Abstract
Neural circuits regulate cytokine production to prevent potentially damaging inflammation. A prototypical vagus nerve circuit, the inflammatory reflex, inhibits tumor necrosis factor–α production in spleen by a mechanism requiring acetylcholine signaling through the α7 nicotinic acetylcholine receptor expressed on cytokine-producing macrophages. Nerve fibers in spleen lack the enzymatic machinery necessary for acetylcholine production; therefore, how does this neural circuit terminate in cholinergic signaling? We identified an acetylcholine-producing, memory phenotype T cell population in mice that is integral to the inflammatory reflex. These acetylcholine-producing T cells are required for inhibition of cytokine production by vagus nerve stimulation. Thus, action potentials originating in the vagus nerve regulate T cells, which in turn produce the neurotransmitter, acetylcholine, required to control innate immune responses.
Neural circuits regulate organ function in order to maintain optimal physiological stability, providing homeostasis to the body’s internal environment. The vagus nerve, named by the Latin word for “wandering,” is a paired structure that arises in the brain stem and travels to visceral organs, where it regulates physiological responses to environmental changes, injury, and infection. In the immune system, electrical stimulation of the vagus nerve inhibits cytokine release; attenuates tissue injury; and ameliorates inflammation-mediated injury in endotoxemia, sepsis, and other cytokine-dependent models of inflammatory disease (1–4). This neural circuit, termed the inflammatory reflex, requires action potentials arising in the vagus nerve, and acetylcholine interacting with the α7 subunit of the nicotinic acetylcholine receptor (nAChR) expressed on cytokine-producing macrophages in spleen (5). Selective cholinergic agonists significantly inhibit cytokine production in spleen and improve outcome in experimental models of inflammatory disease (6–12).
Vagus nerve fibers terminate in the celiac ganglion, the location of neural cell bodies that project axons in the splenic nerve to innervate the spleen (13, 14). Electrical stimulation of either the vagus nerve above the celiac ganglion or the splenic nerve itself significantly inhibits tumor necrosis factor–α (TNF-α) production by red pulp and marginal zone macrophages, the principal cell source of TNF-α released into the circulation during endotoxemia (15–17). Paradoxically, nerve fibers in spleen, originating in the celiac ganglion, are adrenergic, not cholinergic, and utilize norepinephrine as the primary neurotransmitter (18). Thus, although the spleen has been shown to contain acetylcholine (19, 20), the cellular source of this terminal neurotransmitter in the inflammatory reflex is unknown. Because lymphocytes can synthesize and release acetylcholine (21, 22), we reasoned that they might be the source of acetylcholine that relays functional information transmitted by action potentials originating in the vagus nerve to the spleen.
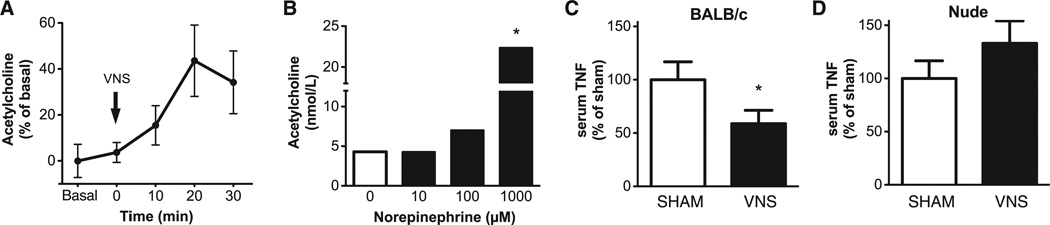
To determine whether vagus nerve stimulation induces increased acetylcholine release in the spleen, we measured acetylcholine in perfusate samples collected by microdialysis. Acetylcholine levels were elevated within minutes after electrical vagus nerve stimulation and reached peak levels within 20 min (Fig. 1A). This indicates that action potentials originating in the vagus nerve can enhance acetylcholine release in the spleen. Previous work indicated that adrenergic nerve endings in the spleen terminate in the T cell region of the white pulp and that splenic nerve stimulation enhances norepinephrine release from spleen (23, 24). To determine the effect of norepinephrine on acetylcholine release, we incubated spleen lymphocytes in the presence of norepinephrine. Norepinephrine significantly stimulated acetylcholine release by spleen lymphocytes (Fig. 1B), which suggested that functional stimulation of adrenergic splenic neurons can stimulate spleen lymphocyte acetylcholine release.
