Abstract
The long term outcome of graft failure after allogeneic stem cell transplantation (SCT) has not been well described. To fill this knowledge gap we performed a retrospective analysis of patients with graft failure over a 10 year time period in a single institution. Cases were included for analysis if they had failed to achieve an absolute neutrophil count (ANC) of 500 per microliter or more by 28 days post SCT or 42 days after cord blood transplantation (1ry graft failure); had a decrease in their ANC to less than 500 per microliter for three consecutive days after having achieved neutrophil engraftment (2ry graft failure) or failed to have evidence of at least 5% or more donor cell engraftment (1ry graft failure with autologous reconstitution). Among 1726 patients who underwent allografts from 1/1/90 through 12/31/2000, we identified 68 patients with graft failure. The 1, 2 and 5 year overall survival (OS) for all patients was 31%, 24% and 15%. A diagnosis of acute leukemia was a significant predictor for poor survival on multivariate analysis. We conclude that graft failure is an uncommon complication post allogeneic SCT and is associated with poor outcomes. Collection of autologous stem cells prior to high risk allografting can salvage a fraction of patients and lead to prolonged survivals.
Keywords: Graft failure, treatment, allogeneic stem cell transplantation
Introduction
Allogeneic hematopoietic stem cell transplantation (SCT) is being increasingly employed as treatment for a variety of malignant and nonmalignant hematologic disorders (1). Allogeneic SCT is used to rescue patients from the myeloablative effects of high dose pre-transplant conditioning therapy. Failure to achieve sustained donor hematopoietic cell engraftment although rare is a life-threatening complication. Various factors such as intensity of the preparative regimen, cell dose, cell manipulation (i.e. T cell depletion), prophylaxis used for prevention of graft versus. host disease (GVHD), human leukocyte antigen (HLA) compatibility, toxicities from infections and administration of drugs that could damage the allograft have all been identified as interfering with effective and sustained reconstitution of hematopoiesis (2,3).
Historically graft failure was a common cause of treatment failure for patients undergoing allogeneic transplants for severe aplastic anemia (4, 5). Graft failure in this setting was thought to be due to low cell doses, relatively low intensity of the conditioning regimen and allosensitization of the recipients (5). Improvements in the conditioning regimen, better understanding of graft cell dose and composition and availability of high resolution HLA matching techniques have reduced graft failure rates, but have not affected recovery from this complication. Treatment strategies of graft failure have generally revolved around re-transplantation and hematopoietic growth factors, however, the efficacy and long term outcomes of these strategies have not been well described (6-14).
The advent of non-myeloablative and reduced intensity regimens has also changed the transplant paradigm. In this setting it is possible to have relatively normal hematopoietic function after transplant without ever having evidence of donor cell engraftment (primary graft failure with autologous reconstitution). Thus graft failure in this situation would not result in poor hematopoietic function, but could result in a high rate of disease recurrence because of the loss of the graft versus tumor effect (15).
To determine the long-term outcomes of patients with graft failure after allogeneic transplantation as well as to define prognostic factors for outcomes and describe the results of different interventions we performed a retrospective analysis of patients with graft failure over a 10 year time period in our institution. The results of this analysis are described herein.
Patient and Methods
A retrospective chart review and waiver of informed consent was approved by the institutional review board at M.D. Anderson Cancer Center. A database review was conducted to identify cases of graft failure among allograft recipients within the dates of 1/1/90 and 12/31/00. Cases were included regardless of the underlying diagnosis, disease status prior to transplant, preparative regimen or stem cell source. A patient was considered to have graft failure if any of these three conditions were met: 1) Primary graft failure: failure to achieve an absolute neutrophil count (ANC) of greater than 500 per microliter by 28 days post bone marrow (BM) or peripheral blood progenitor cell (PB) transplantation or 42 days after cord blood (CB) transplantation 2) Secondary graft failure: loss of neutrophil engraftment as determined by an ANC of less than 500 per microliter for three consecutive days after having achieved neutrophil engraftment with documented donor cell chimerism and no evidence of disease progression in the marrow or 3) Primary graft failure with autologous reconstitution defined as achievement of an ANC of at least 500 per microliter but without evidence of at least 5% or more donor cell chimerism as defined by cytogenetics or molecular techniques (16). Actuarial survival was estimated by the Kaplan-Meier methods and compared according to patients' and transplant characteristics using Cox's proportional hazards model, p values of 0.05 or less were considered statistically significant (17,18). For the purpose of this analysis a reduced intense regimen was defined using the criteria established by Champlin et al. (15).
