TO THE EDITOR
Xerosis is one of the most common cutaneous manifestations in HIV infection. Although reportedly affecting up to 20–30% of patients with HIV (Rowe et al., 1999; Lee et al., 2007), the underlying cause of this xerosis has not yet been elucidated. In a further subgroup, xerosis is complicated by an atopic dermatitis (AD)-like rash or xerotic eczema. Xerosis is reportedly more common in HIV-infected individuals with CD4 cell counts ≤200 μl−1, and treatment with protease inhibitors, such as indinavir, provokes xerosis even in patients with higher CD4 counts (Lee et al., 2007).
Impaired epidermal barrier function is now closely linked to the pathogenesis of AD (Palmer et al., 2006; Weidinger et al., 2006; Sandilands et al., 2007), where defective stratum corneum (SC) barrier function in turn predisposes to repeated antigen penetration, leading to Th2-dominant inflammation (Fallon et al., 2009; Scharschmidt et al., 2009). Accordingly, overexpression of the Th2 cytokine (IL-4) in transgenic mice causes spontaneous AD (Lee and Flavell, 2004). But the chronic barrier abnormality in AD also stimulates production of epidermal cytokines and growth factors that stimulate inflammation (“cytokine cascade”), eventually recruiting Th2 cells, which in turn produce cytokines, such as IL-4, that further compromises barrier function (“outside-inside-back to outside”) hypothesis (Elias and Steinhoff, 2008; Elias et al., 2008).
Although it is widely believed that HIV-associated xerosis and xerotic eczema reflect a worsening of pre-existent AD (Parkin et al., 1987; Cockerell 1991), HIV infection itself provokes a Th2 immunophenotype (Klein et al., 1997), and as noted above, Th2 cytokines themselves downregulate barrier function (Kurahashi et al., 2008), as well as ceramide synthesis (Hatano et al., 2005), and expression of several differentiation-related structural proteins (Howell et al., 2007, 2008). Hence, we hypothesized that a primary, infection-triggered Th2-dominant immune abnormality could drive subsequent epidermal changes in HIV. Accordingly, we assessed here cutaneous permeability barrier status in a cohort of HIV+ subjects, with no previous or current history of either AD or mucosal atopy. These HIV+, non-atopic patients display abnormal basal barrier function that becomes much more prominent in a subgroup of HIV+ patients with xerotic eczema. Moreover, CD4+ cell nadirs (<150 μl−1) correlated significantly with prominent skin dryness, but in this non-atopic cohort, anti-retroviral therapy aggravated neither barrier function, xerosis, nor SC hydration, suggesting that protease inhibitor (PI)-related xerosis and xerotic eczema could occur primarily or only in HIV+ patients with previous or concurrent AD/atopy.
We first assessed basal barrier function over non-lesional skin sites in 21 HIV+ patients (age 47±SD 7.4, two female patients) and six age, sex, and pigment-type matched controls (age 45±SD 9.6, one female patient). The mean duration of HIV infection in these subjects was 12.1±SD 6.4 years. A random group of seven HIV+ patients was genotyped for the three most common filaggrin mutations (that is, R501X, 2282del4, and R2447X), and none carried mutations, despite the fact that several of the patients showed abnormal barrier function and clinical xerosis (Supplementary Table 1). Most of our patients were on treatment with anti-retroviral drugs (n=17, 81%), including a protease inhibitor (n=13, 62%). Nine patients displayed clinically normal skin, whereas nine others had xerosis, and a further three patients displayed xerosis plus generalized xerotic eczema. HIV+ patients with no signs of eczema displayed abnormal basal barrier function (Figure 1a), including subjects without xerosis (≈3- fold increase in transepidermal water loss (TEWL) rates (6.4±0.7 vs 2.2±0.3; P<0.001). Finally, even patients who were off antiretroviral treatment displayed elevated TEWL rates, showing that the barrier abnormality in HIV+ patients cannot be ascribed to antiretroviral therapy.