Fig. 1.
Vagus nerve stimulation increases acetylcholine levels in the spleen and requires T lymphocytes to attenuate TNF-α in endotoxemia. (A) BALB/c mice (n = 5) were subjected to vagus nerve stimulation (5 min), and spleen acetylcholine levels were determined in dialysate samples by mass spectrometry. Results are expressed as a percentage of the average levels of three consecutive samples ± SEM obtained before vagus nerve stimulation (basal). P < 0.05 at 20 min compared with basal [repeated measures analysis of variance (ANOVA) and the Dunnett post hoc test]. VNS, vagus nerve stimulation. (B) Acetylcholine was measured by mass spectrometry in supernatants of nonadherent spleen cells in the presence or absence of norepinephrine at the indicated concentrations. Data were obtained from pooled cells stimulated in duplicate. Results are expressed as the mean of two experiments. *P < 0.05 compared with unstimulated cells (two-tailed t test). (C) BALB/c mice (four or five per group) and (D) BALB/c nude mice (five per group) were subjected to sham surgery or vagus nerve stimulation followed by endotoxin injection. Serum was obtained 90 min after endotoxin administration, and TNF-α was measured by enzyme-linked immunosorbent assay (ELISA). Results are means ± SEM. *P < 0.05 compared with the sham group (two-tailed t test).
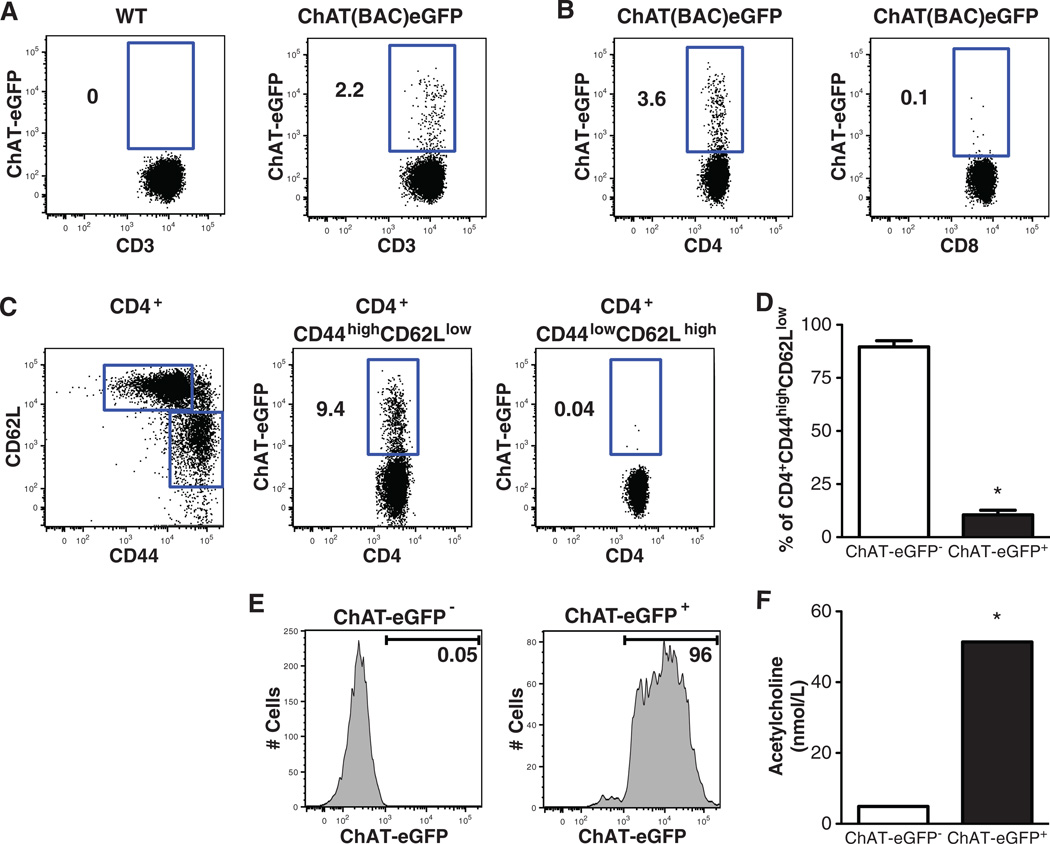
Next, to evaluate the role of T cells in mediating the inflammatory reflex, we studied the effect of vagus nerve stimulation in nude mice, which are devoid of functional T cells. As expected, vagus nerve stimulation in control BALB/c mice significantly suppressed serum TNF-α production during endotoxemia (Fig. 1C). Vagus nerve stimulation failed to attenuate serum TNF-α in nude mice, which indicated that T cell deficiency impairs the inflammatory reflex (Fig. 1D). To