Results
Incidence and Characteristics of Graft Failure
From a total of 1726 allogeneic transplants performed in our institution between 1/1/1990 and 12/31/2000 (1008 BM, 681 PB and 37 CB) a total of 68 patients were identified as having either primary or secondary graft failure. Patient characteristics are summarized in table 1. Twenty nine (43%) patients experienced primary graft failure, 30 (44%) secondary graft failure while 9 (13%) had primary graft failure with autologous reconstitution. The most common assigned cause of graft failure was rejection (48%) followed by infection (225) and persistent disease (19%). Ten patients received no specific therapy for graft failure, 19 patients received growth factors alone (13 of which had secondary graft failure), and 10 patients had autologous cells infused, while 29 had cells from a donor of which 26 were procured from the original donor and 3 from a different donor. Seventeen patients were reconditioned prior to re-infusion of donor hematopoietic cells.
Table 1. Patient Characteristics.
| Variable | |
|---|---|
| N | 68 |
| Median Age in Years (Range) | 37 (4-75) |
| Sex | 45 male/23 female |
| Diagnosis | |
| CML or MPD | 22 |
| AML/ALL | 17/10 |
| Lymphoma/CLL | 13/4 |
| Aplastic Anemia | 2 |
| Median Months from Diagnosis to SCT (range) | 31 (2-369) |
| Donor Type | |
| 6/6 Related | 16 |
| Mismatched Related | 15 |
| 6/6 Unrelated | 31 |
| Mismatched Unrelated | 6 |
| Conditioning Regimen | |
| TBI + Other | 37 |
| Other Ablative | 8 |
| Non Ablative or Reduced Intensity | 23 |
| GVHD Prophylaxis | |
| Tacrolimus +/- other | 40 |
| Cyclosporine +/- other | 20 |
| T cell depletion | 8 |
| Stem Cell Source | |
| Bone Marrow | 36 |
| Peripheral Blood | 29 |
| Cord Blood | 3 |
N: number; CML: Chronic Myelogenous Leukemia; MPD: Myeloproliferative disorder; AML: Acute Myelogenous Leukemia; ALL; Acute Lymphoblastice Leukemia; CLL: Chronic Lymphocytic Leukemia; SCT: Stem cell transplant.
Resolution of Graft Failure
Of the 59 patients who developed neutrophil counts of less than 500 per microliter; 38 (64%) had neutrophil recovery at a median time of 22 days from the time of graft failure diagnosis (range, 8-86). Probability of neutrophil recovery was higher for patients with secondary graft failure (70%) than for those with primary graft failure (59%) but this difference was not statistically significant. The probability of neutrophil recovery was similar for patients receiving growth factor therapy compared to those receiving some form of cellular therapy (67% vs. 53%).
Overall Survival
The 1, 2 and 5 yr overall survival (OS) for all patients was 31%, 24% and 15%. Univariate analysis of patient, disease and transplant characteristics associated with survival are summarized in table 2. A diagnosis of acute leukemia and advanced disease (acute leukemia either not in remission or beyond 2nd remission, accelerated phase CML or chemorefractory lymphoid malignancy) were the only significant predictors of outcome on univariate analysis with hazard ratios (HR) of 1.9 and 2.9 respectively (p values of 0.02 and 0.006). Of these factors, only a diagnosis of acute leukemia remained significant on multivariate analysis (HR=2.6, p value=.01).
Table 2. Patient and Primary Disease Characteristics Associated With Survival After Graft Failure (Univariate Analysis).
