Abstract
Although diabetes is mainly diagnosed based on elevated glucose levels, dyslipidemia is also observed in these patients. Chronic kidney disease (CKD), a frequent occurrence in patients with diabetes, is associated with major abnormalities in circulating lipoproteins and renal lipid metabolism. At baseline, most renal epithelial cells rely on fatty acids as their energy source. CKD, including that which occurs in diabetes, is characterized by tubule epithelial lipid accumulation. Whether this is due to increased uptake or greater local fatty acid synthesis is unknown. We have recently shown that CKD also leads to decreased fatty acid oxidation, which might be an additional mechanism leading to lipid accumulation. Defective fatty acid utilization causes energy depletion resulting in increased apoptosis and dedifferentiation, which in turn contributes to fibrosis and CKD progression. Enhanced fatty acid oxidation in the kidney induced by fenofibrate, a peroxisomal proliferator-activated receptor (PPAR)-α agonist, showed benefit in mouse models of CKD. Fenofibrate treatment also reduced albuminuria in patients with diabetes in multiple clinical trials. Taken together, these findings suggest that further understanding of lipid metabolism in diabetic kidney disease may lead to novel therapeutic approaches.
Keywords: Chronic renal failure, Diabetes, Fatty acid metabolism, Triglyceride
Introduction
Lipids are essential building blocks of cells. They are the structural components of biological membranes, the storage form of most intracellular energy, and the molecules that regulate signaling pathways of a number of biologically essential pathways [1]. Cellular lipid homeostasis is controlled by balancing lipid uptake, synthesis, utilization, and storage. The major lipid classes in the kidney are phospholipids (a major constituent of membranes), triglycerides, and non-esterified or free fatty acids (NEFA). Triglycerides and NEFA are energy substrates for beta-oxidation. While lipids are necessary for normal cellular functions, increasing evidence suggests that abnormal lipid accumulation in non-adipose tissues contributes to injury and organ dysfunction. This has been extensively studied in the heart, where alterations in lipid metabolism and storage have been shown to contribute to development of diabetic cardiomyopathy [2•].
Kidney disease shows the strongest correlation with mortality in patients with diabetes [3]. Close to half of all chronic and end-stage kidney disease can be attributed to diabetic kidney disease (DKD). Because DKD is associated with and likely caused or exacerbated by metabolic disarray, it is paramount to better understand the tightly controlled mechanisms regulating the homeostasis of energetic substrates in renal cells. Evidence is emerging that renal lipid metabolism may play a direct role in DKD progression [4, 5•, 6]. Indeed, lipid accumulation was part of the earliest description of DKD by Kimmelstiel and Wilson [7]. Although they and others focused on glomerular disease, renal tubular lipid deposition was noted in this seminal report. A correlation between lipid deposition and kidney injury has been confirmed in multiple animal models and in human studies [5•, 6–10].
Here, we will review systemic and organ-specific lipid alterations in DKD and chronic kidney disease (CKD), mostly focusing on lipids as energy substrates with less emphasis on lipid secondary messengers and membrane changes.
Lipid Uptake and Metabolism in Renal Epithelial Cells
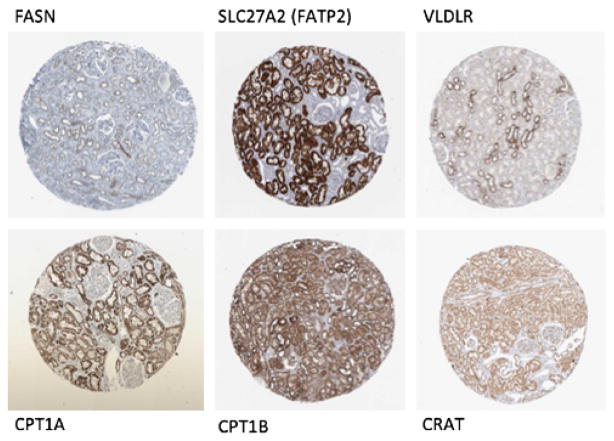
Most organs acquire lipids by de novo fatty acid synthesis and/or uptake of circulating lipoproteins or NEFA. De novo fatty acid synthesis has not been extensively studied in the kidney. According to the Human Protein Atlas, the expression of fatty acid synthase (FASN), the key rate-limiting enzyme in the lipid synthesis pathway, is quite low in the kidney (Fig. 1). In the blood, more than 90 % of fatty acids are esterified and circulate as triglyceride within very low-density lipoprotein (VLDL) and chylomicron particles. Esterified fatty acids are initially catabolized by lipoprotein lipase (LpL), releasing NEFA. In muscle and adipose, much of NEFA are taken up via cell surface receptors, such as cluster of differentiation 36 (CD36), while some enter by simple diffusion. LpL also creates remnant lipoproteins that interact with cell surface lipoprotein receptors. Additionally, core lipids are taken up by lipolysis, which creates small particles that contain surface and core lipids, as well as apoproteins, exclusive of apolipoprotein B (apoB). The pathways responsible for uptake of lipids into the kidney has not been well established. It is interesting to note that VLDL receptor expression seems to be specifically enriched in distal tubule segment. In addition, kidneys have long been known to containe LpL activity [8] and the proximal tubule robustly expresses LpL [9].
