INTRODUCTION
Obesity is associated with an elevated risk of chronic diseases, including diabetes and cardiovascular disease. Accumulation of lipotoxic metabolites in nonadipocyte tissues may be a major cause of dilated cardiomyopathy in patients with obesity or diabetes. Recent studies in humans have related cardiac lipid content with disturbance of cardiac function. Moreover, ectopic disposition of lipids alters substrate metabolism in these tissues and contributes to the development of obesity-mediated diseases.
The heart uses lipids for energy and for cellular structures. Fatty acids supply energy for cardiac muscle contraction and are required substrates for the synthesis of several esters, including phospholipids and sphingolipids. Patients with diabetes and obesity have elevated circulating plasma free fatty acids (FFA) levels. This state might drive greater heart FFA uptake; when uptake exceeds the requirements for energy production, these FFA accumulate as lipid metabolites and neutral lipids. Lipotoxicity is the term used when lipid overload leads to cellular dysfunction and cell death–mediated organ dysfunction.1 Because neutral lipids and other lipid metabolites accumulate in the cardiomyocytes of nonischemic failing hearts,1,2 a hypothesis that lipotoxicity contributes to the development of cardiac dysfunction is gaining credibility.3 In addition, ischemia has been reported to increase heart levels of some lipids.4,5
Of the complex bioactive lipids, ceramide and its metabolites draw particular attention. Studies in animal models of diabetes, obesity, and dilated cardiomyopathy demonstrated that the inhibition of sphingolipid production prevents or delays the onset of the diseases.6,7 In addition, the activation of ceramidase by adiponectin was associated with reduced cardiac injury after apoptosis.5 Thus, there is experimental evidence that ceramide is one of the lipotoxic lipids.
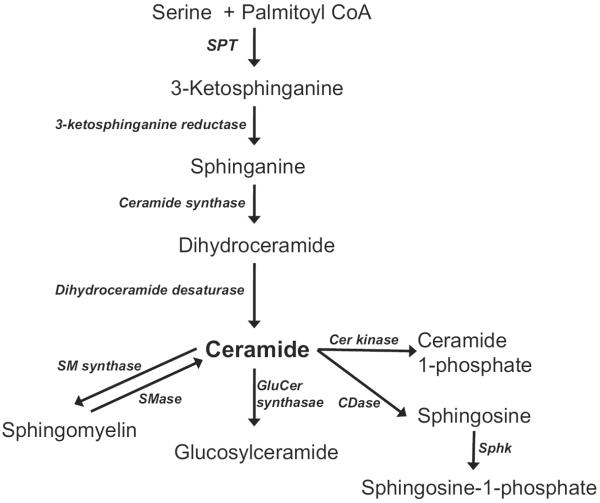
Ceramide is a major molecule in sphingolipid metabolism and has been studied extensively. In addition to its structural role in plasma membranes and lipoproteins, ceramide and its metabolites have profound effects on cellular signaling, such as apoptosis and insulin response.8 Ceramide is also a precursor for other bioactive sphingolipid metabolites, such as ceramide 1-phosphate, sphingosine, and sphingosine 1-phosphate (S1P) (Fig. 1). These sphingolipids regulate cellular processes, including cell proliferation, differentiation, and apoptosis.9 In this review, the authors intend to describe the role of sphingolipids in cardiovascular pathophysiology as a regulator of cardiac energy metabolism and signaling. Understanding the role of sphingolipids in cardiovascular events may lead to strategies to correct the abnormal signaling pathways in failing hearts.
Fig. 1.
Sphingolipid biosynthetic pathway and metabolism. CDase, ceramidase; Cer, ceramide; CoA, coenzyme A; GlucCer, glucosylceramide; SM, sphingomyelin; SMase, sphingomyelinase; Sphk, sphingosine kinase; SPT, serine palmitoyltransferase.
FAILING HEARTS HAVE ABNORMAL LIPID METABOLISM
The heart requires several forms of lipids, including cholesterol and fatty acids. Most circulating plasma fatty acids are esterified and are incorporated into lipoprotein, phospholipids, and triglycerides. Fatty acids supply energy for heart contraction and are used for structural lipids in cells. Tissue uptake of whole lipoproteins by specific receptors leads to intracellular degradation of phospholipids and triglycerides to varying degrees. FFA uptake caused by either albumin-associated FFA or FFA liberated from lipoproteins supplies cardiac energy substrates. Loss of either lipoprotein lipase (LpL)10 or the putative FFA transporter CD3611 in the heart increases cardiac uptake of glucose, suggesting that the heart is fatty acid deprived and needs an alternative source of energy.