| Variable | N | N Died @ 2 years | HR @ 2 years | 95% CI | P value |
|---|---|---|---|---|---|
| Age | |||||
| ≤ 37 years | 34 | 24 | Ref | ||
| > 37 years | 34 | 28 | 1.4 | 0.8-2.4 | 0.2 |
| Gender | |||||
| Male | 45 | 33 | Ref | ||
| Female | 23 | 19 | 1.5 | 0.8-2.6 | 0.2 |
| Disease Risk Group | |||||
| Good | 7 | 3 | Ref | ||
| Intermediate | 10 | 5 | Ref | ||
| Advanced | 51 | 44 | 2.9 | 1.4-6.2 | 0.006 |
| Diagnosis | |||||
| CML/MPD | 22 | 15 | Ref | ||
| NHL/CLL | 17 | 13 | Ref | ||
| AA | 2 | 0 | Ref | ||
| AML/ALL | 27 | 24 | 1.9 | 1.1-3.3 | 0.01 |
| Regimen | |||||
| TBI | 31 | 24 | Ref | ||
| Ablative No TBI | 14 | 12 | 1.2 | 0.6-2.5 | 0.5 |
| Reduced Intensity | 23 | 16 | 0.8 | 0.4-1.4 | 0.4 |
| Donor Type | |||||
| Matched Related | 31 | 25 | Ref | ||
| Matched Unrelated | 16 | 13 | Ref | ||
| Mismatched Related | 15 | 9 | 0.8 | 0.7-2.1 | 0.5 |
| Mismatched Unrelated | 6 | 5 | 0.6 | 0.2-2.9 | 0.6 |
N: number; HR: Hazard Ratios; CI; Confidence intervals; CML: Chronic Myelogenous Leukemia; MPD: Myeloproliferative disorder; AML: Acute Myelogenous Leukemia; ALL; Acute Lymphoblastic Leukemia; CLL: Chronic Lymphocytic Leukemia; SCT: Stem cell transplant. Good Risk: Acute leukemia in 1st remission or CML in 1st Chronic Phase; Intermediate risk: Acute leukemia in 2nd remission or CML in Accelerated phase or Chemosensitive relapse CLL or lymphoma; Advanced disease: Acute leukemia in relapse or beyond 2nd remission or chemorefractory disease.
Outcomes According to Type of Graft Failure
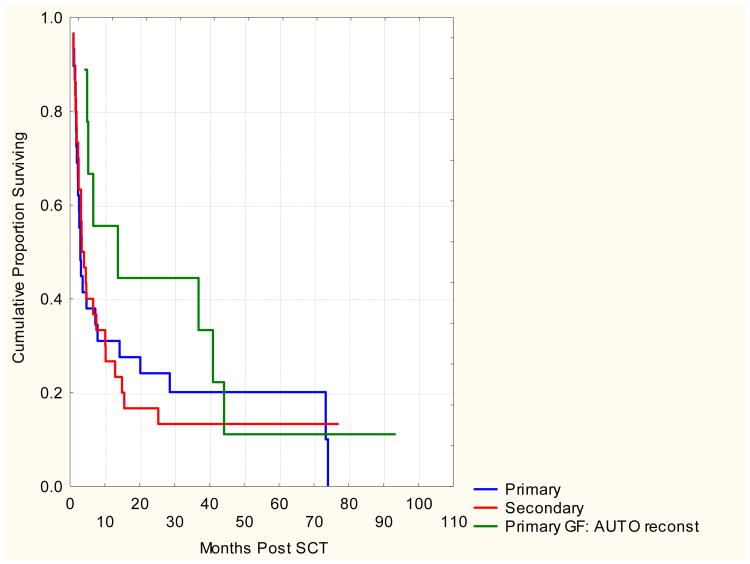
Patients with primary graft failure with autologous reconstitution had a longer median survival than those with primary or secondary graft failure (13.7, 2.9 and 3.7 months respectively, p<0.05). However, 5 year OS rates were similar for all types of graft failure with 18%, 11% and 13% of patients expected to be alive with primary graft failure, primary graft failure with autologous reconstitution and secondary graft failure respectively. Most common causes of death were original malignancy (41%), infection (27%) and graft failure (18%).
