TO THE EDITOR
Acidification of the surface of the stratum corneum (SC), the acid mantle, was initially thought to be important in the defense against infection. The growth of pathogenic microorganisms, such as Staphylococcus aureus and Streptococcus pyogenes, is inhibited by an acidic skin pH whereas the growth of resident (normal) skin flora is stimulated (Puhvel et al., 1975; Korting et al., 1990, 1992). However, recent studies have shown that acidification of the SC has additional functions, including regulating several key SC functions. A major function of the skin is to form a permeability barrier between the dry external environment and the moist interior of the body (Elias, 2007). This permeability barrier resides in the extracellular lipid membranes of the SC, and studies have shown that an acidified SC is required for the formation of a functionally competent permeability barrier (Mauro et al., 1998; Fluhr et al., 2001; Hachem et al., 2003). Specifically, in the SC β-glucocerebrosidase and acid sphingomyelinase metabolize glucosylceramides and sphingomyelin, respectively, to ceramides, which is the major family of lipids in the extracellular membranes that mediate permeability barrier function (Feingold, 2007). Both the enzymes require an acidic milieu for optimal enzymatic activity; hence, when the pH of the SC increases, the metabolism of glucosylceramides and sphingomyelin to ceramides is impaired, resulting in abnormal permeability barrier homeostasis (Holleran et al., 1992, 1993; Feingold, 2007). In addition, an acidic SC pH inhibits the activity of serine proteases thereby maintaining the cohesiveness and integrity of the SC (Hachem et al., 2005). With an increase in SC pH, the activities of these serine proteases are stimulated resulting in the degradation of corneodesmosomes and a decrease in SC integrity and cohesion (Fluhr et al., 2004b; Hachem et al., 2005). Thus, an acidic SC is important in regulating the metabolism and function of the SC, and alterations in SC pH could have numerous adverse effects.
A variety of different pathways are postulated to contribute to the acid mantle of the skin. Exogenous mechanisms, such as free fatty acids of pilosebaceous origin (Puhvel et al., 1975; Bibel et al., 1989), microbial metabolites (Di Marzio et al., 1999), and eccrine gland-derived products, such as lactic acid (Ament et al., 1997; Thueson et al., 1998), are thought to decrease SC pH. Recent studies have shown that endogenous mechanisms also contribute to SC acidification (Fluhr et al., 2001, 2004a; Behne et al., 2002). Both free fatty acid generation from phospholipid hydrolysis catalyzed by one or more isoforms of sPLA2 and a sodium/proton pump antiporter, the sodium/hydrogen antiporter-1 (Behne et al., 2002, 2003; Fluhr et al., 2004a), lower SC pH. A third endogenous mechanism, urocanic acid generation from histidine by the deiminating enzyme, histidase, has been shown to acidify SC in vitro (Krien and Kermici, 2000), but its impact on SC acidification and function in vivo remains uncertain. The histidase pathway of acidification is dependent upon previous proteolysis of filaggrin to histidine, a reaction triggered by a reduction in external humidity (Rawlings and Matts, 2005).
We therefore hypothesized that if the filaggrin–histidine–urocanic acid cascade is crucial in regulating SC pH in vivo, then SC pH should increase with either a reduction in substrate (filaggrin) and/or a decrease in histidase activity. Moreover, as SC acidification occurs over the first few days after birth in parallel with activation of filaggrin proteolysis (Fluhr et al., 2004a), we reasoned that histidase activity should increase simultaneously with SC acidification.
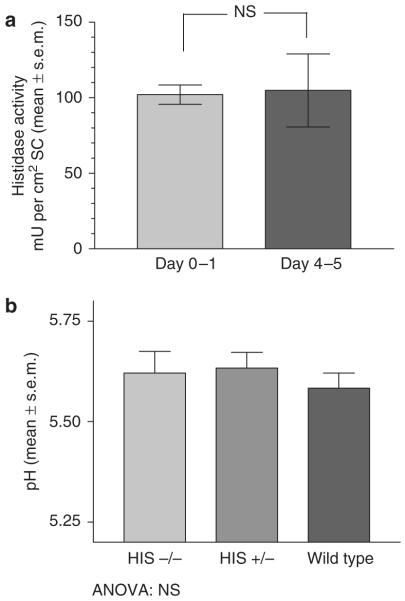
Our initial studies examined histidase activity in newborn albino rats immediately after birth and at 4–5 days after birth (details regarding animals and methods are provided in Supplementary Material online). As reported previously, during this period of time the pH of the SC decreases markedly (Fluhr et al., 2004a). If the filaggrin–histidine–urocanic acid cascade makes a major contribution to this reduction in SC pH, then the activity of histidase in the SC should increase in parallel. As shown in Figure 1a, there was no difference in SC histidase activity at day 0–1 after birth compared to 4–5 days after birth.