identify acetylcholine-producing T cells required for the integrity of the inflammatory reflex, we used ChAT(BAC)-EGFP mice, which express enhanced green fluorescent protein (EGFP) under the control of transcriptional regulatory elements for ChAT, the enzyme that catalyzes the biosynthesis of acetylcholine (25). Flow cytometry revealed that ChAT-EGFP+ cells were 2.6 ± 0.4% of total spleen CD3+ T cells (Fig. 2A), which indicated that only a relatively small subset of total spleen T cells express ChAT. ChAT-EGFP was expressed in 4.4 ± 0.7% of CD4+ cells, but in only a negligible number (0.2 ± 0.1%) of CD8+ T cells (Fig. 2B). When CD4+ cells were further divided into CD44high CD62Llow memory and CD44low CD62Lhigh naïve T cell populations, ChAT-EGFP+ cells were predominantly observed in the CD44high CD62Llow population (Fig. 2C). Among the total memory CD4+ CD44high CD62Llow cells, 10.5 ± 2.1% were ChAT-EGFP+ T cells (Fig. 2D).
Fig. 2.
Spleen acetylcholine-synthesizing T cells express a memory T cell phenotype. (A) ChAT-EGFP expression in spleen CD3+ T cells of wild-type and ChAT(BAC)-EGFP mice. (B) Expression of ChAT-EGFP in CD4+ and CD8+ spleen T cells. (C) CD44 and CD62L expression in spleen CD4+ cells (left), and ChAT-EGFP expression in CD4+ CD44high CD62Llow and CD4+ CD44low CD62Lhigh spleen cells (middle and right, respectively). (D) Percentage of ChAT-EGFP− and ChAT-EGFP+ cells among spleen CD4+ CD44high CD62Llow cells, n = 5. (E) Spleen CD4+ CD44high CD62Llow ChAT-EGFP− and CD4+ CD44high CD62Llow ChAT-EGFP+ cells were obtained by cell sorting. (F) Acetylcholine concentration was determined in supernatants of cells under resting conditions. Data were obtained from pooled cells cultured in duplicate. Results are the means of two experiments. *P < 0.05 compared with CD4+ CD44high CD62Llow ChAT-EGFP− cells (two-tailed t test).