Primary Graft Failure
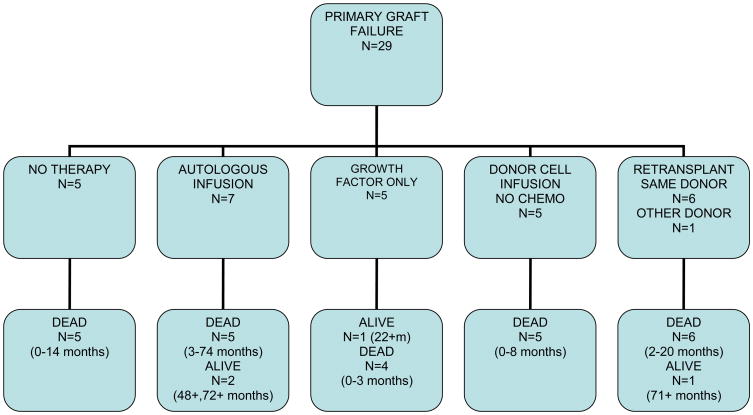
Twenty nine patients had primary graft failure, attributed to graft rejection in 18 patients, persistent disease in 7 patients, infection in 3 patients and an unknown etiology in one patient. Five patients had no specific therapy for graft failure; one of those patients eventually engrafted with donor cells and died 14 months post transplant from disease recurrence. The other 4 patients died within 3 months of initial transplantation either from disease progression or infectious complications. Seven patients received previously cryopreserved autologous stem cells as treatment for their graft failure, six had autologous recovery and one died 14 days post infusion without signs of neutrophil recovery. Five patients were treated with growth factors alone and only one achieved neutrophil recovery with autologous reconstitution resulting in a 22+ month survival. The other four patients died within 3 months of the original transplant. Five patients received cells from the original donor without preceding chemotherapy, four of which had neutrophil recovery with donor cell engraftment. The fifth patient died from progressive disease without hematologic recovery. All five patients died from either GVHD, infection or progressive disease within 8 months of original transplantation (range 2-8 months).
Seven patients were re-transplanted with an allogeneic donor; 6 of those received cells from the original stem cell donor and one from an alternate donor. Four of these had donor cell engraftment, one had autologous reconstitution and 2 patients died without neutrophil recovery. Only two of these patients survived more than 5 months, one died at 20 months from complications of GVHD, and the patient with autologous reconstitution remains alive with active CML at the time of last follow up 72+ months post transplant.
The median survival for patients with primary graft failure in this analysis was 2.9 months post transplant (range, 0.8-72+ months). The outcomes of these patients according to treatment of graft failure is summarized in figure 1-a.
Figure 1-a. Outcomes of Primary Graft Failure According to Treatment.
Primary Graft Failure with Autologous Reconstitution
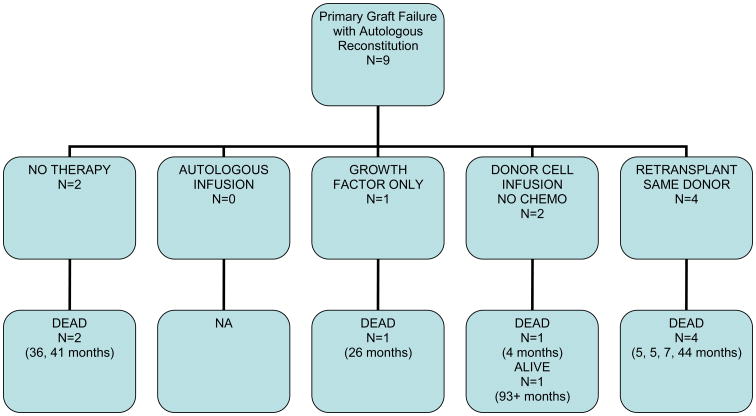
Nine patients had primary graft failure with autologous reconstitution, 7 of these were after RIC regimens and 2 after full dose conditioning. In 5 patients the cause of graft failure was ascribed to rejection, while in 4 it was ascribed to persistent disease. Six patients received subsequent stem cell infusions from the same donor with (n=4) or without (n=2) prior chemotherapy. Two patients received no therapy for graft failure while another received growth factors alone. The median survival of these patients was 13.7 months (range, 4-93+ months). One patient with CML remains alive in hematologic and cytogenetic remission on imatinib therapy 93+ months post initial transplant.
Secondary Graft Failure
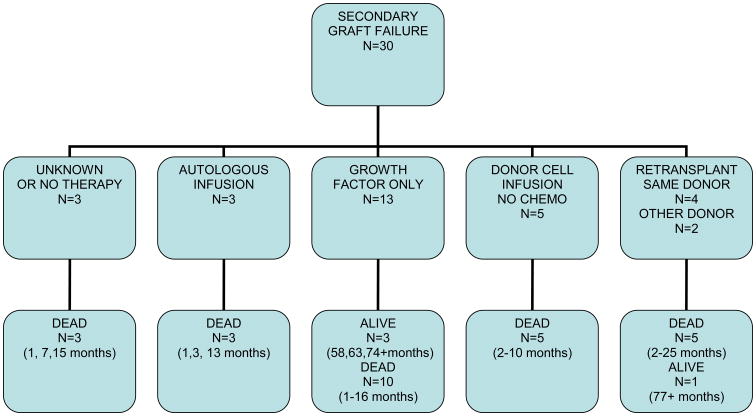
Thirty patients had secondary graft failure, 9 due to rejection, 12 due to infection, and 9 due to other causes (GVHD, drugs, and persistent disease). The median time to secondary graft failure was 51 days (range 14-356 days). Thirteen patients received treatment with growth factor alone. Twelve of these patients had recovery of graft function of which 3 were autologous, 8 were donor derived and one was unknown. Three patients remain alive at 58+, 63+ and 74+ months.
