Abstract
Background:
There is literature that indicates the association of asthma with an increased risk of common and serious microbial infections. We recently reported an increased risk of vaccine-preventable diseases, e.g., herpes zoster (HZ) among children with asthma, defined by predetermined asthma criteria. Little is known about whether this association is persistent if the asthma status is defined by different asthma criteria, e.g., the Asthma Predictive Index, given the heterogeneity of asthma.
Objective:
To assess the consistency of the association between asthma and the risk of HZ in children.
Methods:
This is a population-based case-control study based on all pediatric patients with HZ between 1996 and 2001 in Olmsted County, Minnesota, and 1:1 age- and sex-matched controls without a history of HZ who were enrolled in our previous study. The original Asthma Predictive Index criteria was operationalized by two or more wheezing episodes in a year for the first 3 years of life plus one of the major (physician-diagnosed asthma for a parent or physician-diagnosed eczema for a patient) or two of the minor criteria (physician-diagnosed allergic rhinitis for a patient, wheezing apart from cold, or eosinophilia [≥4%]). Data were fit to traditional logistic regression models to calculate odds ratios and 95% confident intervals.
Results:
Of the original cohort (n = 554), 95 (17%) did not meet the enrollment criteria for this study, which left 459. Of the 221 patients, 53% were female, with a mean (standard deviation) age of 9.7 ± 4.2 years. The risk of HZ was increased in children with asthma defined by the API controlling for a varicella vaccine history and atopic status (adjusted odds ratio 2.56 [95% confidence interval, 1.08–6.56]).
Conclusions:
The association between asthma and increased risk of HZ in children and adolescents is consistent, independent of asthma definitions. Asthma might be an important clinical condition to be considered in HZ vaccine studies.
Keywords: Asthma ascertainment, Asthma Predictive Index (API); predetermined asthma criteria, comorbidity, latent infection, microbial infection, vaccine, herpes zoster (shingles), wheezing, allergy
Asthma affects 4–17% of children in the United States1–5 and 2.8–37% of children worldwide, depending on the country.6 A recent review article highlighted the significant morbidity due to the increased risk of common and serious microbial infections associated with asthma.7 Our recent population-based study showed that children with a history of asthma had a significantly increased risk of herpes zoster (HZ) compared with those without asthma (odds ratio [OR] 2.09 [95% confidence interval [CI], 1.24–3.52]; p = 0.006),8 which indicated the potential impact of asthma on non–airway-related infection. The original study ascertained asthma status by applying predetermined asthma criteria (PAC), which was used for previous studies that showed the association of asthma with increased risks of common and serious microbial infections (e.g., serious pneumococcal diseases, pertussis, Streptococcus pyogenes upper respiratory infection, and recurrent or persistent otitis media).9–12 However, it is unknown whether the original study findings on the association between asthma and the increased risk of HZ are true when a different asthma criteria, e.g., the Asthma Predictive Index (API),13 was applied.
Briefly, the API was developed to predict childhood asthma based on the risk factors during the first 3 years of life13 and is suggested for use in research of childhood asthma by the 2007 National Asthma Education and Prevention Program guideline.14 Because the API has only been used in prospective or cross-sectional studies, 15–19 not a retrospective study, we recently validated use of the API for a retrospective study. The API and PAC showed a high concordance rate (82.9%), and the API showed excellent construct validity in association with known risk factors for asthma, such as other atopic conditions, a family history of asthma, and lower parental education, which indicates suitability of using the API for a retrospective study.20
We hypothesized that the association between asthma and the increased risk of HZ is consistent, independent of asthma criteria (consistency). To test this hypothesis, as a follow-up study to the original study, which showed an increased risk of HZ among children with asthma defined by the PAC, we sought to determine whether asthma defined by different criteria, e.g., API, is still associated with an increased risk of HZ in children. This study was approved by the institutional review board for human subject research at the Mayo Clinic and the Olmsted Medical Center.