Figure 1. Abnormal barrier function in HIV patients, independent of anti-retroviral therapy.
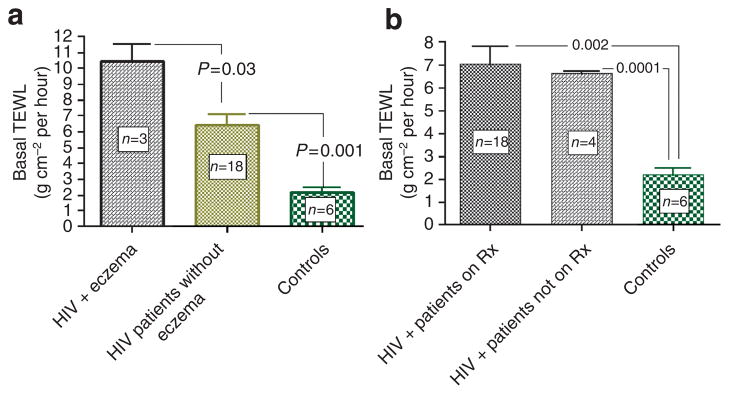
(a) HIV+ patients displayed abnormal basal barrier function that became more prominent in a subgroup with xerotic eczema. Basal TEWL was measured in the interscapular skin using Tewameter at ambient clinic temperatures (22–25°C) and at a relative humidity of 40–60% after 15 minutes acclimatization. HIV patients displayed higher basal TEWL rates, which increased further in patients with xerotic eczema. (b) Abnormal barrier function in HIV+ patients is not attributable to antiretroviral therapy. HIV+ patients displayed abnormal basal barrier function, regardless of their antiretroviral treatment status. Abbreviation: TEWL, transepidermal water loss.
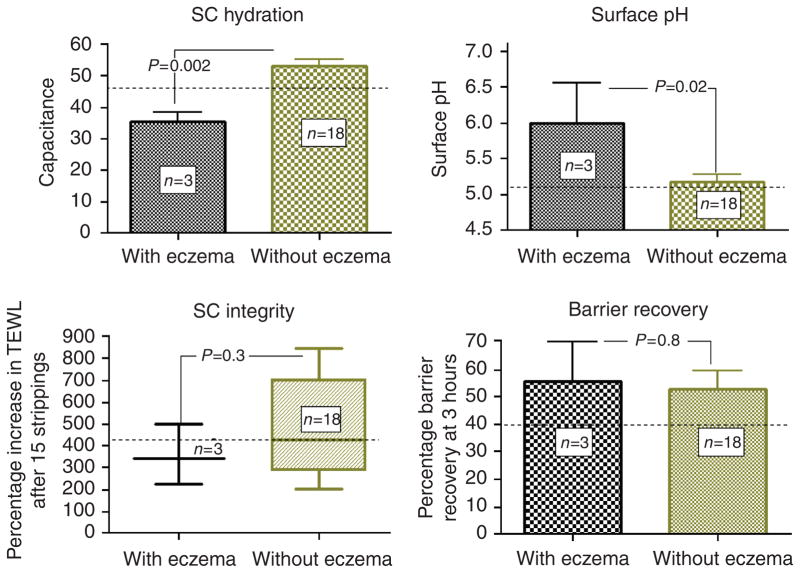
The barrier abnormality became much more prominent in a subgroup of three patients with xerotic eczema (10.5±1.1 vs 2.2±0.3, P<0.001), and these patients displayed significantly reduced SC hydration (35.3±3.2 vs 53.2±2.0, P=0.002) and a higher surface pH (6.0±0.6 vs 5.2±0.1, P<0.05), compared with HIV patients without eczema (Figure 2). Finally, SC integrity/cohesion, surface pH, SC hydration and barrier recovery kinetics did not significantly differ in the HIV+ versus control groups as a whole (Figure 2).
Figure 2. Patients with HIV-related xerotic eczema displayed lower SC hydration and higher pH, but comparable SC integrity and barrier recovery kinetics.
