Abstract
The mammalian epidermis provides both an interface and a protective barrier between the organism and its environment. Lipid, processed into water-impermeable bilayers between the outermost layers of the epidermal cells, forms the major barrier that prevents water from exiting the organism, and also prevents toxins and infectious agents from entering. The secretory phospholipase 2 (sPLA2) enzymes control important processes in skin and other organs, including inflammation and differentiation. sPLA2 activity contributes to epidermal barrier formation and homeostasis by generating free fatty acids, which are required both for formation of lamellar membranes and also for acidification of the stratum corneum (SC). sPLA2 is especially important in controlling SC acidification and establishment of an optimum epidermal barrier during the first postnatal week. Several sPLA2 isoforms are present in the epidermis. We find that two of these isoforms, sPLA2 IIA and sPLA2 IIF, localize to the upper stratum granulosum and increase in response to experimental barrier perturbation. sPLA2F−/− mice also demonstrate a more neutral SC pH than do their normal littermates, and their initial recovery from barrier perturbation is delayed. These findings confirm that sPLA2 enzymes perform important roles in epidermal development, and suggest that the sPLA2IIF isoform may be central to SC acidification and barrier function. This article is part of a Special Issue entitled The Important Role of Lipids in the Epidermis and their Role in the Formation and Maintenance of the Cutaneous Barrier. Guest Editors: Kenneth R. Feingold and Peter Elias.
Keywords: Lipids, pH, Secretory phospholipase, sPLA2, Stratum corneum, Permeability barrier
1. sPLA2 — general aspects
Secretory phospholipases A2 (sPLA2) comprise a large and widely distributed family of enzymes. sPLA2s are distributed throughout the epidermis [1–3]. These enzymes hydrolyze the glycerophospholipid ester bond at the sn-2 position to generate a free fatty acid and a lysophospholipid. A subset of sPLA2s has been shown to initiate and augment arachidonic acid release, which then can be converted to eicosanoids. sPLA2 activity requires micromolar to millimolar Ca2+ concentrations, and is relatively non-selective in the fatty acids that can be released. Two pathways, termed the external plasma membrane pathway and the heparin sulfate (HSPG)-shuttling pathway, have been described (reviewed in Murakami and Kudo 2004) [4]. In the HSPG-shuttling pathway, sPLA2-IIA, -IID, and-V bind to the heparan sulfate chains of HSPG glypican, are endocytosed though caveolae/rafts, and supply arachidonic acid to cyclooxygenases in activated cells. This pathway results in preferential release of cellular arachidonate and is stimulus-dependent. An alternative pathway is the external plasma membrane pathway. In this pathway, sPLA2-V and -X release arachidonic acid from phosphatidylcholine located in the extracellular face of the plasma membrane. The resulting arachidonic acid diffuses or is transported through the cell's plasmamembrane, supplying perinuclear cyclooxygenases. sPLA2-IIF also can utilize the external pathway. This pathway leads to nonselective fatty acid release (see Section 4.0, below), and is stimulus independent, often described as acting on quiescent cells.
sPLA2's act through these, and likely additional pathways that are not yet fully defined to produce a multitude of effects in mammalian tissues. In addition to pancreatic sPLA2-IB, whose role in digestion is well defined, sPLA2 activity controls aspects of lipoprotein metabolism/atherosclerosis; lung surfactant hydrolysis/acute respiratory distress syndrome; bacterial membrane hydrolysis/innate immunity; spermatozoa acrosome reaction; and formation and regulation of the epidermal water permeability barrier (reviewed in Lambeau and Gelb, 2008 and Murakam and Kudo 2004)[4,5].
2. sPLA2 in the skin
Studies examining the presence and localization of sPLA2 subtypes in the skin have not been consistent for some subtypes (notably sPLA2X). The discrepancies noted in Table 1, below, likely result from two factors: 1) expression differs in mouse vs. human skin; and 2) expression differs in cultured keratinocytes vs. native skin. However, studies consistently show that a variety of sPLA2 isoform are expressed in epidermis, and that subtypes vary in location and with differentiation state.
Table 1.
sPLA2 expression in skin (M = mouse; H = human).