In normal adult hearts, ATP is produced via the oxidation of fatty acids, glucose, lactate, and ketone bodies, with glucose and fatty acids being the principal substrates.12 Of these substrates, fatty acids are the major fuel supplying 70% of the ATP for normal cardiac function.13–15 During diabetes, the heart seems to oxidize more fatty acid and less glucose.16 Heart LpL activity is increased, presumably to allow greater lipoprotein-derived fatty acid uptake.16,17 One well-studied model is the isolated working heart of obese db/db mice. These hearts have decreased glucose oxidation rates and increased fatty acid oxidation.18 Although fatty acid oxidation has not been studied in detail, diet-induced obesity rapidly leads to cardiac insulin resistance.19
Diabetic cardiomyopathy is a syndrome in which heart failure is not caused by ischemic heart disease or hypertension.20 The activity of peroxisome proliferator-activated receptor (PPAR) α, a major regulator of fatty acid oxidation in the heart, is increased in diabetic hearts. PPARα overexpression in mice leads to cardiac dysfunction.21 This cardiomyopathy could result from excess fatty acid oxidation, which is postulated to increase reactive oxygen production, or from accumulation of intracellular lipids, which also occurs in this model. Mice with cardiomyocyte PPARα transgenic expression have enhanced activity of enzymes that catalyze the synthesis of triglycerides and, like diabetic hearts, have greater intracellular triglyceride stores.22,23 Hydrolysis of this triglyceride supplies fatty acids to the diabetic hearts.17 In moderately obese individuals, triglyceride accumulation is detected in the myocardium.24
Whether greater reliance on fatty acids is harmful or is an adaptive change is debated. In the setting of ischemia, more specifically hypoxia, the creation of more ATP using less oxygen can occur by the use of glucose and has been proposed as a beneficial therapeutic strategy.25 Whether this strategy is applicable to nonischemic disease is unclear, and some in vivo studies in animal models fail to support a role for reducing fatty acid oxidation in heart failure. Diets with greater amounts of fat and less carbohydrate lead to better function in some failure models.26–29
Another option for the cause of heart failure in diabetic or obese conditions is that toxicity is caused by an alteration of lipid metabolism leading to lipotoxic events. Sphingolipids might be one such lipid.
ELEVATED CERAMIDE CORRELATES WITH LIPOTOXIC CARDIOMYOPATHY
The accumulation of neutral lipids in or around the myocardium is observed in humans or animal models with nonischemic heart failure,1,2,22,23 and it is hypothesized that excess lipid contributes to the development of cardiac dysfunction.3,30,31 Several transgenic animal models of lipotoxic cardiomyopathy have been created to support this notion. When hearts internalize excess lipids or have a defect in lipid oxidation, lipid storage is increased. Cardiomyocyte transgenic expression of long-chain acyl coenzyme A synthetase 1 (myosin heavy chain [MHC]-ACS1),32 fatty acid transport protein 1 (MHC-FATP1),33 and the cell membrane anchored form of LpL (LpLGPI)34 are characterized by elevated myocardial fatty acid uptake and lipid deposition and cardiomyopathy.
How does myocardial lipid accumulation lead to cardiomyopathy? Neutral droplets or fatty acids could produce direct toxic effects on myofibrillar function.35 Fatty acids can induce apoptosis36 in response to increased levels of reactive oxygen species generated as a toxic by-product of lipid oxidation35 and activation of protein kinase C signaling pathway.37 Fatty acids also induce the synthesis of ceramides, which mediate several toxic processes.6 Although it is still unclear which lipid metabolites directly cause cardiomyopathy, in several models, ceramide accumulation in the heart correlates with these pathophysiological events and heart dysfunction (Table 1).6,32,38 When the balance between fatty acid uptake and oxidation is altered, excess fatty acids are directed toward the synthesis of complex lipids, including triglycerides and phospholipids. An alternative route to use fatty acid surplus is via the ceramide biosynthetic pathway. Increased myocardial ceramide levels are associated with diastolic dysfunction in Akita Ins2 (WT/C96Y) mice and Zucker diabetic fatty (ZDF) rats.39 To support this notion of ceramide toxicity, improvement in cardiac dysfunction by pharmacologic and genetic intervention in ZDF rats, Akita Ins2 (WT/C96Y), and MHC-ACS1 mice was accompanied by the reduction of cardiac ceramide.28,36,39 The previously mentioned results suggest that myocardial ceramide levels correlate with the development of cardiac dysfunction found in obese and diabetic hearts.