To examine the capacity for acetylcholine synthesis by T cells, spleen CD4+ CD44high CD62Llow ChAT-EGFP+ and CD4+ CD44high CD62Llow ChAT-EGFP− cells were collected by cell sorting, and acetylcholine was measured in supernatants of these cells under basal conditions. Acetylcholine production was significantly elevated in supernatants of CD4+ CD44high CD62Llow ChAT-EGFP+ cells, as compared with CD4+ CD44high CD62Llow ChAT-EGFP− (Fig. 2E and F). To functionally characterize these acetylcholine-producing T cells, spleen CD4+ CD44high CD62Llow ChAT-EGFP+ cells were stimulated with plate-bound CD3-specific antibody. Levels of interleukin-17A (IL-17A), IL-10, and the T helper 1 cytokine interferon-γ (IFN-γ) were significantly elevated in supernatants 48 hours after stimulation (fig. S1), whereas the T helper 2 cytokines IL-4 and IL-6 were not significantly elevated (fig. S1). This suggested that ChAT expression is not restricted to a discrete functional T cell subset and raised the possibility that T cell acetylcholine-synthesizing capacity might be linked to T cell activation status. To examine this, ChAT-EGFP expression was analyzed on CD4+ CD44high CD62Llow ChAT-EGFP− T cells after stimulation with CD3-specific antibody. Flow cytometry analysis revealed a significant increase in ChAT-EGFP expression within 48 hours after T cell stimulation (fig. S2), which suggested that T cell activation enhances expression of ChAT and acetylcholine release.
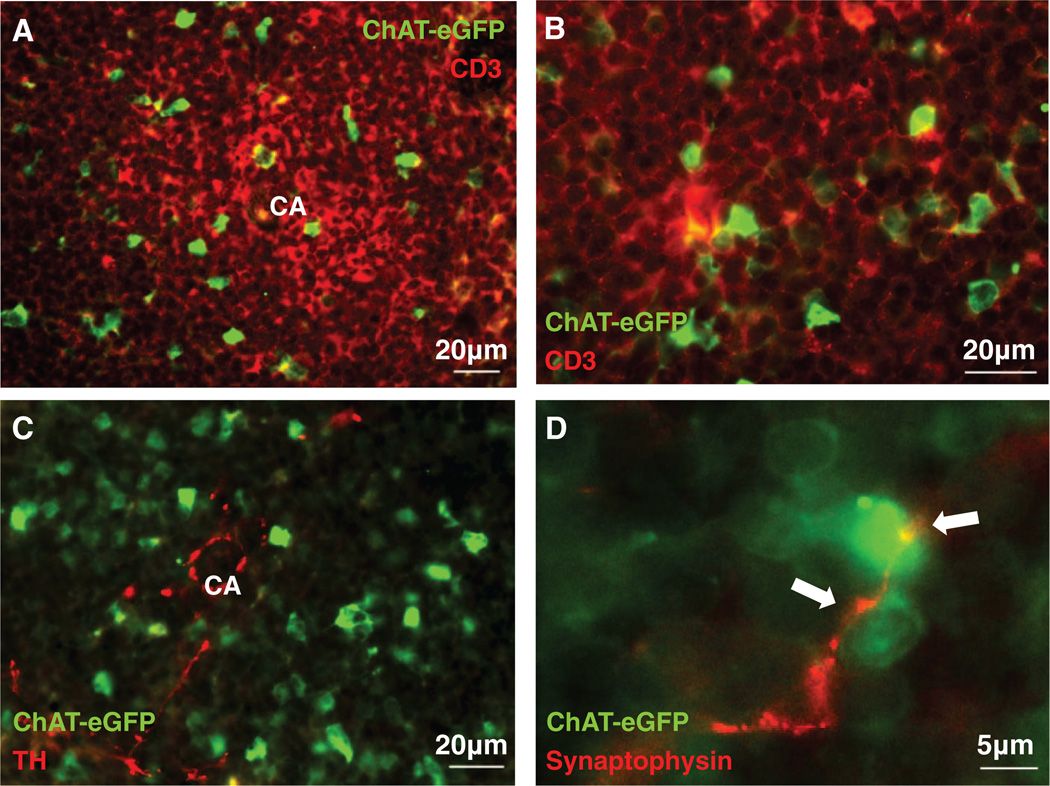
The spatial relation between ChAT-EGFP+ T cells and splenic nerve fibers was explored by immunofluorescence analysis of spleen sections to reveal that ChAT-EGFP expression in T lymphocytes localized primarily in the white pulp (Fig. 3A and B). ChAT-EGFP+ cells in the white pulp were adjacent to splenic nerve fibers expressing tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis (Fig. 3C). As expected, splenic nerve fibers failed to express ChAT-EGFP, in agreement with earlier results, which indicated that splenic nerves are adrenergic and do not produce acetylcholine (15, 18). Synaptophysin, a glycoprotein expressed at neural synapses, was localized adjacent to ChAT-EGFP+ cells in white pulp parenchyma (Fig. 3D). Together with previous results indicating that splenic nerve endings form synapse-like structures on T lymphocytes (23), the termination of these synaptophysin-positive nerve fibers on ChAT-EGFP+ T cells provides an anatomical basis for splenic nerve fibers interacting with acetylcholine-producing T cells. Extensive prior work has established that splenic nerve signals modulate T cell responses by signal transduction through β-adrenergic receptors (26–28). Analysis of mRNA levels of adrenergic receptors β1, β2, and β3 in CD4+ CD44high CD62Llow ChAT-EGFP+ spleen cells revealed expression of adrenergic receptors β1 and β2, but not β3 (fig. S3). This agrees with previous work that β-adrenergic receptors on T cells underlie the mechanism of adrenergic splenic nerve signaling (26–28).