METHODS
Study Setting
Olmsted County, Minnesota, is an excellent setting to conduct a population-based epidemiologic study because medical care is virtually self-contained within the community. In addition, when patients register with any health care provider in the community (e.g., as a newborn), they, or their parents or legal guardian, are asked to grant or refuse authorization of use of their medical records for research. Authorization is granted by more than 95% of all individuals.21 Medical records research by using the geographically defined population of Olmsted County is possible through the Rochester Epidemiology Project (REP), which has been continuously funded by the National Institutes of Health and has been maintained since 1960.22–25 All clinical diagnoses given to an Olmsted County resident who visits nearly any health care facility in the county are electronically indexed to the individual by using a unique REP patient identifying number, and information from every episode of care is contained within the REP data base. By using REP resources, a previous study demonstrated that incidence rates of asthma for this community are similar to other communities.26
Study Design and Subjects
This is a geographically defined population-based case-control study based on all pediatric patients with HZ between 1996 and 2001 in Olmsted County, Minnesota, who were enrolled in our previous study. Details of study subjects were previously described.8,27,28 Briefly, HZ cases were initially identified by the International Classification of Diseases, 9th Revision (ICD-9) codes (053.xx), and medical records for each potential subject were reviewed to verify that the subject was indeed a new case of HZ, based on predetermined criteria for HZ. Confirmation required a characteristic rash (i.e., vesicular rash on a dermatome) and signs or symptoms of pain or itching at the rash site, in addition to a physician's diagnosis of HZ. Exclusion criteria included a lack of authorization for the use of medical records for research, nonresidence in Olmsted County, Minnesota, and another diagnosis that possibly explained the rash, e.g., a culture positive for herpes simplex. Their corresponding 1:1 age- and sex-matched controls without a history of HZ as of the index date were identified from Olmsted County residents. In addition to those for the original study, we also excluded children whose medical records were unavailable to ascertain asthma status by the API (e.g., adopted children or first registration in the clinic after the age of 3 years). Pertinent information on sociodemographic and clinical characteristics of subjects collected for the original study were used in this present study.
Ascertainment of Asthma Status by the API, PAC, and Physician Diagnosis
We conducted comprehensive medical record reviews to determine asthma status by applying (1) the API criteria based on the first 3 years of life (original API), (2) API criteria based on the entire follow-up duration beyond the first 3 years of life (API ever), (3) PAC, and (4) a physician diagnosis of asthma documented in the medical records. The API is summarized in Table 1, and the operational details for applying the API were reported in our recent study.20 We applied two separate API criteria based on the duration of follow-up, as described above (original API versus API ever). The PAC is summarized in Table 2. Briefly, the PAC was developed to be applied to retrospective studies that concerned asthma epidemiology by the asthma researchers, Yunginger and Reed.29 To our knowledge, the PAC is the only predetermined criteria that can be applied to medical records retrospectively without using ICD codes for asthma. Since its development, it has shown high reliability (0.72–0.92) and excellent construct validity to predict various risk factors for asthma.9–12,29,30 The onset date of asthma (asthma index date) by the PAC was defined as the earliest constellation of symptoms found in the medical record that met the PAC for asthma regardless of physician diagnosis of asthma. Because most subjects with probable asthma by the PAC (85%) that became definite asthma over time, we combined both probable and definite asthma.29
Table 1.
API criteria

Positive API: Early frequent wheezing (<3 years of life) plus at least one of the major criteria or two of three minor criteria.
Table 2.
PAC

FEV1= forced expiratory volume in 1 second; FVC = forced vital capacity.
Statistical Analysis
The primary aim of the analysis was to determine the association between asthma defined by various criteria and the risk of HZ. Data were fit to multivariate logistic regression models to calculate ORs and their corresponding 95% CIs for asthma status defined by each definition (i.e., original API, API ever, PAC, and ICD-9 code) in relation to the risk of HZ controlling for pertinent covariates and confounders such as a varicella vaccine history and atopic status, defined as the presence of sensitization against an aeroallergen or a food allergen. We used the Greenland entry criteria (p < 0.20, based on univariate analysis). All analyses were performed by using the JMP statistical software package (version 9.0.1; SAS Institute, Inc., Cary, NC).