Stratum corneum (SC) hydration of the interscapular skin was measured using corneometer. Surface pH was measured with a flat-glass electrode attached to a precision pH meter. SC integrity was assessed as the percentage increase in the TEWL after sequential tape stripping with 15 D-squame tapes. TEWL was read at 3 hours after tape stripping and barrier recovery was calculated as described in text. The dotted lines indicate mean values for control subjects. Abbreviation: TEWL, transepidermal water loss.
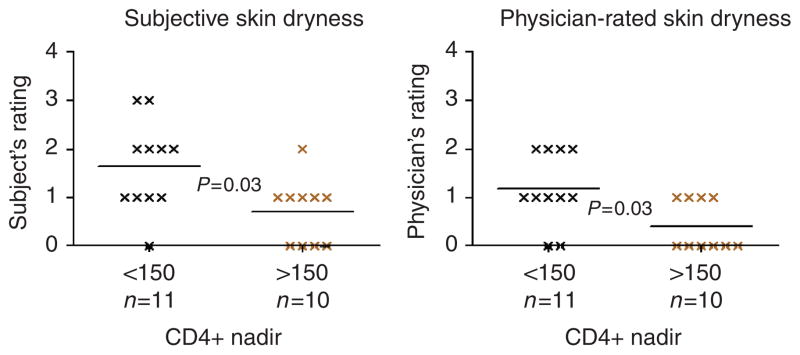
We next assessed epidermal function in relation to patients’ historically lowest CD4 counts (CD4 nadir) in these continuously monitored patients. Barrier function was abnormal in HIV+ patients, whether CD4 nadirs were less than or greater than 150 μl−1 (data not shown). Similarly, SC integrity/cohesion and barrier recovery rates were comparable in patients with low CD4 nadirs versus nadirs >150 μl−1 (not shown). Although HIV patients with CD4+ nadir <150 μl−1 displayed worse subject- and physician-rated visual dryness (Figure 3, P<0.05), SC hydration measurements did not correlate with either subject- or physician-rated skin dryness. But patients with the lowest CD4 nadirs displayed a significantly higher surface pH (5.56±1.8 vs 5.0±0.16, P<0.05). Finally, skin dryness did not correlate with current CD4 cell counts or viral load.
Figure 3. CD4+ nadir <150 μl−1 correlated with higher subject- and physician-rated skin dryness.
Subjects and physicians were asked to rate skin dryness using a visual skin dryness scale (0–4). Subjects with CD4+ nadir <150 μl−1 displayed higher subject- and physician-rated dryness scores.
Although HIV-related xerosis reportedly occurs more commonly in patients receiving antiretroviral treatment with protease inhibitors (Garcia-Silva et al., 2000; Lee et al., 2007), in this non-AD cohort, skin dryness, as rated either by the subjects or by physicians, did not worsen in patients on anti-retroviral therapy, including protease inhibitors. Furthermore, their basal TEWL, SC hydration, SC integrity, surface pH and barrier recovery kinetics did not differ significantly from untreated HIV patients. Although our results could reflect the low numbers of patients in this study, these results suggest that antiretroviral therapy did not adversely affect the cutaneous function in non-atopic HIV.
COMMENTS
Xerosis and xerotic eczema are frequent, troublesome side effects of HIV infection. The Study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM Study) reported a high prevalence of dry skin in a large cohort of HIV+ patients versus controls (Lee et al., 2007). Moreover, xerosis was associated with lower CD4+ counts, current protease inhibitor (PI) use, and recent opportunistic infections. Although xerosis has also been linked to PI use in several other studies (for example, Garcia-Silva et al., 2000), the mechanism(s) that lead(s) to HIV-associated dry skin and xerotic eczema is(are) not known. One prevalent hypothesis holds that PI displace retinoic acid (RA) from its cytosolic receptor, making higher levels of free RA available to provoke xerosis (Lee et al., 2007). Alternatively, depletion of cutaneous peptidergic innervation, affecting either the nutrient supply of the upper dermis, and/or secretory activity of sweat glands, has also been proposed to account for HIV-associated xerosis (Rowe et al., 1999). Interestingly, though increased transcutaneous water loss inevitably leads to xeric stress (lower SC hydration), epidermal permeability barrier function has not yet been examined in HIV infection.
