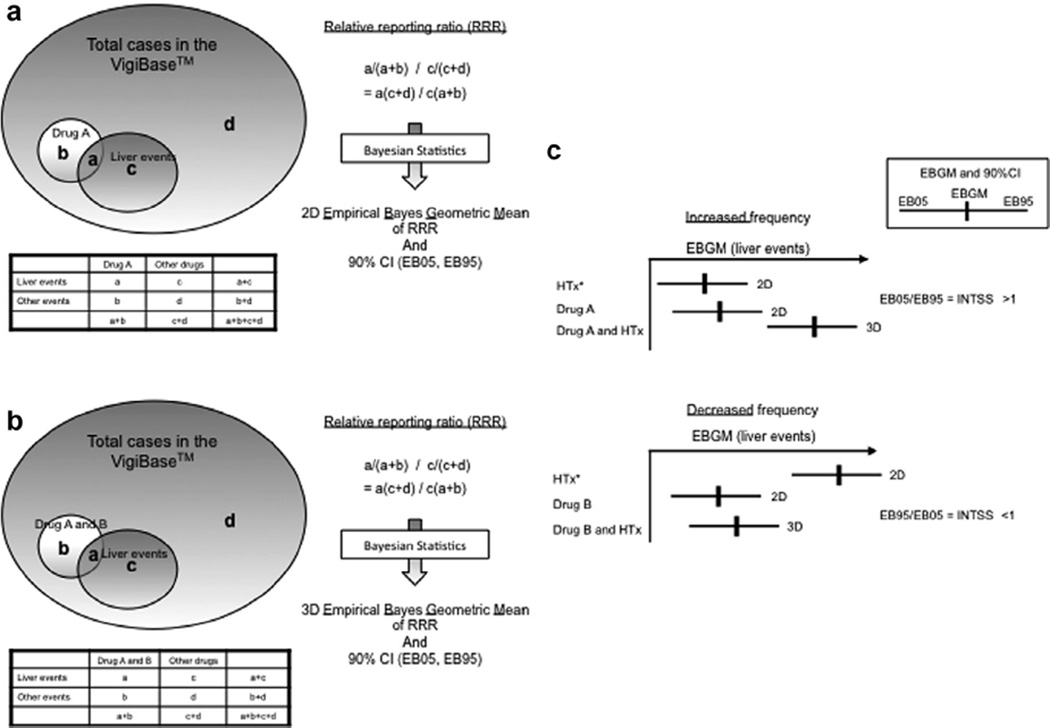
The data were computed using the Empirica™ Signal application (Oracle, Waltham, MA, USA). A relative reporting ratio (RRR) was defined as the observed count divided by the expected count. Empirical Bayes Geometric Mean (EBGM) is defined as the exponential value of log (RRR) under the posterior probability distributions for each true RRR. The lower and upper 90% confidence limits (EB05, EB95) for the RRR were derived from the posterior probability distribution (Fig. 1). Identification of a drug–drug interaction was performed in two ways: based on 90% confidence interval (CI) of EBGM (DuMouchel, 1999, 2001; Hauben, 2003; Almenoff et al., 2005) (unadjusted) and interaction statistics of logistic regression (INT_TOT) (DuMouchel, 2007) (adjusted), described below. Due to the exploratory nature of the analyses, alpha levels were not adjusted for multiple comparisons.
Fig. 1.
(a) Calculation of relative reporting ratio for a drug and a liver event and two-dimensional (2D) empirical Bayes geometric mean of relative reporting ratio (2D EBGM): Relative reporting ratio (RRR) was calculated as the ratio of reporting frequency of a liver event among cases co-reported with Drug A [a/(a + b)] to reporting frequency of a liver event among cases co-reported with other drugs [c/(c + d)]. The RRR, a(c + d)/c(a + b), was then transformed to empirical Bayes geometric mean of relative reporting ratio with 90% confidence interval (CI) while adjusting for sampling variability. (b) Calculation of relative reporting ratio for a pair of drugs and a liver event and three-dimensional (3D) Empirical Bayes Geometric Mean of relative reporting ratio (3D EBGM): The same calculation was applied to pairs of drugs. RRR was calculated as the ratio of reporting frequency of a liver event among cases co-reported with Drug A and Drug B [a/(a + b)] over reporting frequency of a liver event among cases co-reported with other pairs [c/(c + d)]. The RRR, a(c + d)/c(a + b), was then transformed to empirical Bayes geometric mean of relative reporting ratio with 90% CI. (c) Identification of 3D interaction: To identify drugs significantly increasing reporting frequency of liver events, we compared the EB95 of 2D EBGM and the EB05 of 3D EBGM. When the ratio of EB05 to a larger EB95 (i.e., interaction signal score or INTSS) was greater than 1, we considered Drug A significantly increased reporting frequency of liver events co-reported with the drug associated with hepatotoxicity (HTx). To identify drugs significantly decreasing reporting frequency of liver events, we compared the EB05 of the 2D EBGM for the drug associated with hepatotoxicity and the EB95 of the 3D EBGM. When the ratio of the EB95 to the EB05 was lower than 1, we considered Drug B significantly decreased reporting frequency of liver events co-reported with the drug associated with hepatotoxicity.
2.6.1. Identification of significant drug–drug interaction
First, EBGM 3-dimensional (3D) analyses were performed to identify drugs and drug classes which were significantly associated with changes in reporting frequency of the 4 primary study drugs: acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid (i.e., drug–drug interaction). Drug–drug interactions were initially explored using individual drugs (generic names). Then this same analysis was repeated using drug classes (ATC4 category) to strengthen its biological relevance and to better detect a 3D interaction. We computed the reporting frequency of liver events as EBGM with 2-sided 90% CI using the MGPS (Multi-Item Gamma Poisson Shrinker) method (DuMouchel, 1999, 2001; Hauben, 2003; Almenoff et al., 2005). EBGM data were computed for all the following pairs/combinations using the specified custom liver event terms: (1) drug (or drug class) and liver event (i.e., 2D EBGM) and (2) a pair of drugs (or drug classes) and liver event (i.e., 3D EBGM) (Fig. 1a and b). Reflecting the predominant type of injury, the EBGM analyses applied the hepatocellular injury term for acetaminophen, isoniazid, and valproic acid, and the cholestatic term for amoxicillin/clavulanic acid (Chalasani et al., 2014). A significant 3D interaction was identified by comparing 90% CIs of 2D and 3D EBGM using interaction signal score [INTSS] (Almenoff et al., 2003). Fig. 1C outlines detailed methods.