Fig. 1.
Expression of lipid metabolism-related factors in normal human kidney samples. Immunohistochemistry images of normal human kidney samples stained with antibodies against fatty acid synthase (FASN) and solute carrier family 27 (SLC27A2 or fatty acid transport protein 2; FATP2), very low-density lipoprotein receptor (VLDLR), carnitine palmitoyltransferase 1A (CPT1A), carnitine palmitoyltransferase 1B (CPT1B), and carnitine-O-acetyltransferase (CRAT). Images were downloaded from Human Protein Atlas (www.proteinatlas.org)
Most NEFA in the circulation are bound to carrier proteins, mostly albumin, and their uptake requires their dissociation from the carrier protein. CD36 deletion leads to reduced uptake of NEFA in a variety of metabolic tissues [10]. Although there may be other transporters, CD36 is clearly responsible for lipid accumulation in several tissues and is one of the best-known long-chain fatty acid transporters [11, 12]. SLC27A1-6 or FATP are additional fatty acid transport proteins that can aid fatty acid uptake by either allowing transmembrane movement or trapping NEFA by functioning as CoA synthetases. According to the Human Protein Atlas, the renal tubule is one of the highest expression sties for SLC27A2 (Fig. 1); therefore, it could be an important candidate to mediate fatty acid uptake in the kidney.
Renal epithelial cells are special because they obtain NEFA not only from the circulation but also from the filtrate. Although the filtration barrier prevents exposure of the proximal tubules to large lipoproteins, the cells can be exposed to filtered albumin and associated NEFA. In a healthy kidney, the glomerular filtration barrier is largely impermeable to albumin. However, the contribution of albumin-bound fatty acids could be more significant in proteinuric kidney diseases, including DKD, when tubules are exposed to high amount of albumin.
Systemic Lipid Abnormalities in DKD
Diabetes is associated with changes in serum lipid levels and metabolism. The plasma lipoprotein profile in patients with type 2 diabetes (T2DM) is often referred to as diabetic dyslipidemia, which is the triad of low high-density lipoprotein (HDL) cholesterol, raised triglycerides, and small, dense low-density lipoprotein (LDL) particles [13]. Insulin deficiency increases levels of NEFA, which are released from adipocytes via the actions of adipose triglyceride lipase (ATGL) [14] and hormone-sensitive lipase (HSL). ATGL hydrolyses triglyceride to diacylglycerol, which is the primary substrate for HSL. Monoglycerol lipase completes the conversion of triglyceride to NEFA and glycerol within adipocytes. Many of the released NEFA are cleared by the liver, which then recycles them as newly secreted triglyceride. Triglyceride clearance is often reduced in diabetes as a result of reduced activity of LpL, which is also sensitive to insulin [15]. Diabetes-induced hypertriglyceridemia is therefore thought to result from a number of processes including increased triglyceride formation, greater de novo synthesis driven by hyperinsulinemia in T2DM, and defective plasma triglyceride removal [16].
Plasma lipid abnormalities have been linked to development of cardiovascular disease in patients with diabetes and preserved kidney function. Interestingly, non-diabetic patients with CKD also develop similar patterns of dyslipidemia [17, 18].