Table 1.
Cardiac ceramide, cardiomyocytes apoptosis, and cardiac function in animal models of lipotoxic cardiomyopathy, diabetes, and obesity
| Animal Models | References | Cardiac Ceramide Changes | Cardiac Function |
|---|---|---|---|
| MHC-ACS1 mice | Chiu et al32 | Increased | Decreased |
| LpLGPI mice | Yagyu et al34 | Increased | Decreased |
| MHC-PPARγ mice | Son et al38 | Increased | Decreased |
| Akita Ins2 (WT/C96Y) mice | Basu et al39 | Increased | Decreased |
| Zucker diabetic fatty rats | Zhou et al36 | Increased | Decreased |
| ob/ob mice | Torre-Villalvazo et al44 | Increased | No change |
| Rats fed a high-fat diet | Torre-Villalvazo et al44 | Increased | ND |
Abbreviations: LpLGPI, cardiomyocyte-specific overexpression of glycosylphosphatidylinositol-anchored lipoprotein lipase; MHC-ACS1, cardiomyocyte-specific overexpression of long chain acyl coenzyme A synthetase 1; MHC-PPARγ, cardiomyocyte-specific overexpression of peroxisome proliferator-activated receptor γ; ND, not determined.
Methods to reduce ceramide synthesis include the reduction of fatty acid uptake by the heart and conversion of fatty acids to nontoxic triglyceride. Several intervention studies to reduce circulating fatty acids improved cardiac function of lipotoxic animals and reduced cardiac ceramide. These interventions include the administration of the PPARγ agonist troglitazone in ZDF rats, insulin treatment of Akita Ins2 (WT/C96Y) mice, and the overexpression of diacylglycerol acyltransferase 1 in MHC-ACS1 mice.28,36,39
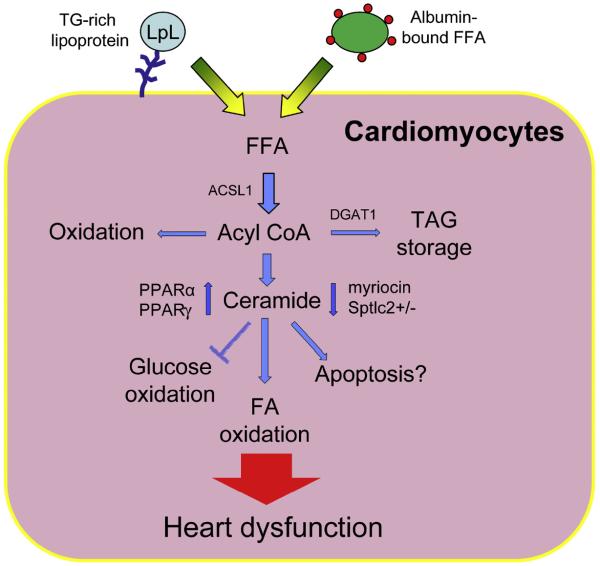
To find a direct connection between ceramide and lipotoxic cardiomyopathy, the authors studied the involvement of ceramide in the development of lipotoxic cardiomyopathy. LpLGPI mice also have increased cardiac ceramide and apoptosis markers, including cytosolic cytochrome c and caspase 3 expression and activity.40 The authors demonstrated that the inhibition of ceramide biosynthesis by myriocin or heterozygous deletion of Sptlc1, a serine palmitoyltransferase (SPT) subunit, decreased the expression of some apoptotic genes and improved cardiac contraction in LpLGPI (Fig. 2).6 In this study, blockage of ceramide biosynthesis seems to modulate mitochondrial substrate oxidation. LpLGPI hearts have increased uptake of FFA and rely on fatty acid oxidation for cardiac energy production. A potential mechanism for the improvement with myriocin is that pharmacologic and genetic inhibition of SPT upregulated pyruvate dehydrogenase kinase-4 and decreased the rate of glucose oxidation but led to greater fatty acid (FA) oxidation. However, glucose uptake was increased in LpLGPI hearts. This paradoxic fate of glucose is explained by the accumulation of glucose as glycogen with increased phosphorylated glycogen synthase kinase 3β.6 In isolated perfused LpLGPI hearts, myriocin restored cardiac efficiency, enhancing myocardial energetics by maintaining cardiac performance at a lower oxygen cost. Even with improved cardiac function and balanced substrate use by myriocin treatment, a long-term treatment of LpLGPI mice with myriocin only partially rescued the survival rate. A potential reason is the involvement of other lipid metabolites in cardiac dysfunction. Other probable candidates for cardiac failure are diacylglycerol, which alters protein kinase C (PKC) signaling, and FFA. More studies are needed to distinguish the role of ceramide from other lipid metabolites.