Fig. 3.
Acetylcholine-synthesizing T cells in spleen are located in the proximity of catecholaminergic nerve endings. (A and B) Immunofluorescent micrographs of ChAT-EGFP (green) expression by T cells in spleen white pulp (CD3, red). (C) Immunofluorescent micrographs of ChAT-EGFP+ cells (green) and nerve fibers stained with tyrosine hydroxylase (TH, red). (D) Fluorescent micrographs of splenic nerve endings (synaptophysin, red) juxtaposed (arrows) to ChAT-EGFP+ (green) cells in the white pulp. CA, central artery. (A) ×400 magnification, (B) ×630 magnification, (C) ×400 magnification, (D) ×630 magnification. Images are representative of spleen sections (n = 3 to 5) from five experiments.
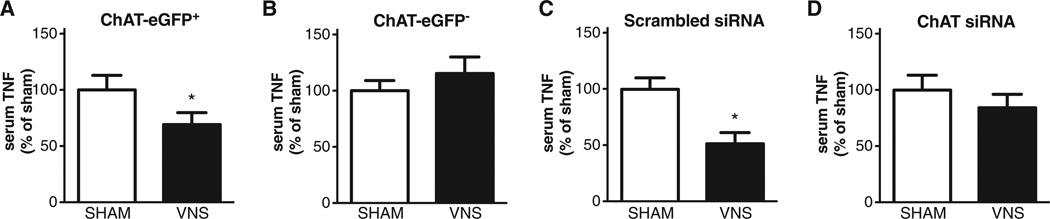
In order to assess the role of acetylcholine-producing T cells in the inflammatory reflex, CD4+ CD44high CD62Llow ChAT-EGFP+ cells obtained by cell sorting were adoptively transferred to nude mice. Vagus nerve stimulation significantly decreased serum TNF-α levels in endotoxemic nude mice reconstituted with CD4+ CD44high CD62Llow ChAT-EGFP+ cells (Fig. 4A) but not in controls reconstituted with CD4+ CD44high CD62Llow ChAT-EGFP− cells (Fig. 4B). The fate of adoptively transferred CD4+ CD44high CD62Llow ChAT-EGFP+ T cells in this nude mouse model was examined by flow cytometry and histological analysis of spleen sections. We found that CD4+ CD44high CD62Llow ChAT-EGFP+ and CD4+ CD44high CD62Llow ChAT-EGFP− T cells harvested from spleens of recipient nude mice remained ChAT-EGFP+ and ChAT-EGFP−, respectively (fig. S4A and B). We also observed significant accumulation of ChAT-EGFP+ cells in the vicinity of synaptophysin-positive nerve fibers in white pulp (fig. S4C). The localization of ChAT-EGFP+ T cells in the white pulp of nude mice after adoptive transfer was indistinguishable from that of ChAT-EGFP+ T cells observed in transgenic ChAT(BAC)-EGFP mice (Fig. 2). Together, these results indicate that acetylcholine-producing T cells populate the spleen after adoptive transfer and localize to the region of splenic neurons under functional control of action potentials originating in the vagus nerve.