Five patients received allogeneic stem cells from the same donor without prior chemotherapy, only two of these patients recovered with donor cells and none survived for more than a year. Four patients were re-transplanted with the same donor; two engrafted with donor cells, and one had autologous reconstitution only after previously cryopreserved autologous stem cells were infused, all of these patients died between 2 and 4 months of initial transplant. Three patients received an infusion of their previously cryopreserved autologous cells, one patient had autologous recovery and died 13 months later from disease progression, while 2 patients died before neutrophil recovery could occur 2 and 6 days after the autologous stem cell infusion. Two patients were re-transplanted with an alternate donor; one recovered with autologous hematopoiesis and is alive 77+ months post initial transplant and the other had donor cell engraftment and died 25 months post initial transplant from complications of GVHD. The median survival for all patients with secondary graft failure was 3.7 months (range, 0.9 – 77+ months).Only 4 patients remain alive at 58+, 63+, 74+ and 77+ months post initial transplant. Outcomes of patients with secondary graft failure according to treatment are summarized in figure 1-b
Figure 1-b. Outcomes of Secondary Graft Failure According to Treatment Failure.
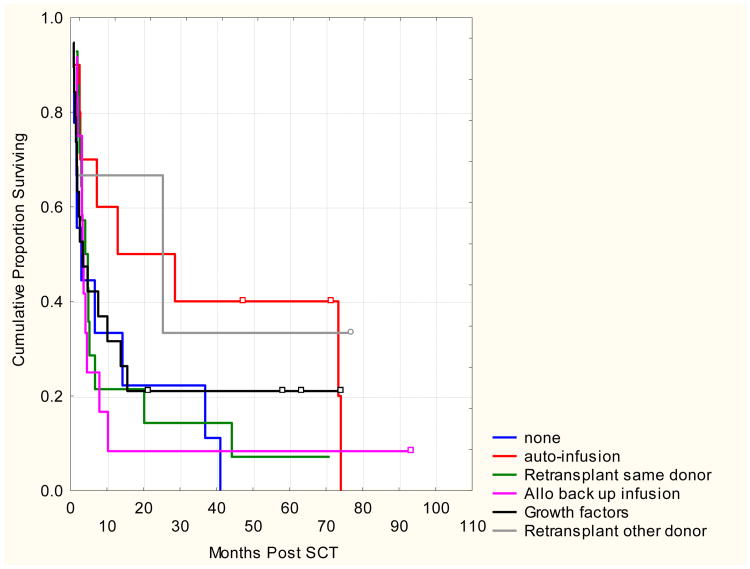
Patients with primary graft failure with autologous reconstitution had a longer median survival than those with primary or secondary graft failure (13.7, 2.9 and 3.7 months respectively). However, the 5 year OS rates were similar for all types of graft failure with 18%, 11% and 13% of patients expected to be alive with primary graft failure, primary graft failure with autologous reconstitution and secondary graft failure respectively. Most common causes of death were graft failure (18%); infection (27%) or original malignancy (41%). Kaplan-Meier survival curves according to graft failure type and treatment are shown in figures 2-a and 2-b.
Figure 2. a and b. Overall Survival According To Treatment Of Graft Failure And Type Of Graft Failure.
Discussion
Graft failure has traditionally been viewed as a complication of allogeneic transplants for aplastic anemia patients who had received multiple prior transfusions, or for patients receiving T-cell depleted transplants (19). However, with the advent of unrelated donor transplantation and particularly before modern allele typing, alternative donor transplantation with or without T cell depletion became the most common situation associate with graft failure occurring in up to 14 % of patients in the initial National Marrow Donor Program experience (20).