Fig. 2.
Lipotoxicity is created by an imbalanced substrate oxidation in heart. Fatty acids are taken up by heart via hydrolysis of triglyceride within lipoproteins by LpL action or transport of albumin-bound free fatty acids. In cardiomyocytes, the free fatty acids are esterified to coenzyme A (CoA) and used for energy or stored as lipid droplets. When lipid uptake exceeds oxidation, more acyl CoAs are shunted to ceramide biosynthesis. Accumulation of ceramide alters the balance of glucose/fatty acid oxidation and leads to cardiac dysfunction. Agonism of PPARα or γ elevates cardiac ceramide levels and leads to cardiac dysfunction. In contrast, myriocin and heterozygous deletion of Sptlc2 prevent cardiac dysfunction. FA, fatty acid; TAG, triacylglycerol; TG, triglyceride.
CERAMIDE-MEDIATED APOPTOSIS OF CARDIOMYOCYTES
Lipotoxic cardiomyopathy is also associated with the loss of cardiomyocytes via apoptosis.41,42 Ceramide is a proapoptotic second messenger that activates several signaling pathways, including PKC, protein phosphatase 1 or 2A, and cathepsin D.43 These signaling pathways are involved in proapoptotic events, including the suppression of Bcl2, the dephosphorylation of protein kinase B (AKT), and the activation of caspases.43 The accumulation of ceramide was reported to be accompanied by cardiomyocyte apoptosis, and pharmacologic inhibition of ceramide biosynthesis reduced cardiomyocyte apoptosis in ZDF rats and MHC-ACS1 mice.28,36 However, a recent report demonstrated that the myocardium of ob/ob mice and rats fed a high saturated-fat diet did not show increased cardiomyocyte apoptosis even with elevation of ceramide.44 These conflicting data suggest that the elevation of cardiac ceramide does not always lead to the activation of apoptosis.
The notion that cardiac dysfunction of LpLGPI hearts results from its dysregulation of substrate use and not from apoptotic loss of cardiomyocytes suggests that ceramide accumulation does not necessarily accompany apoptosis. The incubation of human cardiomyocyte AC16 cells with C6-ceramide downregulated glucose transporter 4 and upregulated pyruvate dehydrogenase kinase 4 gene expression.6 These changes in metabolic genes were consistent with what was found in LpLGPI mice that has elevated ceramide levels in hearts. These findings also suggest that ceramide modulates cardiac energy metabolism via transcriptional regulation of metabolic genes rather than apoptosis.
PPARs REGULATE CARDIAC SPHINGOLIPID METABOLISM
PPAR transcription factors regulate the oxidation of FA and play an important role in the regulation of substrate metabolism in hearts. There are 3 distinct PPAR isoforms: α, β, and γ. Of these isoforms, PPARα and γ are highly expressed in hearts and thought to regulate FA metabolism in cardiomyocytes.45 High fat feeding of cardiac PPARα transgenic mice accelerated the development of cardiomyopathy and was associated with excess FA oxidation and accumulation of ceramide in hearts.46,47 These effects were not observed in wild-type mice and suggest that PPARα is involved in the regulation of ceramide metabolism in hearts. Baranowski and colleagues48,49 demonstrated that activation of PPARα by WY-14643, a PPARα agonist, causes ceramide and sphingomyelin accumulation in the myocardium of high fat–fed rats. This result was caused by the activation of de novo sphingolipid synthesis via elevated SPT activity and increased availability of intracellular palmitate, a substrate of SPT. However, it is unclear whether PPARα regulates SPT expression directly or indirectly by elevating FFA pools. Because PPARα agonist activity did not increase myocardial ceramide levels or SPT activity in regular chow-fed rats, both changes in enzymes and substrates (ie, the high-fat diet) are needed to alter de novo ceramide biosynthesis.48 Alternative pathways for ceramide generation, such as sphingomyelinase and ceramidase, were not affected by PPARα activation.