| Subtype | Epidermal Localization | Citation |
|---|---|---|
| lB | Suprabasal keratinocytes (M) SG — SC junction (H) |
Gurrieri 2003 Ma:ereeuw-Hautier DOW Haas 2005 |
| IIA | Throughout epidermis (M) Upper SC (H) |
Gurrieri 2003 Haas 2005 |
| IIC | Throughout epidermis | Gurrieri 2003 |
| lID | Throughout epidermis (M) | Gurrieri 2003 |
| Perinuclear localization basal keratinocytes (H) | Haas 2005 | |
| IIE | Suprabasal keratinocytes (M) |
Gurrieri 2003 Sato 2009 Haas 2005 |
| IIF | Suprabasal keratinocytes (M, H) |
Sato 2009 Gurrieri 2003 |
| III | Keratinocytes (M) | Sato 2009 |
| Basal keratinocytes (H) | Haas 2004 | |
| Hyperproliferative epidermis (H) | Rys-Sihora 2003 | |
| V | Suprabasal keratinocytes (M) | Gurrieri 2003 |
| Basal and spinous keratinocytes (H) | Haas 2004 Gurrieri 2003 |
|
| X | Basal and suprabasal keratinocytes (M) | Schadolo 2001 |
| Suprabasal layers (H) | Haas 2005 | |
| Present only in hair follicles (M) | Yamamoto 2010 | |
| XII | Suprabasal keratinocytes (M) | Gurrieri 2003 |
3. Key roles of sPLA2 in epidermis
sPLA2 actions are involved in several essential epidermal processes. The most extensively studied is the role of sPLA2s in inflammation. Transgenic mice overexpressing sPLA2-IIA develop epidermal hyperplasia and alopecia [6]. Similarly, transgenic mice overexpressing endogenous group III sPLA2 (sPLA2G3) develop skin inflammation, acanthosis and sebaceous gland hyperplasia [2]. SPLA2IIA also appears to be upregulated in psoriatic skin, along with sPLA2-IID and IB [3]. sPLA-IIA and IID also have been shown to release arachidonic acid [7]. Pharmacologic inactivation of cytosolic phospholipase A2 inhibits TNF-induced NF kappa B activation [8] in HaCat keratinocytes, while TPA-induced inflammation and arachidonic acid release from mouse keratinocytes are modulated by cytosolic and secretory PLA2 [9], further identified as sPLA2-IA, IID and IB [3]. In contrast, transgenic mice overexpressing sPLA2-X develop enhanced differentiation, alopecia and epidermal hyperplasia but no signs of inflammation [10]. Finally, sPLA2-IID is expressed in antigen-presenting skin dendritic cells, where it acts to down-regulate inflammation, in contrast to its role in keratinocytes [11]. In humans, SC acidity influences skin inflammation via various mechanisms. First, impaired SC acidity, via impaired barrier function [12], could lead to increased antigen presentation or microbial invasion. Enhanced antigen presentation, via both the innate and adaptive immune systems, provides inflammatory responses [13]. Impaired SC acidity could act in concert with dysregulation of the immune system to produce a vicious cycle of inflammation and impaired barrier where a leaky barrier leads to antigen ingress and exaggerated inflammation which in turn leads to further barrier deterioration. This has been proposed for skin inflammation in HIV+ patients [14]. Finally, a more neutral pH, acting through increased kallikrein-mediated interleukin 1 activation, can produce increased skin irritation (Nygaard & Egeland).
sPLA2 expression also varies with keratinocyte differentiation state and there is some evidence that sPLA2 can direct keratinocyte differentiation [3,6,10]. Finally, sPLA2 has been shown to control SC acidification (see below).
3.1. Specific sPLA2 isoforms and epidermal barrier development
To assess the role of the various sPLA2 isoforms in skin, we first examined their relative abundance. Because functional studies demonstrate that sPLA2 activity is especially important in barrier homeostasis during the first week of life [15,16], we compared sPLA2 isoform abundance in perinatal mice (1–6 days) with young adult mice (3–6 month). We found that the relative abundance of the different sPLA2 isoforms varied widely, and some isoforms (notably IID, IIE and V) changed as mice matured (Table 2). These data are consistent with earlier studies that show that some sPLA2 isoforms change as keratinocytes differentiate [3].
Table 2.
Relative sPLA2 isoform expression in neonatal and young adult mice.
| Relative gene copy number |
IB | IIA | IIC | IID | IIE | IIF | V | X | XIIA |
|---|---|---|---|---|---|---|---|---|---|
| Neonatal (1–6 day) | 184 ± 517 n = 9 |
704 ± 946 n = 10 |
1674 ± 691 n = 4 |
253,026 ± 97,670 n = 5 |
745,646 ± 286,046 n = 10 |
13,004,451 ± 4,949,792 n = 10 |
674,990 ± 292,527 n = 10 |
7236 ± 3420 n = 10 |
103,215 ± 23,447 n = 9 |
| Young Adult (3–6 month) | 3436 ± 768 n = 5 |
8805 ± 24,646 n = 10 |
1305 ± 2918 n = 5 |
79,291 ± 56,543 n = 5 |
9,948,017 ± 2,220,239 n = 5 |
14,119,680 ± 8,408,130 n = 5 |
23,200 ± 6298 n = 5 |
24,288 ± 16,494 n = 5 |
105,612 ± 17,781 n = 5 |
Real-time RT-PCR was used to determine the expression patterns of genes implicated in skin barrier formation in newborn mice. RT-PCR was performed, using the methods outlined in Ilic et al (2006) [17]. Normalized relative gene copy numbers (GCNs) were then compared for the different sPLA2 isoforms. Most gene expression patterns remained relatively constant during development, with the exceptions of IIE, which increased; which IID and V decreased. sPLA2 IIF was the most abundant isoform expressed, especially during the first week of life.