Fig. 4.
Vagus nerve stimulation requires acetylcholine-synthesizing T cells to attenuate TNF-α in endotoxemia. Indicated groups of mice were subjected to sham surgery or vagus nerve stimulation followed by endotoxin injection. Serum was obtained 90 min after endotoxin administration, and TNF-α was measured by ELISA. (A) BALB/c nude mice receiving spleen CD4+ CD44high CD62Llow ChAT-EGFP+ cells, eight mice per group. (B) BALB/c nude mice receiving spleen CD4+ CD44high CD62Llow ChAT-EGFP− cells, six or seven mice per group. (C) BALB/c nude mice receiving spleen CD4+ cells transfected with control scrambled siRNA, five or six mice per group. (D) BALB/c nude mice receiving spleen CD4+ cells transfected with ChAT siRNA, six or seven mice per group. Results are means ± SEM. *P < 0.05 compared with the respective sham group (two-tailed t test).
It remained theoretically possible that some other unanticipated effect of these cells not related to acetylcholine restored the inflammatory reflex. Accordingly, we next utilized small interfering RNA (siRNA) to deplete (knockdown) ChAT in spleen CD4+ T cells (fig. S5). Scrambled or ChAT siRNA-transfected CD4+ T cells were adoptively transferred into nude mice. Vagus nerve stimulation attenuated serum TNF-α levels in mice that received CD4+ T cells transfected with control siRNA (Fig. 4C), but failed to attenuate TNF-α in nude mice that received CD4+ T cells transfected with ChAT siRNA (Fig. 4D). Vagus nerve stimulation also significantly reduced serum IL-6 and IL-10 levels in nude mice that received CD4+ T cells transfected with control siRNA, but not in nude mice that received ChAT siRNA-treated T cells (fig. S6). Together, these data indicate that T cells with an intrinsic capacity to synthesize acetylcholine are required for the integrity of the inflammatory reflex.
It had been previously established that the inflammatory reflex requires an intact splenic nerve (15) and α7 nAChR expression in effector cytokine-producing cells in spleen (16). These results were previously difficult to rectify with observations that splenic nerves are adrenergic and do not produce the neurotransmitter, acetylcholine, required to interact with α7 nAChR. As early as 1965, investigators had observed that electrical stimulation of the splenic nerve induced acetylcholine release in spleen, but the cell source of this acetylcholine had been contested (29–32). The present findings show that vagus nerve stimulation also increases acetylcholine release from spleen and that spleen lymphocytes release acetylcholine in response to norepinephrine. It is likely that the regulatory effect of acetylcholine-synthesizing T cells described here is not restricted to the spleen, because CD4+ CD44high CD62Llow ChAT-EGFP+ T cells are also present in lymph nodes and Peyer’s patches (fig. S7), which are innervated by adrenergic neurons (33). Further, polyclonal activation of T cells also up-regulates ChAT-EGFP expression (fig. S2) (34) and augments acetylcholine production and release (35), which together make plausible the possibility that T cell activation status modulates the activity of the inflammatory reflex.
A major finding of this study is the surprising functional role for acetylcholine-producing memory T cells as integral components of a neural information system that controls innate immune responses. It should be possible to target these cells as therapeutic modalities for inflammatory and autoimmune diseases.
Supplementary Material
Acknowledgments
This work was supported in part by grants from National Institute of General Medical Sciences, NIH, to K.J.T. and from the Wenner-Gren Foundations in Stockholm to P.S.O. We thank B. Diamond for helpful discussions, and H. Borrero and S. Matheravidathu for technical support. The data reported in this paper are tabulated in the main text and supporting online material.