RESULTS
Of the original 554 subjects, 95 children (56 cases and 39 controls) were excluded (7, withdrawal of research authorization; 11, adoption; 77, unable to apply the API due to insufficient information during the first 3 years of life), and the comparison of basic characteristics between the included and excluded children is summarized in Supplemental Table 1. Overall, the excluded and included subjects were similar except for white race (82% versus 93%; p = 0.001). Characteristics of the subjects with HZ and the controls are summarized in Table 3. Of the remaining 221 subjects, 53% were female and 94% were white. The mean (SD) age at diagnosis of HZ was 9.7 ± 4.2 years. The onset age of primary HZ in children with asthma defined by original API tended to be younger than those without asthma among subjects with HZ only (10.4 ± 4.1 years versus 8.6 ± 4.2 years; p = 0.092).
Table 3.
Characteristics of HZ for cases and controls and factors associated with risk of HZ in children
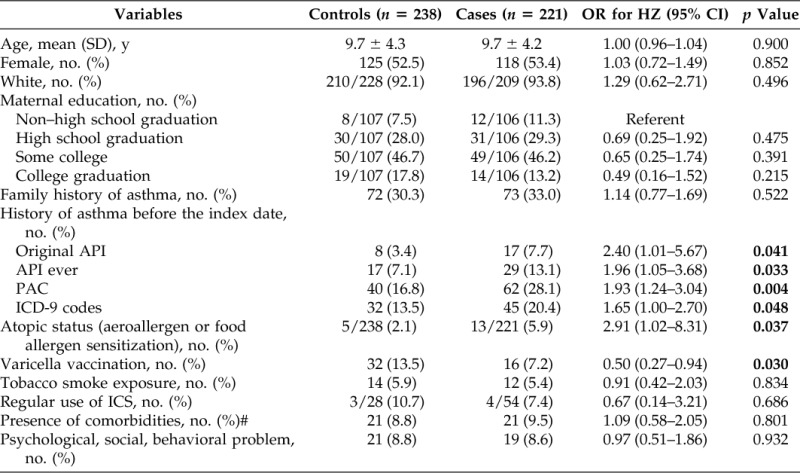
ICS = inhaled corticosteroid.
#Comorbid conditions were based on the Advisory Committee on Immunization Practices–recommended pneumococcal vaccine–eligible conditions.
#p value < 0.05 in bold.
Asthma Defined by Different Asthma Criteria and the Risk of HZ
Results for the association between asthma and HZ are summarized in Table 3. Seventeen of the 221 subjects (7.7%) had asthma by the original API before the index date of HZ compared with 8 of the 238 controls (3.4%) (unadjusted OR 2.40 [95% CI, 1.01–5.67]; p = 0.041). Similarly, asthma status defined by all other criteria before the index date was associated with the increased risk of HZ. The results based on multivariate models to adjust for pertinent covariates and confounders are summarized in Table 4. After adjusting for atopic status, defined as the presence of sensitization against an aeroallergen or a food allergen, and a varicella vaccination history, a history of asthma defined by original API was significantly associated with the increased risk of HZ (adjusted OR 2.56 [95% CI, 1.08–6.56]; p = 0.032) (Table 4). This was true for the association between asthma defined by other criteria and the risk of HZ except a physician diagnosis of asthma that only showed borderline significance. Atopic status was significant or approached statistical significance in multivariate models. A history of varicella vaccination was consistently associated with a decreased risk of HZ. Inhaled corticosteroid use was not associated with the risk of HZ.
Table 4.
Association between asthma and risk of HZ based on a multivariate traditional logistic regression model
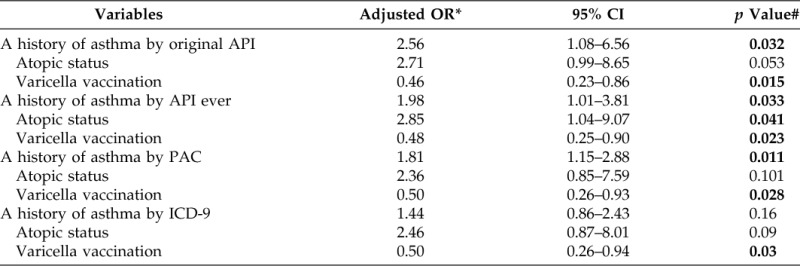
*Adjusted for atopic status, history of varicella vaccination, and history of asthma by each criteria.