The treatment of patients with diabetes with thiazolidinediones, selective PPARγ activators, increases heart failure risk.50 These clinical observations could have resulted from either greater salt or water retention, despite reduced blood pressure and vasodilation, or direct effects of PPARγ agonists on heart metabolism. In support of this latter hypothesis, Son and colleagues38 reported that cardiac transgenic expression of PPARγ led to cardiac dysfunction associated with the induction of FA oxidation genes, the accumulation of glycogen and lipids in mouse myocardium, and the disruption of mitochondrial structure. Cardiac ceramide levels were also elevated modestly. The effects of pharmacologic PPARγ agonists on heart metabolism and function in animal models are mixed. These drugs induce glucose transporters 1 and 4 and increase glucose uptake in cultured rat cardiomyocytes and in the heart of diabetic animal models.51–54 In ZDF rats, the administration of thiazolidinedione reduced cardiac accumulation of ceramide.36 Similarly, PPARγ agonist treatment of LpLGPI mice reduced heart dysfunction and, in this model, was shown to divert circulating lipids to greater adipose and reduced heart uptake.55 So the use of agonists in vivo is likely to reflect the level of cardiac PPARγ expression and the importance of the induction of PPARγ in adipose.
Another possible action of PPARγ agonists is the induction of ceramide synthesis. In one study, the administration of PPARγ agonists elevated SPT activity and intracellular levels of palmitate, whereas the activation of PPARγ did not change the activities of sphingomyelinase and ceramidase.56 Thus, the accumulation of cardiac ceramide is via the activation of de novo ceramide biosynthesis. A modest increase in the expression of SPT protein or mRNA did not match the elevated activity, suggesting SPT activity is regulated by posttranscriptional modification. It has been widely accepted that the increased availability of palmitate, a substrate of SPT reaction, increases SPT activity and expression.57,58 Holland and colleagues59 found that palmitate activates a toll-like receptor pathway and increases intracellular levels of ceramide by activating de novo ceramide synthesis. These findings indicate that palmitate is not only acting as a substrate for SPT-mediated de novo ceramide synthesis but acting as an activator of the rate-limiting enzyme in this biosynthetic pathway. Collectively, PPARs regulate myocardial sphingolipid metabolism mainly via de novo synthesis (see Fig. 2).
CARDIOPROTECTIVE EFFECTS OF S1P
S1P might protect the heart from ischemiareperfusion injury. S1P is synthesized intracellularly and exerts its function by binding to specific plasma membrane G-protein coupled receptors (S1P1~5). Intracellular S1P has a proliferative role in cells and is also secreted to the extracellular space (insideout hypothesis). Secreted S1P binds to the S1P receptors on plasma membrane and elicits its regulatory function. When S1P binds to the S1P receptors, phosphatidylinositol 4-kinase is activated and its downstream targets, AKT and glycogen synthase kinase 3β, are phosphorylated and activate these signaling pathways. Of the 5 subtypes of the S1P receptors, cardiomyocytes express S1P1, S1P2, and S1P3.60 The incubation of rat neonatal cardiomyocytes with S1P or GM1, a ganglioside that induces sphingosine kinase 1 and elevates endogenous S1P production, prevents hypoxia-induced cell death.61 Cardioprotection by S1P and GM1 during ischemia/reperfusion injury was confirmed in vivo.62 The infusion of GM1 reduces cardiac injury through PKCε but S1P exerts cardioprotective effects through the PKCε-independent pathway. Later, it was found that the inactivation of the interaction of G protein and G protein coupled receptor by pertussis toxin or S1P1–3 antagonist removed GM-1 mediated cardioprotection.63 These findings suggest that endogenous S1P is transported from cardiomyocytes and exerts its cardioprotective effects by binding to S1P receptors on the membrane surface. Consistent with these findings, ischemia suppressed sphingosine kinase activity and reduced S1P levels in the heart; these effects were maintained during reperfusion.64 Sphk1-deficient hearts were susceptible to ischemia/reperfusion injury, and adenoviral Sphk1 gene transfer induced cardioprotection and prevented ischemic heart failure.65 Although S1P is one of the major lipid components in high-density lipoprotein (HDL), it has been reported that S1P action is independent of HDL.66