Footnotes
Supporting Online Material
www.sciencemag.org/cgi/content/full/science.1209985/DC1
Materials and Methods
Figs. S1 to S7
References
References and Notes
- 1.Borovikova LV, et al. Nature. 2000;405:458. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 2.Huston JM, et al. Crit. Care Med. 2007;35:2762. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 3.Guarini S, et al. Circulation. 2003;107:1189. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 4.Huston JM, et al. J. Immunol. 2009;183:552. doi: 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, et al. Nature. 2003;421:384. [Google Scholar]
- 6.Kox M, et al. Biochem. Pharmacol. 2009;78:863. doi: 10.1016/j.bcp.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 7.Rosas-Ballina M, et al. Mol. Med. 2009;15:195. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Westerloo DJ, et al. Gastroenterology. 2006;130:1822. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Pavlov VA, et al. Crit. Care Med. 2007;35:1139. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 10.Parrish WR, et al. Mol. Med. 2008;14:567. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeboah MM, et al. Kidney Int. 2008;74:62. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giebelen IA, van Westerloo DJ, LaRosa GJ, de Vos AF, van der Poll T. Shock. 2007;28:700. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- 13.Berthoud HR, Powley TL. J. Auton. Nerv. Syst. 1993;42:153. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger DL, Felten SY, Lorton D, Felten DL. Brain Behav. Immun. 1989;3:291. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 15.Rosas-Ballina M, et al. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11008. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huston JM, et al. J. Exp. Med. 2006;203:1623. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kees MG, Pongratz G, Kees F, Schölmerich J, Straub RH. J. Neuroimmunol. 2003;145:77. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Brain Behav. Immun. 1993;7:191. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 19.Dale HH, Dudley HW. J. Physiol. 1929;68:97. doi: 10.1113/jphysiol.1929.sp002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todman D. Eur. Neurol. 2008;60:162. doi: 10.1159/000145336. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima K, Oohata H, Fujimoto K, Suzuki T. Neurosci. Lett. 1989;104:336. doi: 10.1016/0304-3940(89)90599-5. [DOI] [PubMed] [Google Scholar]
- 22.Rinner I, Schauenstein K. J. Neurosci. Res. 1993;35:188. doi: 10.1002/jnr.490350209. [DOI] [PubMed] [Google Scholar]
- 23.Felten SY, Olschowka J. J. Neurosci. Res. 1987;18:37. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 24.Bellinger DL, Felten SY, Collier TJ, Felten DL. J. Neurosci. Res. 1987;18:55, 126. doi: 10.1002/jnr.490180110. [DOI] [PubMed] [Google Scholar]
- 25.Tallini YN, et al. Physiol. Genomics. 2006;27:391. doi: 10.1152/physiolgenomics.00092.2006. [DOI] [PubMed] [Google Scholar]
- 26.Feldman RD, Hunninghake GW, McArdle WL. J. Immunol. 1987;139:3355. [PubMed] [Google Scholar]
- 27.Kohm AP, Sanders VM. Pharmacol. Rev. 2001;53:487. [PubMed] [Google Scholar]
- 28.Riether C, et al. Brain Behav. Immun. 2011;25:59. doi: 10.1016/j.bbi.2010.07.248. [DOI] [PubMed] [Google Scholar]
- 29.Brandon KW, Rand MJ. J. Physiol. 1961;157:18. doi: 10.1113/jphysiol.1961.sp006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leaders FE, Dayrit C. J. Pharmacol. Exp. Ther. 1965;147:145. [PubMed] [Google Scholar]
- 31.Bulloch K, Damavandy T, Badamchian M. Int. J. Neurosci. 1994;76:141. doi: 10.3109/00207459408985999. [DOI] [PubMed] [Google Scholar]
- 32.Stephens-Newsham LG, Hebb C, Mann SP, Banns H. Gen. Pharmacol. 1979;10:385. doi: 10.1016/0306-3623(79)90076-4. [DOI] [PubMed] [Google Scholar]
- 33.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. J. Immunol. 1985;135(Suppl):755s. [PubMed] [Google Scholar]
- 34.Fujii T, et al. J. Neuroimmunol. 1998;82:101. doi: 10.1016/S0165-5728(97)00195-1. [DOI] [PubMed] [Google Scholar]
- 35.Rinner I, Kawashima K, Schauenstein K. J. Neuroimmunol. 1998;81:31. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.