We performed logistic regression analyses to re-assess the identified drug–drug interaction after adjusting for the effects of other drug classes, age, and gender and estimated the adjusted effects of the drug classes on the liver event reporting frequency of the 4 primary study drugs. Drug classes were considered in the logistic regression analyses when the above EBGM 3-dimensional (3D) analyses were identified to be: (1) associated with decreasing reporting frequency of more than one primary study drug, (2) associated with increasing reporting frequency in more than one primary drug associated with hepatocellular liver injury (i.e., acetaminophen, isoniazid, valproic acid), or 3) associated with increasing reporting frequency in the primary drug associated with cholestatic liver injury (i.e., amoxicillin/clavulanic acid). Interaction statistics [INT_TOT with 90% CI] identified significant drug–drug interaction in the logistic regression models (DuMouchel, 2007). The interaction statistics were defined as a product of both: (1) the predicted ratio of the probability of liver events in the models (i.e. the probability given both the drug classes divided by the probability given one of the drug classes associated with the worst probability) and (2) corrective statistics based on the ratio of observed numbers of 3D cases to predicted numbers of 3D cases using the model without an interaction term, which would adjust for sampling variability and lack of fit to the models (i.e., Empirical Bayes shrinkage estimate of additional interaction) (DuMouchel, 2007); 90% CIs of the calculated interaction statistics were compared with 1. We classified the drug class as associated with a significant decrease in the liver event reporting frequency co-reported with the primary study drug when the upper limit of the 90% CI was less than 1. The drug class was classified as associated with a significant increase in the liver event reporting frequency when the lower limit of the 90% CI was greater than 1.
3. Results
3.1. Exploratory data mining analysis using individual drugs
Liver event reporting frequency was analyzed by data mining for the 4 primary study drugs: acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid. For each of the 4 drugs, total numbers were summarized for: liver event reports, EBGM data with 90% CI, co-medications co-reported, and identified drugs (Table 1). There were ample numbers of liver events for each of the 4 drugs and the various comedications in the database to provide sufficient power for this data-mining analysis: 8215 liver events and 2037 co-medications for acetaminophen, 3302 liver events and 665 co-medications for isoniazid, 3532 liver events and 926 co-medications for valproic acid, and 2804 liver events and 945 co-medications for amoxicillin/clavulanic acid. Overall, potential effects of 2275 co-medications were analyzed. Among these co-medications, liver event reporting frequency was decreased by 245 drugs and increased by 170 drugs in at least one of the 4 primary study drugs (Supplemental Table 2a and b). Folic acid and tramadol were associated with decreased reporting frequency for all the 4 study drugs. Reporting frequency for 3 of the 4 study drugs was decreased in association with bupropion, acetylsalicylic acid, warfarin, furosemide, nifedipine, metoprolol, clozapine, levothyroxine, as well as sulfamethoxazole and trimethoprim. Carbamazepine was associated with increased reporting frequency for all 3 study drugs causing predominantly hepatocellular injury. We then examined the identified drugs by drug classes. Liver event reporting frequency was lower when the primary study drug was co-reported with the following drug classes, including: Tumor Necrosis Factor alpha (TNF-α) inhibitors, angiotensin converting enzyme (ACE) inhibitors, alpha-adrenoreceptor antagonists, mucolytics, monoclonal antibodies (i.e., anti-neoplastic treatment), and histamine-2 (H2)-receptor antagonists. Liver event reporting frequency was higher when the primary study drug was co-reported with several drug classes: sympathomimetics (centrally acting), adrenergic and dopaminergic agents, interferons, macrolides, diphenylpropylamine derivatives, and halogenated hydrocarbons (e.g. anesthetics). Drug classes were listed here only when ≥3 drugs were identified within each category.
Table 1.
Total numbers of reported liver events, 2D EBGM with 90% CI, total numbers of drugs co-reported with the liver events, and numbers of identified drugs for the 4 drugs associated with hepatotoxicity.
| PT (custom terms)* | N | EBGM | EB05 | EB95 | Total N CoMed | N (%) INC | N (%) DEC | |
|---|---|---|---|---|---|---|---|---|
| Acetaminophen | Hepatocellular injury | 8215 | 2.137 | 2.099 | 2.176 | 2037 | 132 (6.5%) |
163 (8.0%) |
| Isoniazid | Hepatocellular injury | 3302 | 5.982 | 5.812 | 6.155 | 665 | 8 (1.2%) |
38 (5.7%) |
| Valproic acid | Hepatocellular injury | 3532 | 2.125 | 2.066 | 2.184 | 926 | 11 (1.2%) |
62 (6.7%) |
| Amoxicillin/clavulanic acid | Cholestatic liver injury | 2804 | 6.588 | 6.386 | 6.795 | 945 | 34 (3.6%) |
41 (4.3%) |
PT: preferred terms of the Medical Dictionary for Regulatory Activity, MedDRA.
N: total numbers of the reported liver events for each drug; 2D EBGM: empirical Bayes geometric mean of relative reporting frequency; EB05: lower limit of 90% CI of 2D EBGM; EB95: upper limit of 90% CI 2D EBGM; Total N CoMed: number of drugs co-reported with liver events related to each of the 4 key drugs; N (%) INC: number (%) of drugs identified as increasing reporting frequency in the EGBM 3D analyses; N (%) DEC: number (%) of drugs identified as decreasing reporting frequency in the EGBM 3D analyses. The numbers of the drugs listed above are not mutually exclusive among the 4 study drugs. Total 2275 unique drugs were analyzed in the EBGM 3D analyses.
Custom terms were used as defined in the methods and Supplemental Table 2.
3.2. Exploratory data mining analysis using drug classes
We next performed data mining analysis using drug classes (i.e., ATC4 categories), examining drug classes co-reported in the primary study drug liver events, to support the biological relevance and enhance the detection of a 3D interaction. Table 2 summarizes the 316 drug classes analyzed (i.e., co-reported with liver events and the 4 study drugs); 122 drug classes of comedications were associated with decreasing reporting frequency, while 82 drug classes were associated with increasing reporting frequency for at least one of the 4 study drugs. Among the 122 drug classes associated with decreased liver event reporting frequency, 15 classes (12.3%) had decreased reporting frequency in all 4 primary drugs and 91 classes (74.6%) were drug-specific. In the 82 drug classes with increased liver event reporting frequency, only 3 (3.7%) had higher reporting frequency in all 4 primary drugs, while 73 (89.0%) were drug-specific. Overall, 47 drug classes were associated with a decreasing or increasing liver event reporting frequency for multiple primary study drugs (Table 3).
Table 2.
Total number of drug classes co-reported with the liver events and the 4 drugs associated with hepatotoxicity, and numbers of identified drug classes for the 4 drugs associated with hepatotoxicity.
| Drug | N 3D | N INC | N DEC |
|---|---|---|---|
| Acetaminophen | 311 | 63 (20.3%) | 81 (26.0%) |
| Isoniazid | 83 | 6 (7.2%) | 22 (26.5%) |
| Valproic acid | 126 | 13 (10.3%) | 44 (34.9%) |
| Amoxicillin/clavulanic acid | 119 | 14 (11.8%) | 26 (21.8%) |
EGBM 3D analyses were repeated pooling the data by drug classes (ATC4). The same liver event terms and reference 2D EBGM data were used for each of the 4 key drugs (Table 1). N 3D: number of drug classes co-reported with liver events and each of the key drugs; N INC: number of drug classes identified as increasing reporting frequency in the EGBM 3D analyses; N DEC: number of drug classes identified as decreasing reporting frequency in the EGBM 3D analyses. The numbers of the drugs listed above are not mutually exclusive among the 4 study drugs. Total 316 unique drug classes (ATC4) were analyzed in the EBGM analyses.
Table 3.
Impact of 48 drug classes (ATC4) on liver events co-reported with acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid in unadjusted and adjusted analyses.