Improved tissue typing techniques as well as improvements in conditioning and patient selection have reduced the risk of graft failure, but this still represents an important complication of allografting particularly after alternative donor transplantation (21, 22). The optimal therapy for patients who develop graft failure has not been defined, and a variety of approaches from growth factors to re-transplantation have been proposed (6-14). In order to develop a rationale approach for the management of this potentially life threatening complication we performed a retrospective review of treatment outcomes among 68 patients who developed this complication during a 10 year period in our institution. This retrospective analysis confirmed that graft failure is a relatively rare complication (only 68 cases among 1726 transplants). Notwithstanding, graft failure was still associated with poor outcomes despite successful recovery of hematologic function.
Of particular interest in this retrospective review was the natural history of patients developing graft failure after reduced intensity or truly non-ablative regimens. It was initially thought that these patients would have autologous reconstitution if donor cell engraftment did not occur (21). Twenty three of the 68 patients with graft failure in this analysis had been conditioned with either a reduced intensity (n=7) or a truly non-ablative regimen (n=16). Of these, 7 died without ever having recovered hematologic function between 0 and 173 days post diagnosis of graft failure. These results underscore that in patients undergoing RIC conditioning who fail to engraft or lose graft function, aggressive treatment of graft failure should be implemented, since autologous reconstitution is far from certain.
Seven of the 8 patients surviving 5 years or more had autologous hematopoietic recovery, three of them because of infusion of previously cryopreserved autologous stem cells. This observation confirms the report by Mehta et al and underscores the importance of autologous stem cell cryopreservation for patients at high risk for graft failure due to stem cell source or graft manipulation, (i.e. alternative donor transplants or T cell depletion)(9). Five of these patients were still alive at last follow up (63+, 71+, 72+, 77+ and 93+ months post initial transplant). Three of these patients had CML, one had aplastic anemia and interestingly one patient with refractory AML remains alive with normal hematologic parameters despite autologous reconstitution with significant cytogenetic abnormalities 74+ months post initial transplant. This long term remission of a patient with a refractory hematologic malignancy despite absence of detectable donor cells has been observed by other investigators and suggests that a graft vs tumor effect may be operative at levels below our current limit of detection of donor cells (22,23).
This 10 year experience suggests that an aggressive approach to patients with graft failure will be needed in order to improve outcomes. This begins by identifying patients at high risk for this complication and developing strategies for early intervention. This retrospective experience supports the recommendation that patients with indolent diseases and patients with acute leukemia in remission should have autologous stem cells cryopreserved if they are to undergo an allogeneic transplant with an alternative source of stem cells (i.e. mismatched or cord blood transplants) or cells are to be manipulated. Seven of the 10 patients that received cryopreserved autologous stem cells for treatment of graft failure had neutrophil recovery and had a median survival of 21 months. Because of the retrospective nature of this review, we were unable to determine why certain patients received certain therapies for graft failure (i.e. infusion of cells with or without preceding chemotherapy, use of alternate donor, etc). In this regard various reports have appeared demonstrating that immunosuppressive conditioning is effective in securing donor cell engraftment in the setting of graft failure with good tolerance and efficacy (14,24)
During the initial years of this analysis no intervention was planned until after day 28 when a bone marrow aspirate confirmed lack of donor cell engraftment. We now recommend intervening as soon as it seems likely that delayed hematologic recovery or graft failure may occur (usually 3 weeks after transplant). Initial interventions include: a) reassessment of all ongoing medications to eliminate all non-essential potentially stem cell toxic drugs (i.e.: linezolid, acyclovir, ganciclovir, ect); b) early assessment of bone marrow aspiration looking for persistent disease or viral infections (i.e.: HHV6, parvovirus, CMV, etc.); c) institution of intensive growth factor therapy if this has not begun already (6, 24,27) and d) establishing a definitive plan if hematologic recovery has not been achieved by 28 days post transplant (i.e.: infusing cryopreserved autologous stem cells; contacting the original stem cell donor or an alternative donor to procure additional stem cells, assessment for investigational strategies, etc.). The issue of early identification and intervention has also been addressed by Mehta et al, who have recommended considering interventions in patients who have not achieved a peripheral blood leukocyte count of greater than 200 per microliter by day 16 post SCT. Re-transplantation using novel immunosuppressive regimens can be successful in achieving donor cell engraftment and can be associated with long term disease control (12,14, 24-26).