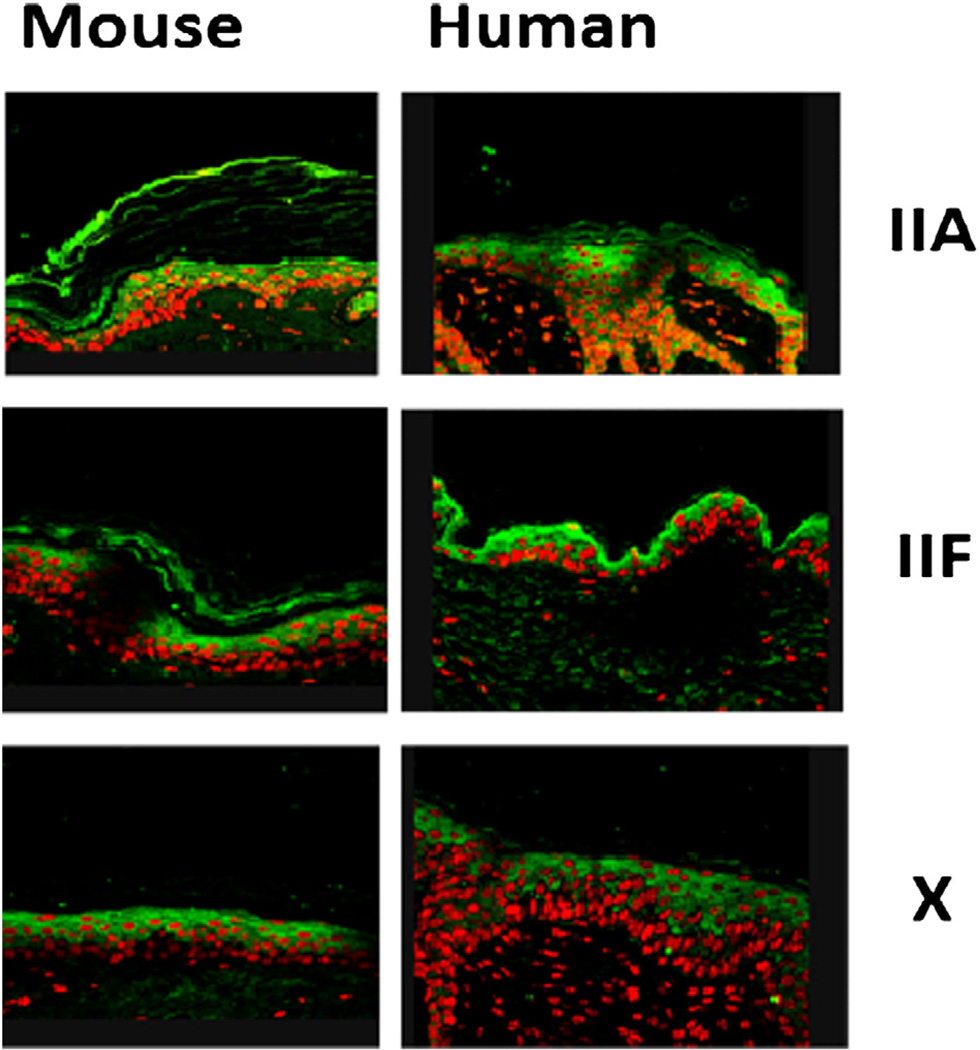
Confirming previous studies [3], we found that the sPLA2 isoform IIA localized to the upper SG/SC (Fig. 1), while isoform X is found throughout much of the epidermis. We also found that IIF localized to the upper SG (Fig 1).
Fig. 1.
Localization of sPLA2 isoforms to the Epidermis. Normal hairless mice and normal human adult skin samples were immunostained with antibodies specific to sPLA2 IIA, IIF and X. All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. Skin samples were fixed in Formalde-Fresh solution (Fisher), then paraffin-embedded. Antibodies directed against specific sPLA2 isoforms were a gift of Dr. Gelb [49]. Immunohistochemical staining for assessing changes in epidermal differentiation was performed as described earlier [50,51]. IIA and IIF localized to the SG and SC of the epidermis, while X was found in the suprabasal levels.