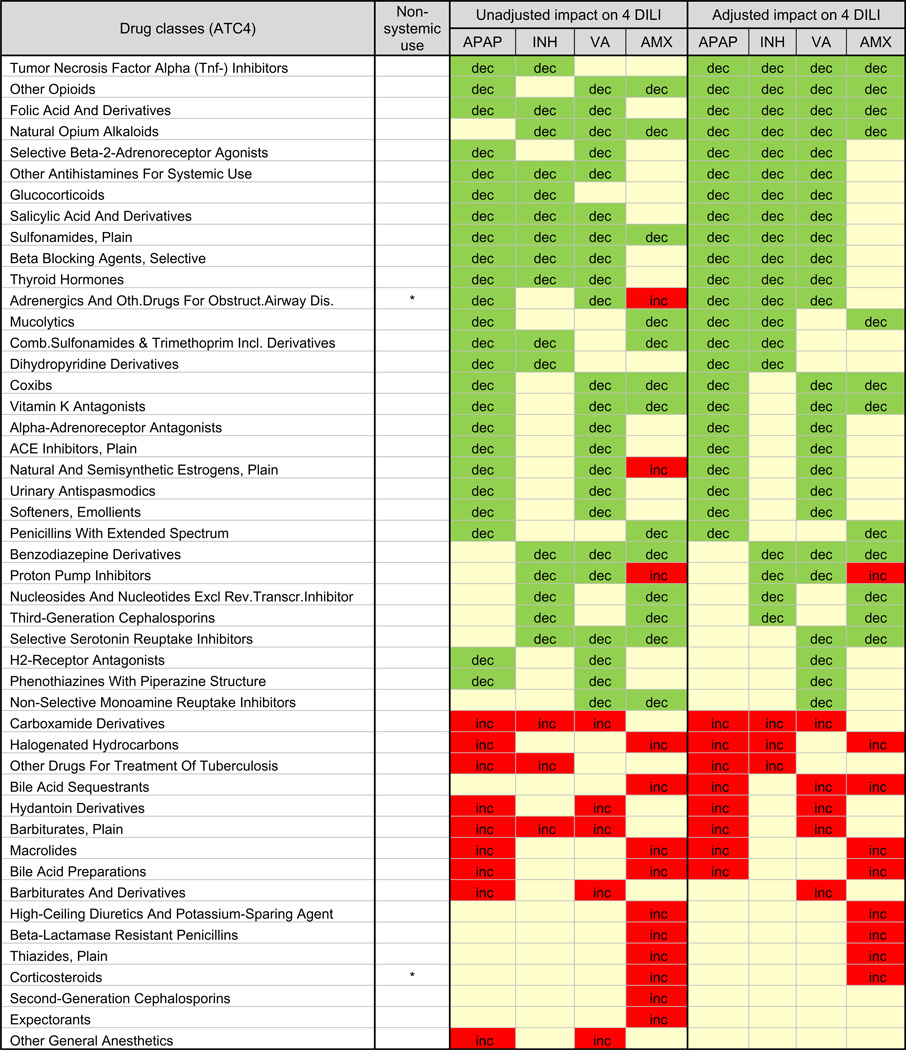 |
APAP, acetaminophen; INH, isoniazid; VA, valproic acid; AMX, amoxicillin/clavulanic acid. The results from unadjusted (3D EBGM analysis) and adjusted analysis (logistic regression models including age, gender, 47 drug classes, and the 4 key drugs) are summarized. ‘dec’ and green indicate negative interaction (i.e., decreased reporting frequency in combination with a drug class) while ‘inc’ and red indicate positive interaction (i.e., increased reporting frequency in combination with a drug class). ‘Blank’ and beige indicate no significant interaction.
The 47 drug classes associated with liver event reporting of more than 1 of the 4 primary drugs were further examined. Logistic regression analysis was performed including age, gender, and the 47 drug classes. After adjusting for these other factors, four drug classes exhibited lower liver event reporting frequency when co-reported with all 4 primary drugs: folic acid, natural opium alkaloids, other opioids and TNF-α inhibitors (Table 3). Several other drug classes were associated with decreased reporting frequency for individual primary drugs. Three drug classes exhibited an increased liver event reporting frequency when co-reported with 3 of 4 primary drugs (after adjustment): halogenated hydrocarbons (e.g. anesthetics), bile acid sequestrants, and carboxamide derivatives (e.g. antiepileptics and antineoplastic alkylating agents). An additional 14 drug classes showed an increased reporting frequency for at least one study drug. Overall, proton pump inhibitors were the only drug class which exhibited different liver event reporting frequency effects for the 4 primary drugs (after adjustment), with decreased reporting for the drugs associated with hepatocellular injury and increased reporting frequency for amoxicillin/clavulanic acid, associated with cholestatic injury.
4. Discussion
Using the global safety database, VigiBase™, data-mining analyses examined how comedications might affect the liver event reporting frequency of 4 drugs highly associated with different forms of hepatotoxicity: acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid. Using quantitative signal detection methods, we first identified liver event reports for the 4 primary drugs and then examined the comedications. We then used data mining to uncover potential drug–drug interactions using frequency data. Drugs and drug classes were identified which significantly decreased or increased liver event reporting frequency when co-reported with the 4 primary drugs. Many drugs and drug classes, when co-reported with these 4 primary drugs, were associated with changes in liver event reporting frequency and clinical outcomes. For example, decreased liver event reporting frequency for all 4 primary drugs was observed when folic acid, TNF-α inhibitors, or opioids were co-reported, suggesting a potential beneficial effect. Decreased liver event reporting frequency was observed for numerous drug classes co-reported with more than one primary study drug associated with hepatotoxicity. Additionally, some drug classes were associated with an increased liver event reporting frequency in a drug specific or nonspecific manner. Based on these findings, we generated hypotheses to explain the mechanisms for the effects of these targeted comedications on drug-induced liver injury.
This developed conceptual framework explains the mechanisms of drug-induced liver injury and the effects of comedications which appeared to ameliorate or exacerbate injury (Figs. 2 and 3). Recent data suggest that liver injury is initiated by a distinct subset of drugs administered at a high daily dose (Lammert et al., 2008; Yu et al., 2014), and lipophilicity (Chen et al., 2014), which may form reactive metabolites (Sakatis et al., 2012), inhibit bile salt export pump (BSEP) or other transporters (Morgan et al., 2013; Aleo et al., 2014), impair mitochondria (Pessayre et al., 2012; Han et al., 2013), alter histone acetylation (Kacevska et al., 2011), and injure biliary epithelium (Cullen et al., 2010). By describing the mechanisms activated by specific medications, one can postulate how comedications impact the initiation or adaptation/resolution of drug-induced liver injury (Pessayre et al., 2012). However, some drugs and drug classes impact liver event reporting frequency for all 4 primary study drugs (even after adjusting for other co-medications), suggesting a more generalized effect. So, perhaps comedications affect the later common phases of drug-induced liver injury, including: cytoprotection, stress response (Han et al., 2013), regulation of injury and/or immune response (Han et al., 2013; Uetrecht and Naisbitt, 2013), and tissue repair. Comedications can favorably modulate the immune system (e.g., TNF-α inhibitors, glucocorticoids, opioids), liver injury/repair (e.g., ACE inhibitors/angiotensin II blockers) (Alisi et al., 2005; Suzuki et al., 2009a,b), and oxidative stress (e.g., folic acid, thyroid hormone) (Huang et al., 2001; Romanque et al., 2011), and thereby decrease liver injury. Other drugs and drug classes may reduce inflammation (e.g., coxibs, aspirin, anti-vitamin K) or protect cells from cellular injury: estrogens (Shimizu and Ito, 2007; Kozlov et al., 2010), mucolytics (Felix et al., 1996), and benzodiazepines (Carayon et al., 1996; Kunduzova et al., 2004).