#p value < 0.05 in bold.
DISCUSSION
Analysis of our study results demonstrated that asthma status defined by different criteria was consistently associated with an increased risk of HZ in children. These results helped affirm the association between asthma and an increased risk of HZ. The results of this present study confirmed the original study findings on the relationship between asthma defined by the PAC and an increased risk of HZ.8 In addition, the direction of the association was consistent, despite the definition of asthma status by four different criteria that controlled for all pertinent covariates. Atopic status was similarly associated with the increased risk of HZ, although the association only approached to statistical significance. Varicella vaccine was consistently protective against HZ, which confirmed our previous study finding. Although 95 children were excluded from the original study, it is unlikely to significantly influence the results and our interpretation of the results because the subjects included and those excluded were similar except race. These results helped establish consistency and coherence in causal inference between asthma and the risk of HZ in children, and are particularly important given the reported heterogeneity of asthma.31–34 Overall, analysis of the results indicated that asthma may have the potential impact on a non–airway-related infection such as HZ.
We recently reported an increased risk of HZ among adults with asthma compared with those without asthma.35 This study finding was corroborated by the two recent studies that indicated associations of asthma with the increased risk of HZ.36,37 The mechanisms that underlie the potential associate between asthma and HZ are unknown. The recent review article on the increased risk of microbial infections associated with asthma covered the potential mechanisms that underlie the relationship between asthma and the increased risk of common and serious microbial infections.7 Both innate and adaptive immune dysfunctions associated with clinical asthma and immunogenic predisposition to asthma might play a role. One noteworthy study showed a more rapid waning of humoral immunity (antimeasles immunoglobulin G levels) over time in children with asthma than in children without asthma,38 which potentially indicates that asthma might affect kinetics or functions of B cells over time in a way that makes the host susceptible to vaccine-induced microbial infections.10 Given that cell-mediated immunity (CMI) is known to decline with age as part of immunosenescence and that decreased CMI is associated with reactivation of varicella zoster virus, our study results might indicate that asthma affects the waning of CMI in addition to the reported impaired innate immunity.38,39 The younger age of HZ onset among children with asthma in this study may support this possibility of more rapidly decreasing CMI function compared with those without asthma. Along these lines, future studies need to address the relationship between asthma and the risk of other latent infections such as herpes simplex virus (HHV-1 and HHV-2), Epstein-Barr virus (HHV-4), cytomegalovirus (HHV-5), and roseolovirus (HHV-6).
Our study results have clinical and research implications. Clinicians and parents of children with asthma need to be aware of our study findings for early detection and treatment for HZ. In HZ vaccine research, asthma might be an important clinical condition to be considered in designing vaccine studies. Because asthma poses an increased risk of microbial infection, including the 2009 novel H1N1 influenza,40–43 it continues to raise a public health concern, as shown in the recent emerging infectious disease, e.g., enterovirus D68, which poses a risk for severe symptoms among children with asthma.44–46
There are inherent limitations in our study as a retrospective study. The original API limited relevant medical information to the first 3 years of life. Asthma-related symptoms continue to occur beyond the first 3 years of life, which affect the ascertainment of asthma status, as suggested by Yunginger et al.29 Thus, we included the API ever, which included all medical information beyond the first 3 years of life. The results based on asthma status by the API ever were similar to those by the original API. Some missing data of major and minor criteria in the API may affect the results of study. For example, in this retrospective study, eosinophilia data were not available for all the subjects. Eosinophilia was defined by ≥4% of the total white blood cells in the test performed before the age of 1 year because blood specimens of the original API study were obtained at the age of 1 year (mean [SD]: 10.9 ± 0.6 months).13 The original API study reported that only 10% of the subjects had eosinophilia and that eosinophilia was seldom used for diagnosing asthma because it is not a specific marker for asthma, particularly in patients with mild asthma.47,48 Further, the proportion of missing eosinophilia data in the present study was not different between the HZ group and the control group (which were 85% versus 83% respectively), which indicates nondifferential misclassification bias. Thus, we believe it is unlikely to influence our study results.