Of the S1P receptors, S1P1 may be most important for cardioprotection. S1P1-specific agonists protected adult mouse cardiomyocytes from hypoxia.67 In contrast, VPC23019 and FTY720, the synthetic antagonists of S1P1, prevented cardioprotection elicited by S1P. However, other groups suggested that S1P2 and S1P3 also exert S1P-mediated cardioprotective action. S1P2/3 double knockout mice have increased myocardial infarct size during ischemia/reperfusion injury,68 suggesting the overlapping role of S1P receptor isoforms. In addition, S1P3 deficiency abolished S1P-mediated cardioprotection, and the pharmacologic inhibition of nitric oxide synthase also caused the disappearance of cardioprotective effects, suggesting an important role of this pathway.69 Recently, it was reported that cardiac-specific S1P1-deficient mice are vulnerable to ischemia/reperfusion injury to the same degree as the wild-type mice.70 These conflicting data may result from the experimental model systems: S1P1 in cardiomyocytes and S1P2/3 in animal hearts. Therefore, the roles of S1P in cardioprotection of nonischemic heart failure deserve further study.
CLINICAL IMPLICATION OF SPHINGOLIPID METABOLISM IN HEART FAILURE
Animal experiments suggest that ceramide is implicated in pathogenesis of cardiac dysfunction associated with obesity and diabetes. However, whether ceramide is relevant to cardiac failure in humans is unclear. Barranowski and colleagues71 found that the enzymes in sphingolipid biosynthesis were upregulated in the right atrial appendage of overweight patients; the tissue was obtained during coronary bypass graft surgery. These genes include Sptlc1/2, Sphk1, alkaline/acid/neutral ceramidases, and neutral ceramidases. When diabetes was present in the obese patients, the expression of some genes was reduced but higher than lean subjects. They also found increased DNA fragmentation in the hearts of obese nondiabetic patients and it was increased further in obese diabetic hearts. Surprisingly, the elevation of cardiac ceramide was not found. The reason for these conflicting data is likely to be coordinated regulation of ceramide synthesis and degradation. These findings suggested that obesity and type 2 diabetes do not induce ceramide accumulation in the human heart or at least in the atrium.
SUMMARY
All tissues, including the heart, need essential lipids. With obesity and diabetes, hearts are likely to have metabolic imbalance and lipid accumulation. A flurry of recent investigations using animal models suggests that ceramide plays important roles in the pathogenesis of heart failure. In contrast, S1P is implicated in cardioprotection during ischemia/reperfusion injury. Further studies will need to first define the lipid abnormalities that occur in human hearts at various stages of failure, and the associated gene/enzyme alterations associated with heart failure from a variety of causes must be determined. Only then can a reasonable plan be devised to alter sphingolipid metabolism as a method to prevent or treat patients.
KEY POINTS.
Sphingolipids, elevated in obesity and type 2 diabetes, may cause cardiomyopathy.
Ceramide alters cardiac energy metabolism and can cause cardiomyocyte apoptosis.
Sphingosine 1-phosphate protects against ischemia/reperfusion injury.
Modulation of sphingolipid metabolism in the heart may become a therapy for cardiac disease in patients with obesity and diabetes.
Acknowledgments
There is no applicable funding support.