A primary barrier abnormality underlies AD in HIV-negative subjects, and it is widely believed that xerosis and xerotic eczema occur primarily in subjects with previous or concurrent AD (Parkin et al., 1987; Cockerell 1991). To examine this issue, we assessed the epidermal function in a small cohort of non-atopic HIV+ patients. We show here that barrier dysfunction and xerosis occur in patients without an atopic background, and barrier function is abnormal even in HIV+ patients without xerosis. As barrier function worsened in a small subgroup of patients with xerotic eczema, barrier dysfunction in HIV-related xerosis and xerotic eczema could represent stages of disease progression along a disease continuum. Thus, all of the functional abnormalities that characterize clinical xerosis, including abnormal barrier function and xerotic eczema occur frequently in patients with non-atopic HIV. Furthermore, we found an elevated SC pH in patients with xerotic eczema, which could contribute to the pathogenesis of barrier dysfunction by multiple downstream mechanisms (Elias et al., 2008; Cork et al., 2009). For example, increased pH is known to activate serine proteases (kallikreins) (Hachem et al., 2002, 2003, 2009), and kallikrein5 itself upregulates an epidermis-initiated cytokine cascade that induces Th2 inflammation (Briot et al., 2009). As visual dryness did not correlate with SC hydration measurements, HIV-related xerosis is not a true ichthyosis, but rather likely a hyperproliferative response in response to impaired barrier function (Proksch et al., 1991). Moreover, SC integrity (cohesion) was normal in these subjects, another distinguishing feature from ichthyosis.
HIV disease, like AD, is itself associated with a Th2 cytokine profile (Klein et al., 1997), which correlates with reduced CD4 counts (Tsunemi et al., 2005), and xerosis was worse in our patients with low CD4 nadirs. As our patients were non-atopic, the putative increase in Th2 cytokines could provoke barrier dysfunction by: (1) reduced epidermal differentiation (Howell et al., 2008), (2) decreased epidermal ceramide production (Hatano et al., 2005), and/or (3) decreased epidermal e-cadherin production (Kobayashi et al., 2004). In contrast to AD, this view of disease pathogenesis suggests an opposite, “inside-to-outside” mechanism as the initial trigger of epidermal dysfunction in HIV. A possible explanation for the progressive worsening of the AD-like phenotype in some HIV patients could be the fact that the defective barrier allows sustained antigen penetration, which further accentuated Th2 cytokine production, (Elias et al., 1999; Taieb, 1999). This sequence would then result in a hypothetical initial “inside-to-outside,” followed by a “back-to-inside” vicious circle in HIV (Supplementary Figure 1). Accordingly, in non-atopic HIV subjects, antiretroviral drugs (including PI) that correct the primary immune abnormality could be expected to improve, rather than worsen xerosis. Yet, if the abnormality in barrier function persists, allowing sustained antigen penetration, cutaneous inflammation would persist, even after T-cell balance has been partially restored by anti-retroviral therapy. This concept could explain why the incidence of HIV-related xerosis has not changed significantly, despite advances in HIV treatment that have reduced other dermatological complications (Hengge et al., 2000).
Written, informed consent was obtained from all participants before enrollment, and all clinical investigations were conducted according to the Declaration of Helsinki principles. The research protocol was approved by the human studies committees at the University of California San Francisco and Veteran Affairs Medical Center, San Francisco.
Complete methods are available online; see figure legends for some details.
Supplementary Material
Acknowledgments
We are indebted to Ms Joan Wakefield for superb editorial assistance. These studies were supported by NIH grant AR051930 and the Medical Research Service, Department of Veterans Affairs.