Lipid Uptake in Chronic and Diabetic Kidney Disease
The effect of systemic lipid alterations on CKD progression is complex and not well understood. In diabetes and CKD, renal tubule cells are exposed to increased NEFA both from the apical and the basal sides because of the high concentration of albumin in the urine, a key feature of DKD. Hyperlipidemia also increases the fatty acid content of albumin [6], further increasing fatty acid delivery. Several studies suggest that albumin-bound long-chain saturated fatty acids might play a role in tubular damage [19, 20]. Earlier work from the Bottinger group suggested that fatty acid uptake via its transporter CD36 induces tubule cell apoptosis via the activation of the p38 MAPK pathway [21]; Fig. 2 shows putative pathways of uptake of NEFA and how they might affect downstream signaling. In humans, renal CD36 expression is upregulated by hyperglycemia, potentially causing a double hit. Studies from the Stadler group indicated that increasing amounts of NEFA bound to albumin lead to defects in mitochondrial respiration and a peroxide-mediated apoptosis of tubular cells [22]. Purified and endotoxin-free albumin bound to palmitate and non-purified albumin products had similar effects on cultured tubule epithelial cells. These effects appear to be exclusive of generation of the toxic lipid ceramide, as the ceramide synthesis inhibitor myriocin had no effect on cell death, indicating that it is not a ceramide-mediated cell death. On the other hand, inhibiting mitochondrial fatty acid entry with etomoxir improved the condition, suggesting a role of mitochondrial beta-oxidation and lipid peroxidation. These studies suggest that free long-chain non-esterified saturated fatty acids added to cultured cells are toxic via activating several different cell death pathways.
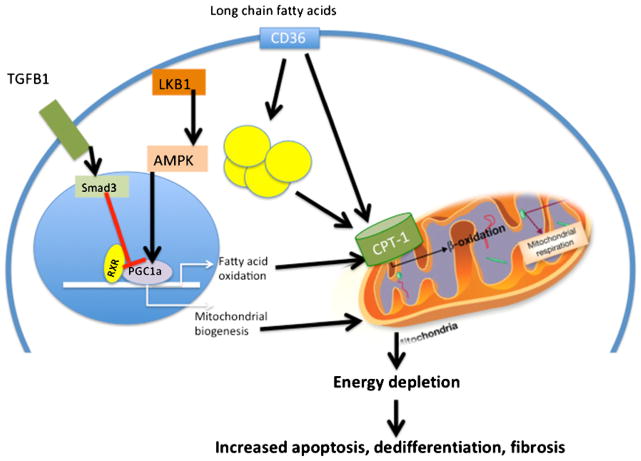
Fig. 2.
Decreased fatty acid oxidation contributes to CKD progression. Schematic model describing lipid abnormalities in renal tubule cells during fibrosis. Renal tubule cells take up long-chain fatty acids taken up via CD36. Fatty acids are then either stored in lipid droplets or oxidized in the mitochondria. The rate-limiting step in fatty acid oxidation is the uptake of fatty acids by mitochondria; this is regulated by CPT1. The RXR/PGC1a complex plays a critical role in transcriptional regulation of fatty acid oxidation and mitochondrial biogenesis. Smad3 and TGFB1 and the LKB1 and AMPK pathway play an important role in regulating the PGC1a/RXR complex. Defective fatty acid oxidation in CKD results in energy depletion and increased apoptosis and dedifferentiation and kidney fibrosis development. CD36 CD36 molecule (thrombospondin receptor); TGFB1 transforming growth factor, beta 1; RXR retinoid X receptor, alpha; PGC1A peroxisome proliferator-activated receptor gamma, coactivator 1 alpha; LKB1 liver kinase B1; AMPK AMPK-activated protein kinase; CPT1 carnitine palmitoyltransferase
Renal Lipid Synthesis
In addition to increased fatty acid uptake, increased renal lipid synthesis might be an important cause of renal lipid accumulation in DKD. Sterol regulatory element-binding proteins (SREBPs) are transcription factors that bind to the sterol regulatory element DNA sequence. Mammalian SREBPs are encoded by the genes SREBF1 and SREBF2. SREBPs regulate cholesterol biosynthesis and uptake and fatty acid biosynthesis. SREBP-1 and fatty acid synthase (FASN) expression are markedly increased in streptozotocin-induced diabetic rats [23, 24]. Cell culture studies showed that high glucose levels increased SREBP1 expression, which in turn led to increased triglyceride accumulation. Treatment of diabetic rats with insulin prevented the increased renal expression of SREBP-1 and the accumulation of triglyceride [4, 25]. These findings led the investigators to hypothesize that renal lipid accumulation is due to increased synthesis and is important for progression of DKD.