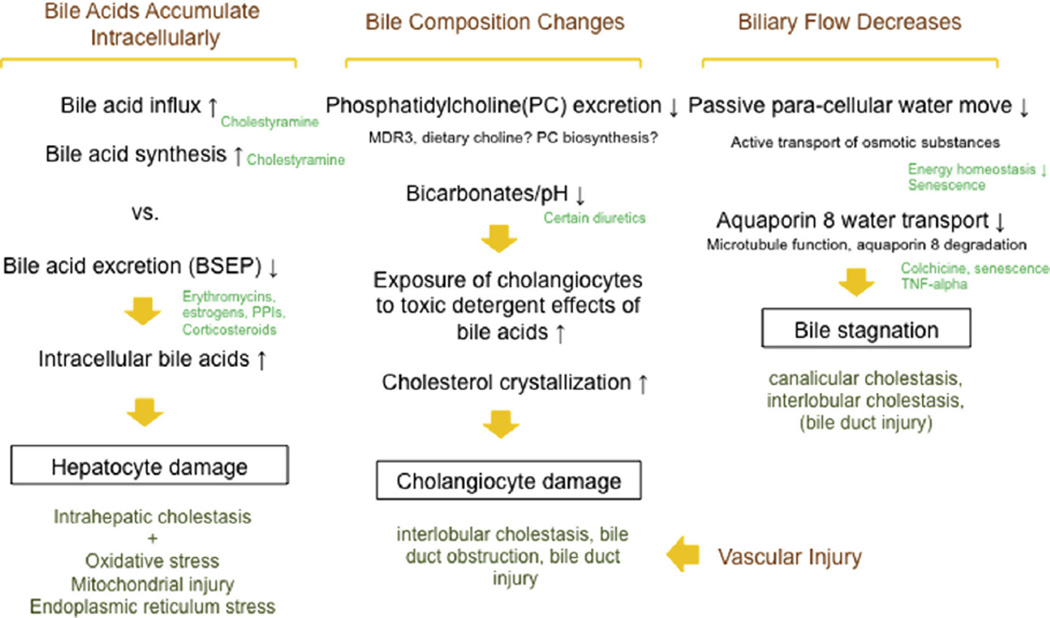
Fig. 2.
Theoretical framework explaining cholestatic features of drug-induced liver injury and the impact of comedications. We theorize that cholestatic features of drug-induced liver injury [i.e., intrahepatocellular cholestasis, canalicular cholestasis, intralobular cholestasis, bile duct injury] are explained by the four depicted mechanisms: intrahepatocellular bile acid accumulation, alteration of bile acid compositions, bile water flow, and vascular injury. In hepatocytes, the quantity of bile acids is tightly regulated by a balance of bile acid influx, synthesis, and excretion. When bile acid excretion is reduced [e.g., via BSEP inhibition] (Stieger et al., 2000; Morgan et al., 2010)], the hepatocellular bile acid accumulation resulting from increased bile acid synthesis and/or increased sinusoidal bile acid transport could exacerbate hepatocellular damage (Perez and Briz, 2009) and intrahepatocellular cholestasis [e.g., cholestyramine] (Jolley et al., 2000; Cheng et al., 2007). Under physiological conditions, cholangiocytes are protected from the detergent effects of bile acids by forming mixed micelles mainly composed of bile acids, phosphatidylcholine and cholesterol (Davit-Spraul et al., 2010). Reduced excretion of phosphatidylcholine into bile (Davit-Spraul et al., 2010) and/or decreasing bicarbonate concentration [e.g., lowering pH] in bile could disturb this micelle formation and/or facilitate cholesterol crystallization, resulting in bile duct injury and obstruction. Although there is limited direct evidence on how diuretics impact susceptibility to drug-induced cholestatic injury, prior clinical studies report diuretic use associated with cholelithiasis (Randall et al., 1992; Leitzmann et al., 2005) and a preclinical study (Hubner et al., 2000) provide indirect support that specific diuretics could alter biliary electrolytes and pH, leading to micelle perturbation and cholesterol crystallization. Bile contains more than 95% water. Although the pathophysiological significance of bile water flow is yet unclear, reduced bile water flow, either passive or active, may contribute to bile stagnation and cholestatic drug-induced liver injury. With decreased bile flow and bile acid secretion, a moderate aging-related decline in biliary function could also predispose to cholestatic features of drug-induced liver injury.
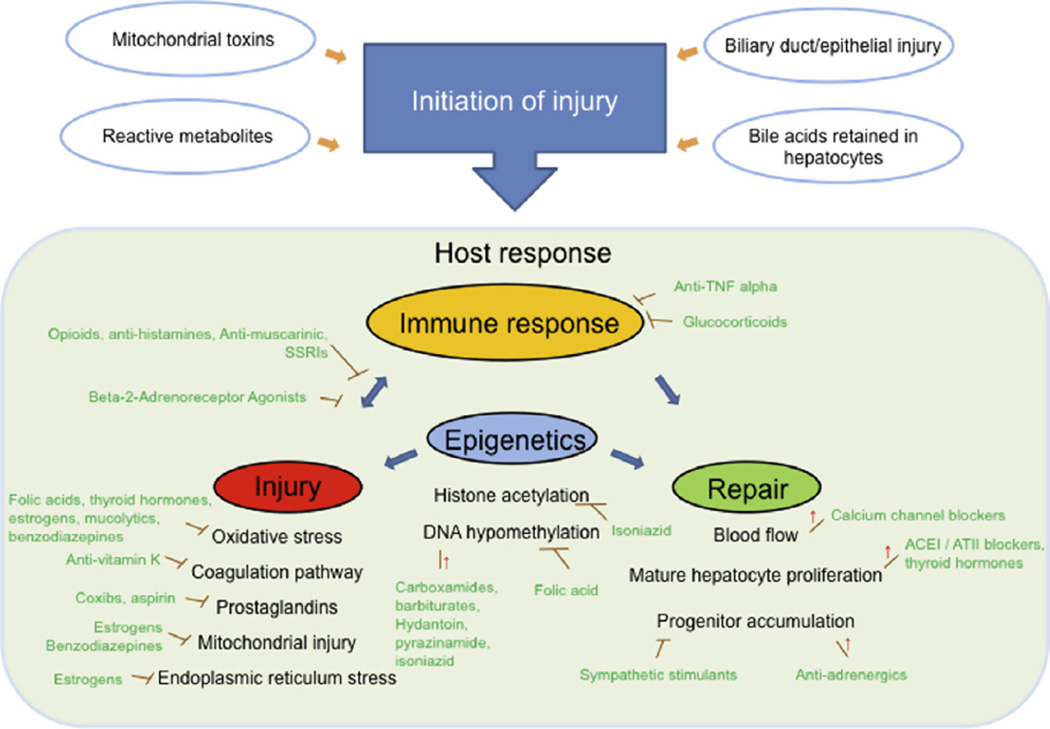
Fig. 3.