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep. 2005;7:412–7. doi: 10.1007/s11906-005-0035-y. [DOI] [PubMed] [Google Scholar]
- 2.Harmancey R, Wilson CR, Taegtmeyer H. Adaptation and maladaptation of the heart in obesity. Hypertension. 2008;52:181–7. doi: 10.1161/HYPERTENSIONAHA.108.110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Perman JC, Bostrom P, Lindbom M, et al. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J Clin Invest. 2011;121:2625–40. doi: 10.1172/JCI43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park TS, Hu Y, Noh HL, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. Journal of lipid research. 2008;49:2101–12. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Guenther GG, Edinger AL. A new take on ceramide: starving cells by cutting off the nutrient supply. Cell Cycle. 2009;8:1122–6. doi: 10.4161/cc.8.8.8161. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Yu Y, Sun S, et al. Ceramide and other sphingolipids in cellular responses. Cell Biochem Biophys. 2004;40:323–50. doi: 10.1385/CBB:40:3:323. [DOI] [PubMed] [Google Scholar]
- 10.Augustus AS, Buchanan J, Park TS, et al. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281:8716–23. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- 11.Hajri T, Ibrahimi A, Coburn CT, et al. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem. 2001;276:23661–6. doi: 10.1074/jbc.M100942200. [DOI] [PubMed] [Google Scholar]
- 12.Stowe KA, Burgess SC, Merritt M, et al. Storage and oxidation of long-chain fatty acids in the C57/BL6 mouse heart as measured by NMR spectroscopy. FEBS Lett. 2006;580:4282–7. doi: 10.1016/j.febslet.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 13.Opie LH. Cardiac metabolism–emergence, decline, and resurgence. Part II. Cardiovasc Res. 1992;26:817–30. doi: 10.1093/cvr/26.9.817. [DOI] [PubMed] [Google Scholar]
- 14.Opie LH. Cardiac metabolism–emergence, decline, and resurgence. Part I. Cardiovasc Res. 1992;26:721–33. doi: 10.1093/cvr/26.8.721. [DOI] [PubMed] [Google Scholar]
- 15.Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues B, Cam MC, Jian K, et al. Differential effects of streptozotocin-induced diabetes on cardiac lipoprotein lipase activity. Diabetes. 1997;46:1346–53. doi: 10.2337/diab.46.8.1346. [DOI] [PubMed] [Google Scholar]
- 17.Pulinilkunnil T, Rodrigues B. Cardiac lipoprotein lipase: metabolic basis for diabetic heart disease. Cardiovasc Res. 2006;69:329–40. doi: 10.1016/j.cardiores.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan J, Mazumder PK, Hu P, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–9. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 19.Ko HJ, Zhang Z, Jung DY, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–46. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Ortega I, Carlos Kaski J. Diabetic miocardiopathy. Med Clin (Barc) 2006;127:584–94. doi: 10.1157/13094003. [DOI] [PubMed] [Google Scholar]
- 21.Park SY, Cho YR, Finck BN, et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–24. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 22.Lewis GF, Carpentier A, Adeli K, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 23.Boden G, Lebed B, Schatz M, et al. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612–7. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 24.Kankaanpaa M, Lehto HR, Parkka JP, et al. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–95. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 25.Jaswal JS, Keung W, Wang W, et al. Targeting fatty acid and carbohydrate oxidation–a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta. 2011;1813:1333–50. doi: 10.1016/j.bbamcr.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Okere IC, Young ME, McElfresh TA, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–23. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 27.Son NH, Yu S, Tuinei J, et al. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010;120:3443–54. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Shi X, Bharadwaj KG, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–23. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haemmerle G, Moustafa T, Woelkart G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–85. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation. 2002;105:1861–70. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 31.Park TS, Yamashita H, Blaner WS, et al. Lipids in the heart: a source of fuel and a source of toxins. Curr Opin Lipidol. 2007;18:277–82. doi: 10.1097/MOL.0b013e32814a57db. [DOI] [PubMed] [Google Scholar]
- 32.Chiu HC, Kovacs A, Ford DA, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–33. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 34.Yagyu H, Chen G, Yokoyama M, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–26. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyntar D, Eppenberger-Eberhardt M, Maedler K, et al. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 2001;50:2105–13. doi: 10.2337/diabetes.50.9.2105. [DOI] [PubMed] [Google Scholar]