Fatty Acid Oxidation in DKD and CKD
Our group has performed genome-wide transcriptome analyses on samples from patients with CKD. We identified cases with hypertensive and diabetic CKD and controls who had diabetes and hypertension in absence of CKD [26••, 27, 28]. Ontology studies of the profiling data indicated that two gene groups showed reproducible and significant changes in these samples: inflammation and metabolism. Changes in metabolism-related genes included the rate-limiting enzymes in fatty acid oxidation and their regulators.
The rate-limiting step in fatty acid oxidation is the transfer of fatty acids into the mitochondria. This step requires linking fatty acid products to carnitine via carnitine palmitoyltransferase 1 (CPT1) and 2. Carnitine-o-acetyltransferase (CRAT) complexes excess acyl groups to carnitine, allowing them to exit the mitochondria [29•]. Renal proximal tubular cells express high levels of CPT1A, 1B, and 2 and CRAT (Fig. 1). While in the liver and skeletal muscle, CRAT can be both mitochondrial and peroxisomal, in the kidney, CRAT is almost exclusively mitochondrial [30]. Any compromise in CPT1 activity will likely have consequences in tubular cell fatty acid metabolism and oxidation. Deletion of CPT1B in the heart causes cardiac hypertrophy and death [31]. Inhibition of CPT1 in the liver leads to steatosis [32]. This suggests that CPT1 control of fatty acid oxidation has profound implications for the energetic balance of the cell. Too little CPT1 accompanied by decreases in other enzymes of fatty acid oxidation leads to energetic crisis.
PPAR Modulation
In addition to changes in CPT1, the rate-limiting enzyme in fatty acid metabolism, we found that in CKD multiple enzymes involved in fatty acid metabolism are affected. This is likely due to the fact that their transcriptional regulatory complex, PPARα and PGC1α, was also significantly reduced [33]. Profiles obtained from transgenic, toxic, and surgical mouse model of kidney fibrosis showed similar reduction in PPARα and PGC1α. In addition to the transcriptional changes, we also observed lipid accumulation analyzed by oil red O and by triglyceride content in all examined mouse models. Tubule epithelial cells in culture almost exclusively depend on fatty acids as their energy source [26••]. But fatty acid oxidation was decreased in fibrotic tubule samples. Blocking fatty acid oxidation in vitro with the use of etomoxir resulted in a similar increase in fatty acid accumulation. In summary, our results indicate that decreased fatty acid oxidation is an additional defect in lipid metabolism that likely also contributes to lipid accumulation.
Lipid Accumulation— Lipotoxicity Versus Lipid Overload
Does increased lipid accumulation contribute to kidney disease development or progression? According to the traditional lipotoxicity theory, accumulation of fatty acid metabolites such as diacyl-glycerols and ceramides in non-adipose organs leads to toxicity and cell death [2•]. Recently, a novel concept called lipid overload has also been proposed. Lipid overload describes the accumulation of non-toxic esterified lipids in lipid droplets [34]; excess lipid accumulation in this manner is thought to be benign.
Initially, lipotoxicity was thought to cause a slowdown of beta-oxidation, leading to accumulation of various lipid products. Contrary recent observations argue that beta-oxidation is accelerated, but is incomplete, due to oversupply leading to accumulation of toxic lipid intermediates. Most of these lipid substrates and incompletely oxidized products typically feed to mitochondrial complex II, fostering a reducing environment on the electron transport chain and a back pressure on complex I leading to excess superoxide production. This concept has been described in skeletal muscle [35••], but it is rather easy to envision it in the kidney as well.
To address the role of lipids in kidney disease development, we generated a new mouse model with tubule-specific over-expression of CD36, the long-chain FA transporter. Animals expressing CD36 presented with increased tubule-specific triglyceride and long-chain fatty acid content [26••]. However, we did not observe significant fibrosis development in this model even at 20 weeks of age. These animals also did not show increased susceptibility to kidney injury. Interestingly, expression of CPT1, the rate-limiting enzyme in fatty acid oxidation, increased in transgenic animals. These observations show similarities with recent studies in the skeletal muscle field. For example, marathon runners and athletes often have large lipid droplets in their muscle, but their fatty acid oxidation capacity is high, a phenomenon described as the Bathlete’s paradox.^ Our results indicate that lipid accumulation alone (lipid overload) in the presence of continued fatty acid oxidation capacity does not necessarily cause kidney dysfunction [36].