Theoretical framework explaining clinical drug-induced liver injury and the impact of comedications. As depicted, liver injury can be initiated by various mechanisms. Once initiated, the severity and clinical outcome of drug-induced liver injury is affected by the individual’s response. We theorize that such responses are largely determined by host immune response and the balance between injury/inflammation and repair. Our study identified numerous drugs/drug classes which are known to modulate immune response and inflammation. Such drugs/drug classes include anti-TNFα, glucocorticoids, opioids (Roy and Loh, 1996; Peterson et al., 1998), anti-histamines (Okamoto et al., 2009), anti-muscarinic (Vacca et al., 2011), SSRIs (Maes, 2001), and beta2-adrenoreceptor agonists (Wang et al., 2009). Other drugs/drug classes are known to reduce inflammation [e.g., coxibs, aspirin, anti-vitamin K, TNF-alpha inhibitors, glucocorticoids] or protect cells from cellular injury [e.g., folic acids] (Huang et al., 2001), thyroid hormones (Romanque et al., 2011), estrogens (Shimizu and Ito, 2007; Kozlov et al., 2010), mucolytics (Felix et al., 1996), and benzodiazepines (Carayon et al., 1996; Kunduzova et al., 2004). Several drugs/drug classes may exert their impact via modulating repair [e.g., ACE-inhibitors, angiotensin II blockers, calcium blockers, antiadrenergics, thyroid hormones] (Garcia-Pagan et al., 1994; Alisi et al., 2005; Suzuki et al., 2009a,b) or epigenetic modification [i.e., hypo-methylation and/or histone acetylation, e.g., carboxamides, hydantoin, barbiturates, pyrazinamide, and isoniazid] (Poirier and Wise, 2003; Murata et al., 2007).
In contrast, some comedications appear to enhance liver injury. This could be explained by a comedication enhancing metabolism to a toxic metabolite or reducing detoxification mechanisms, which are well known to enhance hepatotoxicity of classic hepatotoxins like acetaminophen (Zimmerman, 1999). As carbamazepine induces several cytochrome P450s, it could enhance hepatotoxicity through this mechanism. Other compounds, including sympathetic stimulants, deplete glutathione (James et al., 1993) and inhibit liver repair mechanisms (Oben et al., 2003) thus increasing susceptibility. Carboxamides, pyrazinamide, and isoniazid are also known to induce epigenetic modifications (i.e., hypo-methylation and/or histone acetylation); decreased histone acetylation with isoniazid administration impairs liver regeneration after injury (Poirier and Wise, 2003; Murata et al., 2007). The use of proton pump inhibitors decreased reporting of the drugs associated with hepatocellular injury, while increasing the reporting frequency of amoxicillin/- clavulanic acid associated with cholestatic injury, postulated due to enhanced allergenicity as proton pump inhibitors increase gastric pH (Ramirez et al., 2013). These findings align with an earlier analysis of fatal acetaminophen-associated liver injury, in which comedications that exacerbate injury or reduce repair increased injury while those that reduce injury or enhance repair were protective (Suzuki et al., 2009a,b).
In addition to the effect of comedications on hepatocellular injury, the effect of comedications on cholestatic injury was examined for amoxicillin/clavulanic acid (Fig. 3). Hepatocytes tightly regulate the quantity of intracellular bile acids through balancing bile acid influx, synthesis, and excretion. Cholestatic injury can result from a reduction in bile acid excretion (e.g., macrolide-associated BSEP inhibition (Stieger et al., 2000; Morgan et al., 2010), increased bile acid synthesis, and/or increased sinusoidal bile acid transport (e.g., cholestyramine (Jolley et al., 2000; Cheng et al., 2007). Additional mechanisms may contribute to cholestatic drug-induced liver injury, including: alteration of bile composition, biliary water flow, and vascular injury (Fig. 3).
Based on the rationale provided above, it is plausible that co-medications that modulate immune response, drug metabolism, cytoprotection, adaptation, injury, cholestasis, and repair may modify susceptibility to a wide range of drugs associated with hepatotoxicity (Fig. 2). Related to these mechanisms, genetic or acquired risk factors could similarly be generalizable among different drugs. With hepatotoxicants sensitizing hepatocytes to TNF-alpha, the innate and adaptive immune response critically determine the course of drug-induced liver injury (Han et al., 2013). For example, compared to rats receiving the hepatotoxicant amiodarone or nonlethal lipopolysaccharide (LPS) alone, both TNF-alpha concentration and liver injury were increased with the coadministration of LPS and amiodarone (Lu et al., 2012). When the TNF-alpha inhibitor, etanercept, was administered one hour prior to the LPS and amiodarone, liver injury significantly decreased (Lu et al., 2012). In the current analysis, TNF-alpha inhibitor coadministration decreased liver event reporting for all four clinical hepatotoxicants; this suggests the importance of TNF-alpha in clinical drug-induced liver injury and the translational relevance of the nonclinical models (Lu et al., 2012). These theories can be systematically evaluated in a well-characterized drug-induced liver injury cohort, linked to genetic information, or a large electronic medical record system to assess clinical and disease association (Kohane, 2011).
Using electronic medical records, a retrospective UK population study reported a 6-fold increased risk of drug-induced liver injury with coadministration of two or more drugs associated with hepatotoxicity, in comparison to single drug use (de Abajo et al., 2004). Similarly, a systematic review reported an increased risk of severe or fatal hepatotoxicity when amoxicillin: clavulanate is coadministered with drugs associated with hepatotoxicity, including antimicrobials, analgesics and hormonal therapy (Yazici et al., 2014). Examining clinical factors and preclinical mechanisms, most (67%) drugs withdrawn from the market or displaying black box warnings for drug-induced liver injury exhibit inhibition of both liver mitochondrial and bile salt export pump function (Aleo et al., 2014). Aside from these studies, there are very limited data on the effect of comedications on drug-induced liver injury, due to the low frequency of drug-induced liver injury (requiring very large databases) and the detailed clinical information required to accurately evaluate events. However, there is a compelling clinical need to understand how medications or supplements may affect clinical outcomes and influence susceptibility to specific drugs. For example, numerous chemotherapy agents result in hepatotoxicity (McWhirter et al., 2013); addition of a carboxamide (e.g. dacarbazine) could potentially further increase injury or result in the interruption or termination of otherwise effective therapy.
We present a mechanism-based approach to examine how comedications can modulate the liver safety of 4 drugs commonly associated with hepatotoxicity. Our search for published drug: drug interactions related to the 4 study drugs in the publicly available resources revealed metabolic interactions for acetaminophen and isoniazid, as well as isoniazid or valproic acid and carbamazepine, which could increase hepatotoxicity. However, the large majority of drug pairs identified by data mining were not predicted by published drug: drug interactions. Using this unbiased (as opposed to hypothesis-driven) approach to evaluate comedications in a global database, we analyzed all relevant drug pairs, as liver injury mechanisms are incompletely understood.