Our study has important strengths. This is a population-based study design, which minimizes a selection bias. In addition, ascertainment of asthma status by using the API was performed independent of asthma status by a physician diagnosis of asthma, which minimized an observational bias. Our study setting has unique advantages, including a self-contained health care environment and a medical record linkage system that links patients and their medical records to health care providers.
In conclusion, asthma is consistently associated with an increased risk of HZ in children and adolescents, despite ascertainment of asthma status by different criteria. The mechanisms that underlie this association should be explored in future studies.
ACKNOWLEDGMENTS
We thank the staff of the Pediatric Asthma Epidemiology Research Unit for research support. We thank Elizabeth Krusemark for her administrative assistance.
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (R21 AI101277) and Scholarly Clinician Award from the Mayo Foundation. Also, this study was made possible by using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award R01AG034676.
Presented at Pediatric Academic Societies, Vancouver, Canada, May 3–6, 2014
B.P. Yawn is a consultant for Merck and GlaxoSmithKline and has received grants from Merck. The remaining authors have no conflicts of interest to declare pertaining to this article
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1. Azad MB, Coneys JG, Kozyrskyj AL, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: Systematic review and meta-analysis. BMJ 347:f6471, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Vital signs: Asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep 60:547–552, 2011. [PubMed] [Google Scholar]
- 3. Lethbridge-Çejku M, Vickerie J. Summary health statistics for U.S. adults: National Health Interview Survey, 2003. National Center for Health Statistics. Vital Health Stat 10(225). 2005. Hyattsville, Maryland. [PubMed] [Google Scholar]
- 4. Stanton MW. The High Concetration of US Health Care Expenditures. 2006. Available online at http://www.ahrq.gov/research/ria19/expendria.htm; accessed May 19, 2012.
- 5. Schiller JS, Lucas JW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2011. National Center for Health Statistics. Vital Health Stat 10(256). 2012. Hyattsville, Maryland. [PubMed] [Google Scholar]
- 6. Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 368:733–743, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): Is asthma more than a chronic airway disease? J Allergy Clin Immunol 134:247–257, quiz 258–259, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim BS, Mehra S, Yawn B, et al. Increased risk of herpes zoster in children with asthma: A population-based case-control study. J Pediatr 163:816–821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juhn YJ, Kita H, Yawn BP, et al. Increased risk of serious pneumococcal disease in patients with asthma. J Allergy Clin Immunol 122:719–723, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Capili CR, Hettinger A, Rigelman-Hedberg N, et al. Increased risk of pertussis in patients with asthma. J Allergy Clin Immunol 129:957–963, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frey D, Jacobson R, Poland G, et al. Assessment of the association between pediatric asthma and Streptococcus pyogenes upper respiratory infection. Allergy Asthma Proc 30:540–545, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Bjur KA, Lynch RL, Fenta YA, et al. Assessment of the association between atopic conditions and tympanostomy tube placement in children. Allergy Asthma Proc 33:289–296, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 162(pt. 1):1403–1406, 2000. [DOI] [PubMed] [Google Scholar]
- 14. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 120(suppl.):S94–S138, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Ater D, Bar BE, Fireman N, et al. Asthma-predictive-index, bronchial-challenge, sputum eosinophils in acutely wheezing preschoolers. Pediatr Pulmonol 49:952–959, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Castro-Rodriguez JA, Sardon O, Perez-Yarza EG, et al. Young infants with recurrent wheezing and positive asthma predictive index have higher levels of exhaled nitric oxide. J Asthma 50:162–165, 2013. [DOI] [PubMed] [Google Scholar]
- 17. Moeller A, Diefenbacher C, Lehmann A, et al. Exhaled nitric oxide distinguishes between subgroups of preschool children with respiratory symptoms. J Allergy Clin Immunol 121:705–709, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol 114:1282–1287, 2004. [DOI] [PubMed] [Google Scholar]
- 19. Brand PL, Luz Garcia-Garcia M, Morison A, et al. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respir Med 105:1588–1595, 2011. [DOI] [PubMed] [Google Scholar]