Figure 1. Role of histidase in SC acidification.
(a) Histidase activity in the stratum corneum (SC) was measured in newborn rats and rats at age 4–5 days (n = 6). (b) The SC surface pH in Peruvian and matched controls was measured with a flat, glass surface electrode (Mettler-Toledo, Giessen, Germany), attached to a pH meter (Skin pH Meter PH 900; Courage & Khazaka, Cologne, Germany). HIS−/−, n = 8; HIS +/−, n = 14; wild type, n = 6.
To determine the role of histidase in SC acidification more definitively, we next studied mice that were deficient in histidase activity, the Peruvian mouse (Selden et al., 1995). As shown in Figure 1b, SC pH was similar in animals deficient in histidase activity, suggesting again that the histidase pathway is not essential for SC acidification.
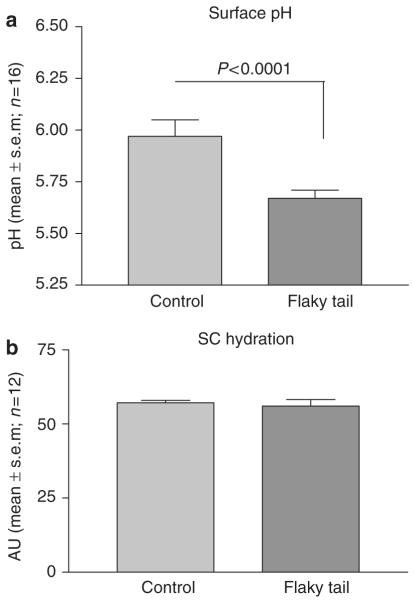
We next studied animals deficient in filaggrin production due to impaired proteolytic processing of profilaggrin to filaggrin, the flaky tail (ft/ft) mouse (Presland et al., 2000). As shown in Figure 2a, SC pH was actually slightly decreased, not increased, in flaky tail mice, indicating that filaggrin is not essential for SC acidification. In addition, SC hydration (Figure 2b) is also not altered in the flaky tail mouse, suggesting that this additional function also does not absolutely require filaggrin.
Figure 2. Effect of filaggrin deficiency on SC function.
(a) In flaky tail (ft/ft) mice, surface pH was measured with a flat, glass surface electrode as described in Figure 1 legend (n = 16; P<0.0001). (b) Stratum corneum (SC) hydration was measured with corneometer (Corneometer CM 820; Courage & Khazaka); n = 12; t-test, NS.
We next asked whether other acidifying mechanisms are upregulated in ft/ft mice to compensate for reduced urocanic acid generation. As shown in Supplementary Figure S1 (Supplementary Material), the immunostainable protein content of both NHE1 and sPLA2A is increased in ft/ft mice whereas two other isoforms of sPLA2, sPLA2F and sPLA2X1, do not change (data not shown). These results suggest that compensatory upregulation of these alternate acidifying pathways could account for the normal to decreased SC pH in ft/ft mice.
Taken together, the above results do not provide support for the hypothesis that the filaggrin–histidine–urocanic acid cascade is essential for SC acidification. Increases in sPLA2 activity and/or NHE1 levels, or other yet to be elucidated mechanisms, appear to acidify the SC in the absence of the filaggrin–histidine–urocanic acid cascade. Yet, when either the sPLA2 or NHE1 pathways of acidification are compromised, the bulk pH of SC rises (Fluhr et al., 2001, 2004a; Behne et al., 2002), indicating that other acidifying mechanisms cannot compensate for them. One can easily envision that because of the importance of an acid mantle in SC function (Fluhr et al., 2001, 2004b; Schmid-Wendtner and Korting, 2006) that numerous pathways contribute to its formation, and that the absence of any particular pathway will result in minor or no changes in SC pH. Thus, although both sPLA2 and NHE1 activities are required for the formation of the acid mantle (Fluhr et al., 2001, 2004a; Behne et al., 2002), the filaggrin–histidine–urocanic acid cascade is not essential for SC acidification.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grants AR39448, HD029706, and AR049932, and the Research Service, Department of Veterans Administration at San Francisco. The study has received institutional approval for animal experiments.