The use of spontaneously reported safety data in pharmacovigilance is limited by reporting bias, variable data quality and diagnostic accuracy, confounding effects of co-medications and/or other comorbidities, duplicate reports, and reporting influenced by heightened awareness of adverse drug events in the media or due to regulatory actions (Almenoff et al., 2005). Furthermore, incomplete case data frequently preclude adjudication of drug-induced liver injury reports, as well as the reliable identification of acute liver failure. We applied logistic regression models to adjust for potential confounding effects by co-reported drugs; however, numerous other factors, not evaluated in this study, may also influence reporting frequency (e.g. country-specific mandatory reporting, etc.). Interpretation of pharmacovigilance data requires a thoughtful and comprehensive assessment of disease mechanisms, clinical pharmacology, clinical science, and potential biases. Data mining is particularly useful for generating hypotheses, which must be further examined in electronic medical records, drug-induced liver injury (or acute liver failure) registries (Suzuki et al., 2009a,b), clinical trials or through preclinical mechanistic investigations. Such an approach has recently identified a pravastatin:paroxetine pharmacodynamic interaction in the Food and Drug Administration (FDA) Adverse Event Reporting System (Tatonetti et al., 2011).
In addition to using spontaneously reported safety data, this study has several other limitations. The analysis deliberately overlooked drug–drug interactions caused by drug-specific properties, due to its focus on the class effect of drugs. For example, increased liver event reporting was an anticipated drug:drug interaction with coadministration of carboxamides or barbiturates (CYP2C9 inducers) with valproic acid (a CYP2C9 substrate) (Indiana University, 2015). However, reported drug:drug interactions were otherwise uncommon among the comedications significantly altering liver event reporting frequency of the four hepatotoxicants (Law et al., 2014; Indiana University, 2015). In addition, the analysis did not evaluate the effect of drug–dose, –age or –gender interactions on effect of comedications, although these have previously been reported (Suzuki et al., 2009a,b). As underlying disease can modify the impact of comedications or susceptibility to liver injury, this should be investigated in future studies. Lastly, the analysis may not have effectively identified the impact of herbal medicines or dietary supplements, as the chemical and biological properties of these agents are not well characterized, despite being increasingly recognized in drug-induced liver injury and acute liver failure cases (Estes et al., 2003; Takikawa et al., 2009; Reuben et al., 2010). The potential impact of these agents merits future investigation.
Despite its limitations, our approach is valuable in investigating the impact of comedications on specific drug-induced liver injury events. The global VigiBase™ includes millions of adverse drug reports for over-the-counter drugs, dietary supplements, and herbal medicines, in addition to prescription drugs. So, this database provides a valuable resource for exploratory analyses on the impact of comedications and theory generation. Additionally, comedications may alter susceptibility to injury by diverse mechanisms: altering drug absorption, distribution, metabolism and excretion; modulating cell biology, stress response, and regeneration; and influencing neural, endocrine, and immune systems. Thus, assessing the impact of comedications on epidemiological endpoints (e.g., frequency, clinical outcomes) has the great advantage of examining diverse mechanisms in a holistic manner. In summary, this is the first study to systematically investigate the effect of comedications on liver injury events co-reported with 4 drugs commonly associated with hepatotoxicity (acetaminophen, isoniazid, valproic acid, and amoxicillin/clavulanic acid) by applying quantitative signal detection methods to a global safety reporting system. We constructed a holistic, evidence-based conceptual framework to explain how the identified drugs and drug classes might influence liver events using established mechanisms of liver injury and adaptation/repair. Due to the limitations of pharmacovigilance data, these findings are preliminary. Yet, we believe our results and theories have broad implications for future drug-induced liver injury research, and provide a foundation to investigate drug–drug interactions in electronic medical records or large clinical databases containing well-characterized drug-induced liver injury cases, as well as in nonclinical experimental models. The specific theories developed in this framework are being independently validated currently. These efforts will enhance patient safety, yield preventive strategies to avoid deleterious drug combinations and promote interdisciplinary research to delineate clinical mechanisms of drug-induced liver injury. Once the approach has been verified and validated, it may be applied to other key toxicities (such as cardiovascular, renal, and dermatologic adverse drug events) as a valuable tool to investigate drug safety in the setting of polypharmacy.
Supplementary Material
Acknowledgments
The authors would like to thank Annie Lin, PhD and L. Lan, PhD, Department of Biostatistics, Duke University, Durham, NC, for their insight regarding statistical approaches used in this analysis, Kim Adkison, PharmD, Clinical Pharmacology, GlaxoSmithKline, for her insight on drug transporters and clinical pharmacology contributed in data interpretation and theory generation in this project, and Julie Papay, PharmD, Clinical Safety, GlaxoSmithKline, for her insight on clinical drug safety and critical revision of the paper. This publication was made possible by Grant Number UL1RR024128 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIH, or the World Health Organization. VigiBase™ is a registered trademark of The Uppsala Monitoring Centre. At the time of this research, Katarina Ilic was sponsored by GSK Academy and the Ministry of Education and Science of Republic of Serbia (Grant No. 172 009).
Abbreviations
- ACEI
angiotensin converting enzyme inhibitor
- ADE
adverse drug events
- AERS
adverse event reporting system
- APAP
acetaminophen
- ATC classification
Anatomical Therapeutic Chemical Classification
- ATIIA
angiotensin II receptor antagonist
- BSEP
bile salt export pump
- COX
cyclooxygenase
- DILI
drug induced liver injury
- EBGM
Empirical Bayes Geometric Mean of Relative Reporting Frequency
- FDA
Food and Drug Administration
- ICSR
individual case safety report
- INTSS
interaction signal score
- MedDRA
Medical Dictionary for Regulatory Activity
- NSAID
non-steroidal anti-inflammatory drug
- OTC
over the counter
- SSRI
selective serotonin reuptake inhibitor
- 2D
two-dimensional
- 3D
three-dimensional
- TNF
Tumor Necrosis Factor
- WHO
World Health Organization.
Footnotes
Author contributions (all authors can attest to the intellectual content of the paper)
Ayako Suzuki: Study development, analysis, implementation, manuscript preparation.
Nancy A. Yuen: Study development, analysis, implementation, manuscript review.
Katarina Ilic, Richard T. Miller, Melinda J. Reese, H. Roger Brown, Jeffrey I. Ambroso, and J. Gregory Falls: Study development, implementation, manuscript review.
Christine M. Hunt: Study development, implementation, manuscript preparation.
Conflict of interest
N.A. Yuen, R.T. Miller, M.J. Reese, H.R. Brown, J.I. Ambroso, and J.G. Falls are employees and/or shareholders of GSK; C.M. Hunt was a GSK employee and shareholder at the time of this work. Ayako Suzuki currently possesses a consulting agreement with GSK.
Transparency Document
The Transparency document associated with this article can be found in the online version.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.yrtph.2015.05.004.