- 20. Wi CI, Park MA, Juhn YJ. Development and initial testing of Asthma Predictive Index for a retrospective study: An exploratory study. J Asthma 26:1–8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yawn BP, Yawn RA, Geier GR, et al. The impact of requiring patient authorization for use of data in medical records research. J Fam Pract 47:361–365, 1998. [PubMed] [Google Scholar]
- 22. Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am 245:54–63, 1981. [DOI] [PubMed] [Google Scholar]
- 23. Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: Half a century of medical records linkage in a US Population. Mayo Clinic Proc 87:1202–1213, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: Assessing the most prevalent conditions in a defined American population. Mayo Clinic Proc 88:56–67, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester epidemiology project. Am J Epidemiol 173:1059–1068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yawn BP, Wollan P, Kurland M, Scanlon P. A longitudinal study of the prevalence of asthma in a community population of school-age children. J Pediatr 140:576–581, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 82:1341–1349, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Yawn BP, Itzler RF, Wollan PC, et al. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc 84:787–794, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yunginger JW, Reed CE, O'Connell EJ, et al. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis 146:888–894, 1992. [DOI] [PubMed] [Google Scholar]
- 30. Beard CM, Yunginger JW, Reed CE, et al. Interobserver variability in medical record review: An epidemiological study of asthma. J Clin Epidemiol 45:1013–1020, 1992. [DOI] [PubMed] [Google Scholar]
- 31. Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 178:218–224, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 181:315–323, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fitzpatrick AM, Teague WG, Meyers DA, et al. Heterogeneity of severe asthma in childhood: Confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 127:382–389.e1–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lazic N, Roberts G, Custovic A, et al. Multiple atopy phenotypes and their associations with asthma: Similar findings from two birth cohorts. Allergy 68:764–770, 2013. [DOI] [PubMed] [Google Scholar]
- 35. Juhn YJ, Kwon HJ, Bang DW, et al. Asthma as an unrecognized risk factor for Herpes zoster in adults: a population-based case-control study. J Allergy Clin Immunol 133(suppl 2), AB 243, 2014. [Google Scholar]
- 36. Esteban-Vasallo MD, Dominguez-Berjon MF, Gil-Prieto R, et al. Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: A population-based study from primary care in Madrid (Spain). Hum Vaccin Immunother 10:1650–1660, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forbes HJ, Bhaskaran K, Thomas SL, et al. Quantification of risk factors for herpes zoster: Population based case-control study. BMJ 348:g2911, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoo KH, Jacobson RM, Poland G, Weaver A, Lee L, Chang T, Juhn YJ. Asthma status and waning of measles antibody levels. Pediatr Infect Dis J 33:1016–1022, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gershon AA, Gershon MD, Breuer J, et al. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 48(suppl. 1):S2–S7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santillan Salas CF, Mehra S, Pardo Crespo MR, Juhn YJ. Asthma and severity of 2009 novel H1N1 influenza: A population-based case-control study. J Asthma 50:1069–1076, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kloepfer KM, Olenec JP, Lee WM, et al. Increased H1N1 infection rate in children with asthma. Am J Respir Crit Care Med 185:1275–1279, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. ANZIC Influenza Investigators, Webb SA, Pettila V, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 361:1925–1934, 2009. [DOI] [PubMed] [Google Scholar]
- 43. Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med 361:1935–1944, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Centers for Disease Control and Prevention. What Parents Need to Know about Enterovirus D68. Available online at http://www.cdc.gov/features/evd68/; accessed November 17, 2014.
- 45. Wark PA, Grissell T, Davies B, et al. Diversity in the bronchial epithelial cell response to infection with different rhinovirus strains. Respirology 14:180–186, 2009. [DOI] [PubMed] [Google Scholar]
- 46. Owens B. Rare enterovirus continues to circulate in North America. Lancet 384:1250, 2014. [DOI] [PubMed] [Google Scholar]
- 47. McGrath KW, Icitovic N, Boushey HA, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med 185:612–619, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med 323:1033–1039, 1990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