Cord blood transplantation is being used increasingly in adults and is associated with a higher risk of graft failure than bone marrow or peripheral blood stem cell transplantation. Chan et al. recently reviewed their experience with graft failure after cord blood transplantation. Seventy one of 110 pediatric patients had achieved a neutrophil count of 400 per microliter or greater by day 28, of the remaining 33 patients who were still neutropenic, 20 eventually attained donor myeloid recovery and 10 survived without donor-derived hematopoiesis. These patients received a second UCBT with 9 patients engrafting. Six of these patients remained alive at the time of the report with a median survival of over 2 years (24). Thus waiting until day 42 post cord blood transplant before intervening may be reasonable for the stable patient with autologous stem cells cryopreserved, but may not be appropriate for other patients.
In conclusion, graft failure is a rare complication post allogeneic stem cell transplantation, but is associated with poor outcomes. Collection of autologous stem cells prior to high risk allografting, as well as aggressive interventions can salvage a fraction of patients and lead to prolonged survivals. Disease recurrence after resolution of graft failure remains an important cause of treatment failure suggesting that once the patient is stabilized serious consideration to definitive therapy should once again be considered.
Figure 1-c. Outcomes of Primary Graft Failure with Autologous Reconstitution According to Treatment Failure.
Table 3. Outcomes of Graft Failure According to Treatment.
| Intervention | N | Overall Survival @ 2 years |
|---|---|---|
| None | 10 | 22% |
| Growth Factors | 19 | 21% |
| Autologous Infusion | 10 | 50% p=0.08 |
| Allogeneic Back up infusion | 12 | 8% |
| Re-transplant same donor | 14 | 14% |
|
| ||
| Re-transplant different donor | 3 | |
References
- 1.Armitage J. Bone Marrow Transplantation. N Engl J Med. 1994;330:827–838. doi: 10.1056/NEJM199403243301206. [DOI] [PubMed] [Google Scholar]
- 2.Tabbara I, Zimmermann K, Morgan C, Nahleh Z. Allogeneic hematopoietic cell transplantation; Complications and results. Arch Int Med. 2002;162:1558–1562. doi: 10.1001/archinte.162.14.1558. [DOI] [PubMed] [Google Scholar]
- 3.Thomas ED, Fefer A, Buckner CD, Storb R. Current status of bone marrow transplantation for aplastic anemia and acute leukemia. Blood. 1977;49:671–81. [PubMed] [Google Scholar]
- 4.Storb R. Graft rejection and graft versus host disease in marrow transplantation. Transplantation Proceedings. 1989;23(1 Pt 3):2915–2918. [PubMed] [Google Scholar]
- 5.Storb R, Thomas ED, Buckner CD, et al. Marrow transplantation for aplastic anemia. Seminars in Hematology. 1984;21:27–35. [PubMed] [Google Scholar]
- 6.Weisdorf D, Verfaillie CM, Davies SM, et al. Hematopoietic growth factors for graft failure after bone marrow transplantation: a randomized trial of granulocytemacrophage colony- stimulating factor (GM-CSF) versus sequential GM-CSF plus granulocyte- CSF. Blood. 1995;85:3452–3456. [PubMed] [Google Scholar]
- 7.Davies SM, Weisdorf DJ, Haake RJ, et al. Second infusion of bone marrow for treatment of graft failure after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;4:73–77. [PubMed] [Google Scholar]
- 8.Remberger M, Ringden O, Ljungman P, et al. Booster marrow or blood cells for graft failure after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;22:73–78. doi: 10.1038/sj.bmt.1701290. [DOI] [PubMed] [Google Scholar]
- 9.Mehta J, Powles R, Singhal S, et al. Outcome of autologous rescue after failed engraftment of allogeneic marrow. Bone Marrow Transplant. 1996;17:213–217. [PubMed] [Google Scholar]
- 10.Woodard P, Tong X, Richardson S, et al. Etiology and outcome of graft failure in pediatric hematopoietic stem cell transplant recipients. J Pediatr Hematol Oncol. 2003;25:955–959. doi: 10.1097/00043426-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Nemunaitis J, Singer JW, Buckner CD, Durnam D, Epstein C, Hill R, et al. Use of recombinant granulocyte-macrophage colony-stimulating factor in graft failure after bone marrow transplantation. Blood. 1990;76:245–253. [PubMed] [Google Scholar]
- 12.Grandage VL, Cornish JM, Pamphilon DH, Potter MN, Steward CG, Oakhill A, et al. Second allogeneic bone marrow transplants from unrelated donors for graft failure following initial unrelated bone marrow transplantation. Bone Marrow Transplant. 1998;21:687–690. doi: 10.1038/sj.bmt.1701146. [DOI] [PubMed] [Google Scholar]