Energy Depletion in CKD
The kidney is a high-energy demand organ, which along with the heart has the greatest energy use per gram of tissue [37]. Therefore, it is logical that renal epithelial cells use fatty acids as their energy source at baseline. Several old studies indicate that about 60 % of the energy in the kidney comes from burning fatty acids [38]. Fatty acids hold up to four times more energy per mass than glucose. In addition, fatty acids can enter via diffusion while glucose uptake requires insulin. We found that the expression of enzymes and transcription factors needed for fatty acid metabolism is decreased in kidney fibrosis. Utilization of fatty acids was also decreased in cells cultured in vitro following profibrotic stimuli. Most organs, for example the heart, switch to glucose oxidation when fatty acid oxidation is decreased. We found that following injury, the decreased fatty acid oxidation was not associated with increased glucose metabolism resulting in energy depletion. The energy depletion then resulted in increased apoptosis and dedifferentiation of epithelial cells, a phenotype observed in CKD.
Next, we wanted to know whether lower fatty acid oxidation plays a role in fibrosis and CKD development. In renal tubule epithelial cells, just like in many other cell types, the PPARα/PGC1α pathway is one of the key transcriptional regulators of fatty acid utilization. We found that animals with transgenic expression of PGC1α in renal tubule cells showed significant protection from fibrosis development. Furthermore, we were also able to improve fatty acid oxidation with the use of fenofibrate, a PPARα agonist. Fenofibrate treatment not only improved fatty acid oxidation but also significantly reduced renal fibrosis development in two different models: folic acid precipitation and unilateral urethral obstruction. These findings are consistent with earlier studies showing fibrosis amelioration in tubule-specific PPARα transgenic animals.
Our recent studies identified several important upstream regulators of fatty acid utilization in renal epithelial cells. We found that liver kinase B (LKB1) is upstream of PPARα and PGC1α. Genetic deletion of Lkb1 in distal tubule epithelial cells (using the Kspcre/Lkb1flox/flox animals) resulted in not only decreased fatty acid oxidation but also development of severe kidney fibrosis at 27 weeks of age, indicating the key role of this pathway in fibrosis development (unpublished result, Drs. Susztak and Han). In summary, these studies indicate that defective fatty acid oxidation plays an important role in tubule degeneration and fibrosis development.
It is not fully clear whether the decreased fatty acid oxidation is already present in early DKD. Kidney biopsy samples from patients with DKD show a strong reduction in fatty acid oxidation-related enzymes and transcriptional regulators. In contrast, most mouse models of DKD do not develop progressive kidney fibrosis and do not show changes in PPARα and PGC1α levels. Recent microarray studies of the diabetic eNOS knockout animals, the only DKD model with significant renal function decline, highlighted decreased regulators of fatty acid metabolism. In addition, the diabetes picture is further complicated by the fact that circulating lipid levels are increased, which could favor increased passive lipid influx even at early stages of the disease. Whether these changes in lipid metabolism are causal or secondary can be tested using animal models.
Lipotoxicity and Lipid Peroxidation
We propose that energy depletion from lower FA oxidation is an important contributor of CKD development and/or progression. On the other hand, we cannot exclude the possibility that the decreased expression of fatty acid oxidation-related enzymes resulted in the generation of potentially harmful lipid intermediates. Some of these toxic intermediates are diacylglycerol, ceramides, and fatty acyl-CoAs and have been described in other organs. Diacylglycerol is generated by the conjugation of glycerol-3-phosphate with acetyl-CoA. Ceramides are made through breakdown of more complex sphingolipids. Our preliminary analysis did not show a significant change in ceramide levels in CKD.
Altered expression of CRAT could be an excellent potential candidate to generate some of these toxic intermediates. CRAT transports the short chain non-membrane permeable acyl-CoA products out of the mitochondria by linking them with carnitine and converting them to membrane permeable acetylcarnitines [29•]. The enzyme therefore could act as a Brelief valve^ to export excess lipids from the mitochondria; in its absence, incompletely oxidized fatty acids accumulate within the mitochondria. In addition, pyruvate oxidation is compromised and a reducing environment conductive to superoxide formation created. Conditional deletion of CRAT in animal models will be essential to test this specific hypothesis.