- 36.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drosatos K, Bharadwaj KG, Lymperopoulos A, et al. Cardiomyocyte lipids impair beta-adrenergic receptor function via PKC activation. Am J Physiol Endocrinol Metab. 2011;300:E489–99. doi: 10.1152/ajpendo.00569.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son NH, Park TS, Yamashita H, et al. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basu R, Oudit GY, Wang X, et al. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009;297:H2096–108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama M, Yagyu H, Hu Y, et al. Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem. 2004;279:4204–11. doi: 10.1074/jbc.M311995200. [DOI] [PubMed] [Google Scholar]
- 41.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–71. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 43.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–25. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 44.Torre-Villalvazo I, Gonzalez F, Aguilar-Salinas CA, et al. Dietary soy protein reduces cardiac lipid accumulation and the ceramide concentration in high-fat diet-fed rats and ob/ob mice. J Nutr. 2009;139:2237–43. doi: 10.3945/jn.109.109769. [DOI] [PubMed] [Google Scholar]
- 45.Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J Mol Med (Berl) 2007;85:697–706. doi: 10.1007/s00109-007-0170-9. [DOI] [PubMed] [Google Scholar]
- 46.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baranowski M, Blachnio A, Zabielski P, et al. PPAR-alpha agonist induces the accumulation of ceramide in the heart of rats fed high-fat diet. J Physiol Pharmacol. 2007;58:57–72. [PubMed] [Google Scholar]
- 49.Burkart EM, Sambandam N, Han X, et al. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–9. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 51.Bahr M, Spelleken M, Bock M, et al. Acute and chronic effects of troglitazone (CS-045) on isolated rat ventricular cardiomyocytes. Diabetologia. 1996;39:766–74. doi: 10.1007/s001250050509. [DOI] [PubMed] [Google Scholar]
- 52.Sidell RJ, Cole MA, Draper NJ, et al. Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the Zucker fatty rat heart. Diabetes. 2002;51:1110–7. doi: 10.2337/diabetes.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 53.Carley AN, Semeniuk LM, Shimoni Y, et al. Treatment of type 2 diabetic db/db mice with a novel PPARgamma agonist improves cardiac metabolism but not contractile function. Am J Physiol Endocrinol Metab. 2004;286:E449–55. doi: 10.1152/ajpendo.00329.2003. [DOI] [PubMed] [Google Scholar]
- 54.Liu LS, Tanaka H, Ishii S, et al. The new antidiabetic drug MCC-555 acutely sensitizes insulin signaling in isolated cardiomyocytes. Endocrinology. 1998;139:4531–9. doi: 10.1210/endo.139.11.6310. [DOI] [PubMed] [Google Scholar]
- 55.Vikramadithyan RK, Hirata K, Yagyu H, et al. Peroxisome proliferator-activated receptor agonists modulate heart function in transgenic mice with lipotoxic cardiomyopathy. J Pharmacol Exp Ther. 2005;313:586–93. doi: 10.1124/jpet.104.080259. [DOI] [PubMed] [Google Scholar]
- 56.Baranowski M, Blachnio A, Zabielski P, et al. Pioglitazone induces de novo ceramide synthesis in the rat heart. Prostaglandins Other Lipid Mediat. 2007;83:99–111. doi: 10.1016/j.prostaglandins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Shimabukuro M, Higa M, Zhou YT, et al. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–90. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 58.Blazquez C, Geelen MJ, Velasco G, et al. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001;489:149–53. doi: 10.1016/s0014-5793(01)02089-0. [DOI] [PubMed] [Google Scholar]
- 59.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–70. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol. 2009;53:189–97. doi: 10.1097/FJC.0b013e3181926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karliner JS, Honbo N, Summers K, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–7. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 62.Jin ZQ, Zhou HZ, Zhu P, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–7. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 63.Tao R, Zhang J, Vessey DA, et al. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Vessey DA, Kelley M, Li L, et al. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12:BR318–24. [PubMed] [Google Scholar]
- 65.Duan HF, Wang H, Yi J, et al. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007;18:1119–28. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 66.Kennedy S, Kane KA, Pyne NJ, et al. Targeting sphingosine-1-phosphate signalling for cardioprotection. Curr Opin Pharmacol. 2009;9:194–201. doi: 10.1016/j.coph.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Honbo N, Goetzl EJ, et al. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–8. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 68.Means CK, Xiao CY, Li Z, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–51. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 69.Theilmeier G, Schmidt C, Herrmann J, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–9. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 70.Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82:193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baranowski M, Blachnio-Zabielska A, Hirnle T, et al. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. J Lipid Res. 2010;51:74–80. doi: 10.1194/jlr.M900002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]