References
- Aleo MD, et al. Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology. 2014;60(3):1015–1022. doi: 10.1002/hep.27206. [DOI] [PubMed] [Google Scholar]
- Alisi A, et al. Thyroid status affects rat liver regeneration after partial hepatectomy by regulating cell cycle and apoptosis. Cell. Physiol. Biochem. 2005;15(1–4):69–76. doi: 10.1159/000083639. [DOI] [PubMed] [Google Scholar]
- Almenoff JS, et al. Disproportionality analysis using empirical Bayes data mining: a tool for the evaluation of drug interactions in the post-marketing setting. Pharmacoepidemiol. Drug Saf. 2003;12(6):517–521. doi: 10.1002/pds.885. [DOI] [PubMed] [Google Scholar]
- Almenoff J, et al. Perspectives on the use of data mining in pharmaco-vigilance. Drug Saf. 2005;28(11):981–1007. doi: 10.2165/00002018-200528110-00002. [DOI] [PubMed] [Google Scholar]
- Argikar UA, Remmel RP. Effect of aging on glucuronidation of valproic acid in human liver microsomes and the role of UDP-glucuronosyltransferase UGT1A4, UGT1A8, and UGT1A10. Drug Metab. Dispos. 2009;37(1):229–236. doi: 10.1124/dmd.108.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig. Liver Dis. 2006;38(1):33–38. doi: 10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Carayon P, et al. Involvement of peripheral benzodiazepine receptors in the protection of hematopoietic cells against oxygen radical damage. Blood. 1996;87(8):3170–3178. [PubMed] [Google Scholar]
- Carey EJ, et al. Inpatient admissions for drug-induced liver injury: results from a single center. Dig. Dis. Sci. 2008;53(7):1977–1982. doi: 10.1007/s10620-008-0250-x. [DOI] [PubMed] [Google Scholar]
- Caster O, et al. Methylprednisolone-induced hepatotoxicity: experiences from global adverse drug reaction surveillance. Eur. J. Clin. Pharmacol. 2014;70(4):501–503. doi: 10.1007/s00228-013-1632-3. [DOI] [PubMed] [Google Scholar]
- Chalasani NP, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. quiz 967. [DOI] [PubMed] [Google Scholar]
- Chen M, et al. A testing strategy to predict risk for drug-induced liver injury in humans using high-content screen assays and the ‘rule-of-two’ model. Arch. Toxicol. 2014;88(7):1439–1449. doi: 10.1007/s00204-014-1276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, et al. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem. Pharmacol. 2007;74(11):1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CK, et al. Use of complementary and alternative therapies in community-dwelling older adults. J. Altern. Complement. Med. 2007;13(9):997–1006. doi: 10.1089/acm.2007.0527. [DOI] [PubMed] [Google Scholar]
- Cullen JM, et al. Time course gene expression using laser capture microscopy-extracted bile ducts, but not hepatic parenchyma, reveals acute alpha-naphthylisothiocyanate toxicity. Toxicol. Pathol. 2010;38(5):715–729. doi: 10.1177/0192623310373774. [DOI] [PubMed] [Google Scholar]
- Davit-Spraul A, et al. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin. Liver Dis. 2010;30(2):134–146. doi: 10.1055/s-0030-1253223. [DOI] [PubMed] [Google Scholar]
- de Abajo FJ, et al. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br. J. Clin. Pharmacol. 2004;58(1):71–80. doi: 10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictionary. World Health Organization, Anatomical Therapeutic Chemical Classification. 2014 [Google Scholar]
- DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA Spontaneous Reporting System (with discussion) Am. Stat. 1999;53:177–202. [Google Scholar]
- DuMouchel W, P D. Empirical Bayes Screening for Multi-item Associations the Seventh ACM SIGKDD International Conference on Knowledge Discovery and Data Mining San Francisco; California Association for Computing Machinery; 2001. [Google Scholar]
- DuMouchel W. A new family of link functions extending logistic regression Joint Statistical Meetings; July 30, 2007; Salt Lake City. 2007. (Invited presentation) [Google Scholar]
- Estes JD, et al. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch. Surg. 2003;138(8):852–858. doi: 10.1001/archsurg.138.8.852. [DOI] [PubMed] [Google Scholar]
- Felix K, et al. The antioxidative activity of the mucoregulatory agents: ambroxol, bromhexine and N-acetyl-l-cysteine. A pulse radiolysis study. Life Sci. 1996;59(14):1141–1147. doi: 10.1016/0024-3205(96)00431-6. [DOI] [PubMed] [Google Scholar]
- Fontana RJ, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147(1):96–108. e104. doi: 10.1053/j.gastro.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pagan JC, et al. Nicardipine increases hepatic blood flow and the hepatic clearance of indocyanine green in patients with cirrhosis. J. Hepatol. 1994;20(6):792–796. doi: 10.1016/s0168-8278(05)80151-5. [DOI] [PubMed] [Google Scholar]
- Gurwitz JH, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- Han D, et al. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol. Sci. 2013;34(4):243–253. doi: 10.1016/j.tips.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben M. A brief primer on automated signal detection. Ann. Pharmacother. 2003;37(7–8):1117–1123. doi: 10.1345/aph.1C515. [DOI] [PubMed] [Google Scholar]
- Huang RF, et al. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. J. Nutr. 2001;131(1):33–38. doi: 10.1093/jn/131.1.33. [DOI] [PubMed] [Google Scholar]
- Hubner C, et al. Sodium, hydrogen exchange type 1 and bile ductular secretory activity in the guinea pig. Hepatology. 2000;31(3):562–571. doi: 10.1002/hep.510310303. [DOI] [PubMed] [Google Scholar]
- Indiana University Division of Clinical Pharmacology P450 Drug Interaction Table. [accessed 09.02.15];2015 Available at: < http://medicine.iupui.edu/clinpharm/ddis/main-table/>. [Google Scholar]
- James RC, et al. Phenylpropanolamine potentiation of acetaminopheninduced hepatotoxicity: evidence for a glutathione-dependent mechanism. Toxicol. Appl. Pharmacol. 1993;118(2):159–168. doi: 10.1006/taap.1993.1021. [DOI] [PubMed] [Google Scholar]
- Jolley CD, et al. Induction of bile acid synthesis by cholesterol and cholestyramine feeding is unimpaired in mice deficient in apolipoprotein AI. Hepatology. 2000;32(6):1309–1316. doi: 10.1053/jhep.2000.19811. [DOI] [PubMed] [Google Scholar]
- Kacevska M, et al. Perspectives on epigenetics and its relevance to adverse drug reactions. Clin. Pharmacol. Ther. 2011;89(6):902–907. doi: 10.1038/clpt.2011.21. [DOI] [PubMed] [Google Scholar]
- Kohane IS. Using electronic health records to drive discovery in disease genomics. Nat. Rev. Genet. 2011;12(6):417–428. doi: 10.1038/nrg2999. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, et al. Effect of estrogen on mitochondrial function and intracellular stress markers in rat liver and kidney following trauma-hemorrhagic shock and prolonged hypotension. Mol. Med. 2010;16(7–8):254–261. doi: 10.2119/molmed.2009.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunduzova OR, et al. Involvement of peripheral benzodiazepine receptor in the oxidative stress, death-signaling pathways, and renal injury induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 2004;15(8):2152–2160. doi: 10.1097/01.ASN.0000133563.41148.74. [DOI] [PubMed] [Google Scholar]
- Lammert C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: search for signals. Hepatology. 2008;47(6):2003–2009. doi: 10.1002/hep.22272. [DOI] [PubMed] [Google Scholar]
- Law V, et al. DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 2014;42(D1):D1091–D1097. doi: 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitzmann MF, et al. Thiazide diuretics and the risk of gallbladder disease requiring surgery in women. Arch. Intern. Med. 2005;165(5):567–573. doi: 10.1001/archinte.165.5.567. [DOI] [PubMed] [Google Scholar]