Lipid peroxidation is another potentially important contributor of tubule epithelial damage. While increased reactive oxygen generation has been shown to play a role in the development of DKD, little is known about lipid peroxidation. Lipid peroxidation requires potent hydroxyl radical for forming lipid radicals (LOO•). Lipid peroxidation can be terminated by annihilation, when lipid radicals react with one another or with protein radicals. After a series of chain reactions, reactive lipid-end products (LOOH) start to accumulate. These lipid peroxidation end products, such as 4-hydroxynonenal and isoprostanes, are known biomarkers in human diabetes, but their origin and role in renal physiology and pathology are not well known. Pathologically high concentrations of lipid peroxidation products (4-HNE, F-isoprostanes) are often correlated with the development of cardiovascular disease and diabetes [39–41]. A specific subclass of reactive lipids (electrophile lipids) can be important cell signaling molecules; this raises the possibility that not all lipid peroxides are toxic, especially at lower levels [42•].
Reactive lipids are electrophilic in nature, and therefore, they can react with nucleophilic residues on proteins. Reaction with lysine and histidine are often non-specific or toxic [43]. On the other hand, signaling functions are usually related to their reaction with cysteine residues; Cys residues contain thiol groups that act as redox-sensitive switches in signal transduction [44]. This concept could open up new avenues of research in DKD. First of all, it will be essential in the future to carefully catalogue lipid intermediates, including reactive lipid products in healthy and diseased kidney samples. Next, we will need test whether there is a qualitative or quantitative defect in lipid intermediates in CKD.
Clinical Implications
There are two issues to consider when deciding whether to treat lipid metabolism defects in patients with CKD and DKD. Will treatment of systemic lipid abnormalities prevent or improve renal function? Does correction of circulating lipid abnormalities reduce risk of cardiovascular disease? The association between serum lipid levels and kidney disease development has been extensively studied. The most common abnormality is increased serum triglyceride and HDL levels. Total cholesterol levels can vary quite significantly. The Action in Diabetes and Vascular Disease (ADVANCE) study in T2DM showed that low concentrations of HDL cholesterol are associated with a significantly increased risk of microalbuminuria and macroalbuminuria [45]. In type 1 diabetes mellitus (T1DM) altered lipid profiles were observed in participants of the Diabetes Control and Complications Trial (DCCT) who progressed to diabetic nephropathy [46]. In the Finnish Diabetic Nephropathy Study (FinnDiane) cohort of patients with T1DM, increased levels of triglycerides were associated with progressive albuminuria, and increased total cholesterol concentrations were associated with progression to renal failure. However, there are limited data on the effects of treatment in such patients as most trials of triglyceride-reducing agents did not specifically target patients with hypertriglyceridemia (triglyceride levels >200 mg/dl).
Results on cholesterol reduction in CKD using statins are relatively controversial. The Study of Heart and Renal Protection (SHARP) trial indicated significant reduced cardiovascular disease in patients treated with simvastatin plus ezetimibe compared to placebo [47]. Such benefit cannot be demonstrated for end-stage patients on dialysis. Statins did not alter renal function decline for diabetic kidney disease patients. Several recent studies indicated that we do not fully understand lipid abnormalities in end-stage renal disease. Current results have been analyzed by the Kidney Disease| Improving Global Outcomes (KDIGO). The group recommends statin treatment for patients with CKD who are older than 50 years of age and suggests use of statins for patients who are younger than 50. On the other hand, they suggest that statin treatment should not be initiated for patients who are already on dialysis. These recommendations are almost exclusively based on cardiovascular risk analysis.
Mechanistic studies using mouse and cell models indicate that agonists of PPARα and PPARγ could reverse defects in fatty acid oxidation and could ameliorate kidney disease progression [48]. PPARα agonists effectively reduce serum triglyceride levels. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study demonstrated that fenofibrate reduced albuminuria in patients with diabetes [49]. Unfortunately, fibrates increase serum creatinine levels, most likely due to an interference with creatinine excretion. In most patients, serum creatinine levels return to normal after discontinuation of fibrates. Fibrates also decreased albuminuria in the larger Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [50•, 51]. The post hoc analysis of ACCORD indicates a small but significant benefit for reduction for GFR decline for patients in the fenofibrate arm compared to placebo. Interestingly, this effect was independent of serum triglyceride levels, potentially indicating the benefit of intrarenal targeting of the PPAR system. Future studies with more specific compounds shall determine the role of serum lipid and lipid metabolic alterations in development and progression of CKD.