Abbreviations
- AD
atopic dermatitis
- KLK
kallikrein
- PI
protease inhibitor
- RA
retinoic acid
- SC
stratum corneum
- TEWL
transepidermal water loss
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–47. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerell CJ. Seborrheic dermatitis-like and atopic dermatitis-like eruptions in HIV-infected patients. Clin Dermatol. 1991;9:49–51. doi: 10.1016/0738-081x(91)90114-z. [DOI] [PubMed] [Google Scholar]
- Cork MJ, Danby SG, Vasilopoulos Y, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–43. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–70. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Silva J, Almagro M, Juega J, et al. Protease inhibitor-related paronychia, ingrown toenails, desquamative cheilitis and cutaneous xerosis. Aids. 2000;14:1289–91. doi: 10.1097/00002030-200006160-00035. [DOI] [PubMed] [Google Scholar]
- Hachem J, Schurer N, Roelandt T, et al. Acute acidification of stratum corneum membrane domains using polyhydroxyl acids improves lipid processing and inhibits degradation of comeodesmosomes. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.249. e-pub ahead of print 10 September 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–53. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Fowler A, Behne M, et al. Increased stratum corneum pH promotes activation and release of primary cytokines from the stratum corneum attributable to activation of serine proteases. J Invest Dermatol. 2002;119:258. [Google Scholar]
- Hatano Y, Terashi H, Arakawa S, et al. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786–92. doi: 10.1111/j.0022-202X.2005.23651.x. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Franz B, Goos M. Decline of infectious skin manifestations in the era of highly active antiretroviral therapy. Aids. 2000;14:1069–70. doi: 10.1097/00002030-200005260-00025. [DOI] [PubMed] [Google Scholar]
- Howell MD, Fairchild HR, Kim BE, et al. Th2 cytokines act on S100/A11 to down-regulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–58. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SA, Dobmeyer JM, Dobmeyer TS, et al. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. Aids. 1997;11:1111–8. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Inai T, Morita K, et al. Reciprocal regulation of permeability through a cultured keratinocyte sheet by IFN-gamma and IL-4. Cytokine. 2004;28:186–9. doi: 10.1016/j.cyto.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008;128:1329–31. doi: 10.1038/sj.jid.5701138. [DOI] [PubMed] [Google Scholar]
- Lee D, Benson CA, Lewis CE, et al. Prevalence and factors associated with dry skin in HIV infection: the FRAM study. Aids. 2007;21:2051–7. doi: 10.1097/QAD.0b013e3282eea51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GR, Flavell RA. Transgenic mice which overproduce Th2 cytokines develop spontaneous atopic dermatitis and asthma. Int Immunol. 2004;16:1155–60. doi: 10.1093/intimm/dxh117. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- Parkin JM, Eales LJ, Galazka AR, et al. Atopic manifestations in the acquired immune deficiency syndrome: response to recombinant interferon gamma. Br Med J (Clin Res Ed) 1987;294:1185–6. doi: 10.1136/bmj.294.6581.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch E, Feingold KR, Man MQ, et al. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991;87:1668–73. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe A, Mallon E, Rosenberger P, et al. Depletion of cutaneous peptidergic innervation in HIV-associated xerosis. J Invest Dermatol. 1999;112:284–9. doi: 10.1046/j.1523-1747.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Smith FJ, Irvine AD, et al. Filaggrin’s fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol. 2007;127:1282–4. doi: 10.1038/sj.jid.5700876. [DOI] [PubMed] [Google Scholar]
- Scharschmidt T, Man M, Hatano Y, et al. Filaggrin deficiency confers a para-cellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009;124:496–506. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb A. Hypothesis: from epidermal barrier dysfunction to atopic disorders. Contact Dermatitis. 1999;41:177–80. doi: 10.1111/j.1600-0536.1999.tb06125.x. [DOI] [PubMed] [Google Scholar]
- Tsunemi S, Iwasaki T, Imado T, et al. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. Aids. 2005;19:879–86. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- Weidinger S, Illig T, Baurecht H, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–9. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.