- Lu J, et al. Amiodarone exposure during modest inflammation induces idiosyncrasy-like liver injury in rats: role of tumor necrosis factor-alpha. Toxicol. Sci. 2012;125(1):126–133. doi: 10.1093/toxsci/kfr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena MI, et al. Determinants of the clinical expression of amoxicillin– clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44(4):850–856. doi: 10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- Lucena M, Isabel, et al. Susceptibility to amoxicillin–clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141(1):338–347. doi: 10.1053/j.gastro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. The immunoregulatory effects of antidepressants. Hum. Psychopharmacol. 2001;16(1):95–103. doi: 10.1002/hup.191. [DOI] [PubMed] [Google Scholar]
- McWhirter D, et al. Chemotherapy induced hepatotoxicity in metastatic colorectal cancer: a review of mechanisms and outcomes. Crit. Rev. Oncol. Hematol. 2013;88(2):404–415. doi: 10.1016/j.critrevonc.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Moore TJ, et al. Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch. Intern. Med. 2007;167(16):1752–1759. doi: 10.1001/archinte.167.16.1752. [DOI] [PubMed] [Google Scholar]
- Morgan RE, et al. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci. 2010;118(2):485–500. doi: 10.1093/toxsci/kfq269. [DOI] [PubMed] [Google Scholar]
- Morgan RE, et al. A multifactorial approach to hepatobiliary transporter assessment enables improved therapeutic compound development. Toxicol. Sci. 2013;136(1):216–241. doi: 10.1093/toxsci/kft176. [DOI] [PubMed] [Google Scholar]
- Murata K, et al. A novel mechanism for drug-induced liver failure: inhibition of histone acetylation by hydralazine derivatives. J. Hepatol. 2007;46(2):322–329. doi: 10.1016/j.jhep.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Navarro VJ, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399–1408. doi: 10.1002/hep.27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oben JA, et al. Sympathetic nervous system inhibition increases hepatic progenitors and reduces liver injury. Hepatology. 2003;38(3):664–673. doi: 10.1053/jhep.2003.50371. [DOI] [PubMed] [Google Scholar]
- Okamoto T, et al. Histamine H1-receptor antagonists with immunomodulating activities: potential use for modulating T helper type 1 (Th1)/Th2 cytokine imbalance and inflammatory responses in allergic diseases. Clin. Exp. Immunol. 2009;157(1):27–34. doi: 10.1111/j.1365-2249.2009.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009;15(14):1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessayre D, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab. Rev. 2012;44(1):34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- Peterson PK, et al. The opioid–cytokine connection. J. Neuroimmunol. 1998;83(1–2):63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier LA, Wise CK. On the chemical causation of methyl deficiency and its attendant pathologies. Regul. Res. Perspect. 2003;3:1–31. [Google Scholar]
- Qato DM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300(24):2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez E, et al. Proton pump inhibitors are associated with hypersensitivity reactions to drugs in hospitalized patients: a nested case-control in a retrospective cohort study. Clin. Exp. Allergy. 2013;43(3):344–352. doi: 10.1111/cea.12034. [DOI] [PubMed] [Google Scholar]
- Randall LH, et al. Cholelithiasis in infants receiving furosemide: a prospective study of the incidence and one-year follow-up. J. Perinatol. 1992;12(2):107–111. [PubMed] [Google Scholar]
- Reuben A, et al. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52(6):2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanque P, et al. Thyroid hormone administration induces rat liver nrf2 activation: suppression by N-acetylcysteine pretreatment. Thyroid. 2011;21(6):655–662. doi: 10.1089/thy.2010.0322. [DOI] [PubMed] [Google Scholar]
- Roy S, Loh HH. Effects of opioids on the immune system. Neurochem. Res. 1996;21(11):1375–1386. doi: 10.1007/BF02532379. [DOI] [PubMed] [Google Scholar]
- Sakatis MZ, et al. Preclinical strategy to reduce clinical hepatotoxicity using in vitro bioactivation data for >200 compounds. Chem. Res. Toxicol. 2012;25(10):2067–2082. doi: 10.1021/tx300075j. [DOI] [PubMed] [Google Scholar]
- Senior JR. Drug hepatotoxicity from a regulatory perspective. Clin. Liver Dis. 2007;11(3):507–524. vi. doi: 10.1016/j.cld.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Sgro C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36(2):451–455. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol. Res. 2007;37(4):239–247. doi: 10.1111/j.1872-034X.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- Stieger B, et al. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118(2):422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- Suzuki A, et al. Co-medication with adrenoreceptor antagonists is associated with lower MELD scores at admission in patients with acetaminophen-induced acute liver failure. Gastroenterology. 2009a;136(5) Suppl. 1:A-810. [Google Scholar]
- Suzuki A, et al. Co-medications modulating liver injury and repair influence clinical outcome of acetaminophen-associated liver injury. Clin. Gastroenterol. Hepatol. 2009b;7(8):882–888. doi: 10.1016/j.cgh.2009.03.034. [DOI] [PubMed] [Google Scholar]
- Suzuki A, et al. Drugs associated with hepatotoxicity and their reporting frequency of liver adverse events in VigiBase: unified list based on international collaborative work. Drug Saf. 2010;33(6):503–522. doi: 10.2165/11535340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Takikawa H, et al. Drug-induced liver injury in Japan: an analysis of 1676 cases between 1997 and 2006. Hepatol. Res. 2009;39(5):427–431. doi: 10.1111/j.1872-034X.2008.00486.x. [DOI] [PubMed] [Google Scholar]
- Tatonetti NP, et al. Detecting drug interactions from adverse-event reports: interaction between paroxetine and pravastatin increases blood glucose levels. Clin. Pharmacol. Ther. 2011;90(1):133–142. doi: 10.1038/clpt.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J, Naisbitt DJ. Idiosyncratic adverse drug reactions: current concepts. Pharmacol. Rev. 2013;65(2):779–808. doi: 10.1124/pr.113.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca G, et al. Inhibition of granulocyte migration by tiotropium bromide. Respir. Res. 2011;12:24. doi: 10.1186/1465-9921-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, et al. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol. Sin. 2009;30(11):1522–1528. doi: 10.1038/aps.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, Paul B, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296(1):87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- Yazici C, et al. Risk factors for severe or fatal drug-induced liver injury from amoxicillin–clavulanic acid. Hepatol. Res. 2014 doi: 10.1111/hepr.12410. http://dx.doi.org/10.1111/hepr.12410. [DOI] [PubMed] [Google Scholar]
- Yu K, Geng X, Chen M, Zhang J, Wang B, Ilic K, Tong W. High daily dose and being a substrate of cytochrome P450 enzymes are two important predictors of drug-induced liver injury. Drug Metab. Dispos. 2014;42(4):744–750. doi: 10.1124/dmd.113.056267. [DOI] [PubMed] [Google Scholar]
- Zimmerman HJ. Vulnerability of the liver to toxic injury,. Chapter 3 in Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Philadelphia, PA, USA: Lippincott Williams and Wilkins; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.