- 13.Guardiola P, Kuentz M, Garban F, Blaise D, Reiffers J, Attal M, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukemia, chronic myeloid leukaemia and aplastic anemia. French Society of Bone Marrow Transplantation. Br J Haematol. 2000;111:292–302. doi: 10.1046/j.1365-2141.2000.02306.x. [DOI] [PubMed] [Google Scholar]
- 14.Jabbour E, Rondon G, Anderlini P, et al. Treatment of donor graft failure with nonmyeloablative conditioning of fludarabine, antithymocyte globulin and a second allogeneic hematopoietic transplantation. Bone Marrow Transplant. 2007;40:431–435. doi: 10.1038/sj.bmt.1705760. [DOI] [PubMed] [Google Scholar]
- 15.Champlin R, Khouri I, Kornblau S, Molldrem, Giralt S. Reinventing bone marrow transplantation. Nonmyeloablative preparative regimens and induction of graft-vs-malignancy effect. Oncology. 1999;13(5):621–628. [PubMed] [Google Scholar]
- 16.Antin JH, Childs R, Filipovich AH, et al. Establishment of complete and mixed donor chimerism after allogeneic lymphohematopoietic transplantation: recommendations from a workshop at the 2001 Tandem Meetings of the International Bone Marrow Transplant Registry and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2001;7:473–85. doi: 10.1053/bbmt.2001.v7.pm11669214. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–81. [Google Scholar]
- 18.Cox DR. Regression models and life tables (with discussion) J R Statistical Soc B. 34:187. [Google Scholar]
- 19.Storb R, Prentice RL, Thomas ED, et al. Factors associated with graft rejection after HLA identical marrow transplantation for aplastic anemia. Br J Haematol. 1983;55:573–585. doi: 10.1111/j.1365-2141.1983.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 20.Kernan NA, Bartsch G, Ash RC, et al. congenital disorders of the lymphohematopoietic system and congenital metabolic disorders. N Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 21.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after non myeloablative conditioning. Blood. 2004;104:2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 22.Ballen KK, Colvin G, Porter D, Quesenberry PJ. Low dose total body irradiation followed by allogeneic lymphocyte infusion for refractory hematologic malignancy—an updated review. Leukemia & Lymphoma. 2004;45:905–10. doi: 10.1080/10428190310001628167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballen KK, Becker PS, Emmons RV, et al. Low-dose total body irradiation followed by allogeneic lymphocyte infusion may induce remission in patients with refractory hematologic malignancy. Blood. 2002;100:442–50. doi: 10.1182/blood.v100.2.442. [DOI] [PubMed] [Google Scholar]
- 24.Chan KW, Grimley MS, Taylor C, Wall DA. Early identification and management of graft failure after unrelated cord blood transplantation. Bone Marrow Transplant. 2008;10:1–7. doi: 10.1038/bmt.2008.40. [DOI] [PubMed] [Google Scholar]
- 25.Heinzelmann F, Lang PJ, Ottinger H, et al. Immunosuppressive total lymphoid irradiation-based reconditioning regimens enable engraftment after graft rejection or graft failure in patients treated with allogeneic hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2008;70:523–8. doi: 10.1016/j.ijrobp.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Lang P, Mueller I, Greil J, et al. Retransplantation with stem cells from mismatched related donors after graft rejection in pediatric patients. Blood Cells Mol Dis. 2008;40:33–39. doi: 10.1016/j.bcmd.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Hachem RY, Hicks K, Huen A, Raad I. Myelosuppression and serotonin syndrome associated with concurrent use of linezolid and selective serotonin reuptake inhibitors in bone marrow transplant recipients. Clinical Infectious Diseases. 2003;37:e8–11. doi: 10.1086/375689. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Law P, Ball E. Failure of engraftment. In: Ball E, Lister J, Law P, editors. Hematopoietic Stem Cell Therapy. 1st. Churchill-Livingston; Philadelphia: 2000. pp. 521–531. [Google Scholar]
- 29.Mehta J, Powles R, Singhal S, et al. Early identification of patients at risk of death due to infections, hemorrhage, or graft failure after allogeneic bone marrow transplantation on the basis of the leukocyte count. Bone Marrow Transplant. 1997;19:349–355. doi: 10.1038/sj.bmt.1700657. [DOI] [PubMed] [Google Scholar]