Conclusion
Cellular metabolism of renal cells is an underappreciated research area. Fatty acids are important for the kidney as they represent their baseline energy source. In CKD and DKD, there are several alterations in systemic and intrarenal lipid metabolism; lipids accumulate within the tubules. Recent reports indicate that changes in tubule fatty acid oxidation may alter progression. Further research is needed to understand the contribution of systemic and intrarenal lipid abnormalities to DKD development.
Acknowledgments
Research in the Stadler and Susztak laboratories are supported by DiaComp Pilot and Feasibility grants (14GHSU1393, through Georgia Regents University/NIH) and the Pennington Foundation (K.St), NIH (K.Su) DK076077, DK087635 and HL45095, HL073029, and DK095684 (IJG).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Krisztian Stadler, Ira J. Goldberg, and Katalin Susztak declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors
This article is part of the Topical Collection on Microvascular Complications— Nephropathy
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Aon MA, Bhatt N, Cortassa SC. Mitochondrial and cellular mechanisms for managing lipid excess. Front Physiol. 2014;5:282. doi: 10.3389/fphys.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Goldberg IJ, Trent CM, Schulze PC. Lipid metabolism and toxicity in the heart. Cell Metab. 2012;15(6):805–12. doi: 10.1016/j.cmet.2012.04.006. This paper provides a thorough review of fatty acid metabolism in the heart and highlights diabetic or obese conditions, alterations in lipid metabolism and possible treatments to alleviate lipid-related pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1(1):8–15. doi: 10.1161/JAHA.111.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang T, et al. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280(37):32317–25. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 5•.Wang W, et al. Deletion of scavenger receptor A protects mice from progressive nephropathy independent of lipid control during diet-induced hyperlipidemia. Kidney Int. 2012;81(10):1002–14. doi: 10.1038/ki.2011.457. This study highlights the important role of a transmembrane receptor in hyperlipidemic kidney and tubular cell injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70(9):1560–6. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 7.Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Pathol. 1936;12(1):83–98. [PMC free article] [PubMed] [Google Scholar]
- 8.Chajek T, Stein O, Stein Y. Pre- and post-natal development of lipoprotein lipase and hepatic triglyceride hydrolase activity in rat tissues. Atherosclerosis. 1977;26:549–61. doi: 10.1016/0021-9150(77)90122-8. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg IJ, et al. Localization of lipoprotein lipase mRNA in selected rat tissues. J Lipid Res. 1989;30(10):1569–77. [PubMed] [Google Scholar]
- 10.Coburn CT, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275(42):32523–9. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 11.Bobulescu IA, Dubree M, Zhang J, McLeroy P, Moe OW. Effect of renal lipid accumulation on proximal tubule Na+/H+ exchange and ammonium secretion. Am J Physiol Renal Physiol. 2008;294(6):F1315–22. doi: 10.1152/ajprenal.00550.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. 2010;19(4):393–402. doi: 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg HN. Diabetic dyslipidemia: basic mechanisms underlying the common hypertriglyceridemia and low HDL cholesterol levels. Diabetes. 1996;45 (Suppl 3):S27–30. doi: 10.2337/diab.45.3.s27. [DOI] [PubMed] [Google Scholar]
- 14.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312(5774):734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 15.Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989;320(16):1060–8. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- 16.Taskinen MR, et al. Dual metabolic defects are required to produce hypertriglyceridemia in obese subjects. Arterioscler Thromb Vasc Biol. 2011;31(9):2144–50. doi: 10.1161/ATVBAHA.111.224808. [DOI] [PubMed] [Google Scholar]
- 17.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290(2):F262–72. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan V, Vaid M. Dyslipidemia in chronic kidney disease: managing a high-risk combination. Postgrad Med. 2009;121(6):54–61. doi: 10.3810/pgm.2009.11.2077. [DOI] [PubMed] [Google Scholar]
- 19.Arici M, et al. Fatty acids carried on albumin modulate proximal tubular cell fibronectin production: a role for protein kinase C. Nephrol Dial Transplant. 2002;17(10):1751–7. doi: 10.1093/ndt/17.10.1751. [DOI] [PubMed] [Google Scholar]