Abbreviation
- SC
stratum corneum
Footnotes
CONFLICT OF INTEREST The authors state no conflict interest in relation to this study except JS (who has a research contract with Procter and Gamble on an unrelated project).
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Ament W, Huizenga JR, Mook GA, et al. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise. Int J Sports Med. 1997;18:35–9. doi: 10.1055/s-2007-972592. [DOI] [PubMed] [Google Scholar]
- Behne MJ, Barry NP, Hanson KM, et al. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J Invest Dermatol. 2003;120:998–1006. doi: 10.1046/j.1523-1747.2003.12262.x. [DOI] [PubMed] [Google Scholar]
- Behne MJ, Meyer JW, Hanson KM, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem. 2002;277:47399–406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- Bibel DJ, Miller SJ, Brown BE, et al. Antimicrobial activity of stratum corneum lipids from normal and essential fatty acid-deficient mice. J Invest Dermatol. 1989;92:632–8. doi: 10.1111/1523-1747.ep12712202. [DOI] [PubMed] [Google Scholar]
- Di Marzio L, Cinque B, De Simone C, et al. Effect of the lactic acid bacterium Streptococcus thermophilus on ceramide levels in human keratinocytes in vitro and stratum corneum in vivo. J Invest Dermatol. 1999;113:98–106. doi: 10.1046/j.1523-1747.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- Feingold KR. The importance of lipids in cutaneous function. J Lipid Res. 2007;48:2529–30. doi: 10.1194/jlr.E700004-JLR200. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Behne MJ, Brown BE, et al. Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter-1 acidify neonatal rat stratum corneum. J Invest Dermatol. 2004a;122:320–9. doi: 10.1046/j.0022-202X.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Kao J, Jain M, et al. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001;117:44–51. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Mao-Qiang M, Brown BE, et al. Functional consequences of a neutral pH in neonatal rat stratum corneum. J Invest Dermatol. 2004b;123:140–51. doi: 10.1111/j.0022-202X.2004.22726.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Crumrine D, Fluhr J, et al. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J Invest Dermatol. 2003;121:345–53. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Man MQ, Crumrine D, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–20. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Imokawa G, et al. beta-Glucocerebrosidase activity in murine epidermis: characterization and localization in relation to differentiation. J Lipid Res. 1992;33:1201–9. [PubMed] [Google Scholar]
- Holleran WM, Takagi Y, Menon GK, et al. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Invest. 1993;91:1656–64. doi: 10.1172/JCI116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korting HC, Hubner K, Greiner K, et al. Differences in the skin surface pH and bacterial microflora due to the long-term application of synthetic detergent preparations of pH 5.5 and pH 7.0. Results of acrossover trial in healthy volunteers. Acta Derm Venereol. 1990;70:429–31. [PubMed] [Google Scholar]
- Korting HC, Lukacs A, Vogt N, et al. Influence of the pH-value on the growth of Staphylococcus epidermidis, Staphylococcus aureus and Propionibacterium acnes in continuous culture. Zentralbl Hyg Umweltmed. 1992;193:78–90. [PubMed] [Google Scholar]
- Krien PM, Kermici M. Evidence for the existence of a self-regulated enzymatic process within the human stratum corneum—an unexpected role for urocanic acid. J Invest Dermatol. 2000;115:414–20. doi: 10.1046/j.1523-1747.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290:215–22. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- Presland RB, Boggess D, Lewis SP, et al. Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J Invest Dermatol. 2000;115:1072–81. doi: 10.1046/j.1523-1747.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- Puhvel SM, Reisner RM, Sakamoto M. Analysis of lipid composition of isolated human sebaceous gland homogenates after incubation with cutaneous bacteria. Thin-layer chromatography. J Invest Dermatol. 1975;64:406–11. doi: 10.1111/1523-1747.ep12512337. [DOI] [PubMed] [Google Scholar]
- Rawlings AV, Matts PJ. Stratum corneum moisturization at the molecular level: an update in relation to the dry skin cycle. J Invest Dermatol. 2005;124:1099–110. doi: 10.1111/j.1523-1747.2005.23726.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- Selden C, Calnan D, Morgan N, et al. Histidinemia in mice: a metabolic defect treated using a novel approach to hepatocellular transplantation. Hepatology. 1995;21:1405–12. [PubMed] [Google Scholar]
- Thueson DO, Chan EK, Oechsli LM, et al. The roles of pH and concentration in lactic acid-induced stimulation of epidermal turnover. Dermatol Surg. 1998;24:641–5. doi: 10.1111/j.1524-4725.1998.tb04221.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.