- 20.Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol. 2002;283(4):F640–7. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- 21.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2(2):e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggiero C, et al. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am J Physiol Renal Physiol. 2014;306(8):F896–906. doi: 10.1152/ajprenal.00484.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277(21):18919–27. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54(8):2328–35. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 25.Jiang T, Liebman SE, Lucia MS, Li J, Levi M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005;68(6):2608–20. doi: 10.1111/j.1523-1755.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 26••.Kang HM, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21(1):37–46. doi: 10.1038/nm.3762. This novel study provides evidence for the first time that it is not necessarily the increase in fatty acid levels but its defective oxidation that may play a key role in kidney fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ledo N, et al. Functional genomic annotation of genetic risk loci highlights inflammation and epithelial biology networks in CKD. J Am Soc Nephrol. 2015;26(3):692–714. doi: 10.1681/ASN.2014010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–40. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Muoio DM, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764–77. doi: 10.1016/j.cmet.2012.04.005. This study provides key evidence for the lipid overload theory in skeletal muscle in contrast to the lipotoxicity theory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973;248(10):3426–32. [PubMed] [Google Scholar]
- 31.Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab. 2014;16(8):757–60. doi: 10.1111/dom.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vickers AE. Characterization of hepatic mitochondrial injury induced by fatty acid oxidation inhibitors. Toxicol Pathol. 2009;37(1):78–88. doi: 10.1177/0192623308329285. [DOI] [PubMed] [Google Scholar]
- 33.Son NH, et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120(10):3443–54. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284(52):36312–23. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15(5):595–605. doi: 10.1016/j.cmet.2012.04.010. This paper discusses mitochondrial lipid overload and its potential role in insulin sensitivity and emphasizes the advances of this hypothesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, et al. Resting energy expenditure-fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metab. 2000;279(3):E539–45. doi: 10.1152/ajpendo.2000.279.3.E539. [DOI] [PubMed] [Google Scholar]
- 38.Nieth H, Schollmeyer P. Substrate-utilization of the human kidney. Nature. 1966;209(5029):1244–5. doi: 10.1038/2091244a0. [DOI] [PubMed] [Google Scholar]
- 39.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomark Biochem Indic Expo Response Susceptibility Chem. 2005;10(1):S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 40.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25(2):279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 41.Stadler K, et al. Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic Biol Med. 2008;45(6):866–74. doi: 10.1016/j.freeradbiomed.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442(3):453–64. doi: 10.1042/BJ20111752. This study highlights that reactive lipids are not only deleterious, but they play major roles in signaling pathways as well due to their reactive, electrophile nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper CE, Patel RP, Brookes PS, Darley-Usmar VM. Nanotransducers in cellular redox signaling: modification of thiols by reactive oxygen and nitrogen species. Trends Biochem Sci. 2002;27(10):489–92. doi: 10.1016/s0968-0004(02)02191-6. [DOI] [PubMed] [Google Scholar]
- 44.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2(90 re7) doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton J, et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care. 2012;35(11):2201–6. doi: 10.2337/dc12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anonymous. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 47.Baigent C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Portilla D, Mandel LJ, Bar-Sagi D, Millington DS. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 1992;262(3 Pt 2):F354–60. doi: 10.1152/ajprenal.1992.262.3.F354. [DOI] [PubMed] [Google Scholar]
- 49.Davis TM, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54(2):280–90. doi: 10.1007/s00125-010-1951-1. [DOI] [PubMed] [Google Scholar]
- 50•.Ting RD, et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: the FIELD Study. Diabetes Care. 2012;35(2):218–25. doi: 10.2337/dc11-1109. The studies described in this paper collectively summarize the advantages and potential disadvantages of fenofibrate therapy in diabetic kidney disease patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kostapanos MS, Florentin M, Elisaf MS. Fenofibrate and the kidney: an overview. Eur J Clin Investig. 2013;43(5):522–31. doi: 10.1111/eci.12068. [DOI] [PubMed] [Google Scholar]