To further delineate the roles of sPLA2 isoforms IIA and IIF, we examined their behavior after barrier disruption in normal hairless mouse skin. As assessed by immunostaining, both isoforms increased after barrier disruption, peaking at 6 h and returning to baseline by 24 h. Occlusion blocked the increases for both isoforms seen after barrier disruption demonstrating that this increase is a specific response to barrier requirements. Nuclear hormone receptor agonists such as LXR and PPARα are known to activate sPLA2 [18–20]. We therefore assessed whether application of LXR or PPAR agonists changed sPLA2 IIA or IIF expression in vivo. We found that sPLA2 IIF expression was increased by LXR and PPAR alpha, beta and gamma agonists, while sPLA2 IIA expression was unchanged after agonist applications. These findings suggest that sPLA2 isoform expression is controlled by epidermal barrier status, and that sPLA2 IIF, in particular, might be important in epidermal barrier homeostasis.
3.2. sPLA2 enhances epidermal barrier formation via lipid metabolism and SC acidification
The epidermal barrier is critical for terrestrial life, preventing water and ion loss, and also preventing toxins, infectious agents and bacteria from entering [21]. Pathological states in which this barrier is lost, such as prematurity or burns, are associated with high mortality, stressing the importance of this barrier [22].
The epidermal barrier is formed by the outermost layer of the skin, the stratum corneum (SC). A two-compartment complex formed by the anucleate SC corneocytes and the interspersed lipid bilayers, originally likened to ‘bricks and mortar’ [23,24], constitute the major permeability barrier. Several coordinated processes are required to develop this barrier. First, the epidermis must develop completely, forming normal anucleate SC corneocytes. Second, lipid must be secreted form the stratum granulosum (SG) cells before they transition into SC corneocytes [25,26]. In contrast to the phospholipid-containing plasma membrane of the viable epidermal keratinocytes, lamellar membranes are composed of ceramides, cholesterol and fatty acids. These lipids are delivered to the SC by the secretion of lamellar body contents. sPLA2 activity polar epidermal lipids into less polar species. Together with ceramides, these apolar lipids are major components of the lipid found between corneocytes in the SC. sPLA2 enzymes have been localized within the lamellar bodies, and pharmacologic sPLA2 inhibition reduces free fatty acid content, increases phospholipid content, inhibits barrier recovery and compromises epidermal barrier function [27]. Even though these studies use relatively non-selective pharmacologic inhibitors, they suggest that sPLA2might play an important role in generating the SC lipids required for establishing a competent epidermal permeability barrier. We were able to test this using more specific deletion of the sPLA2 IIF isoform in mice (see 4.0 below).
In addition to generating lipids required for structural barrier formation, sPLA2 activity also generates fatty acids that acidify the SC. Two enzymes, sphingomyelinase (SM) and beta-glucocerebrosidase (GCS) generate ceramides destined for the lamellar bilayers. While differentiation and lipid secretion are controlled by a number of factors, including Ca2+ [28] the SM and GCS enzymes are activated by acidity [29–31]. SC lipid metabolism and acidity, produced partly by sPLA2 lipid hydrolysis, regulates lipid processing in the SC, producing a barrier that protects the organism from toxins, water loss and infection [29,32].
3.3. Other functions of SC acidity
SC acidity also functions independently as a deterrent to skin infections. An acidic SC inhibits skin colonization with skin pathogens such as Staphylococcus aureus and Streptococcus pyogenes [33,34]. Skin alkalinity, as seen in infant skin exposed to urea in the diaper area, predisposes to bacterial and yeast infections [35]. Finally, SC acidity controls SC integrity. Loss of SC acidity increases serine protease activity, leading to abnormal corneodesmosome degradation [12,32], and loss of SC integrity and cohesion. The importance of SC acidity for antimicrobial function has been recognized since 1892, when Heuss coined the term “acid mantle” [36].
4. sPLA2 subtypes and SC acidity
Although acidification is essential for normal epidermal barrier function, it takes place within a narrow, strictly defined area of the epidermis. The viable epidermis maintains a neutral pH. Discrete microdomains of acidity, localized between cells of neutral pH, begin to appear at the base of the SC [37], both at equilibrium and as acidity develops after birth and is restored during barrier recovery [38]. Overall, the SC progressively acidifies, with the outermost layers 1–2 pH units more acid than the viable epidermis [39]. Increased acidity in the upper SC is due to more numerous acidic microdomains rather than increasing acidity in a fixed number of acidic microdomains [37,38]. SC acidity varies among groups, with men generally having a more acidic SC than women, post-pubertal skin more acidic than pre-pubertal skin, and certain areas of the body more acidic than others [40–46]. As noted in Table 1, above, sPLA2 subtypes are distributed throughout the epidermis. sPLA2 activity increases in the first week after birth, and its distribution broadens to include all SC layers by five days after birth [15]. Pharmacologic inhibition of sPLA2 increases SC pH in the first week after birth [16]. The most likely candidates for SC acidification, based on distribution, are sPLA2-1B, IIA [8,47,48], and IIF, as noted above.
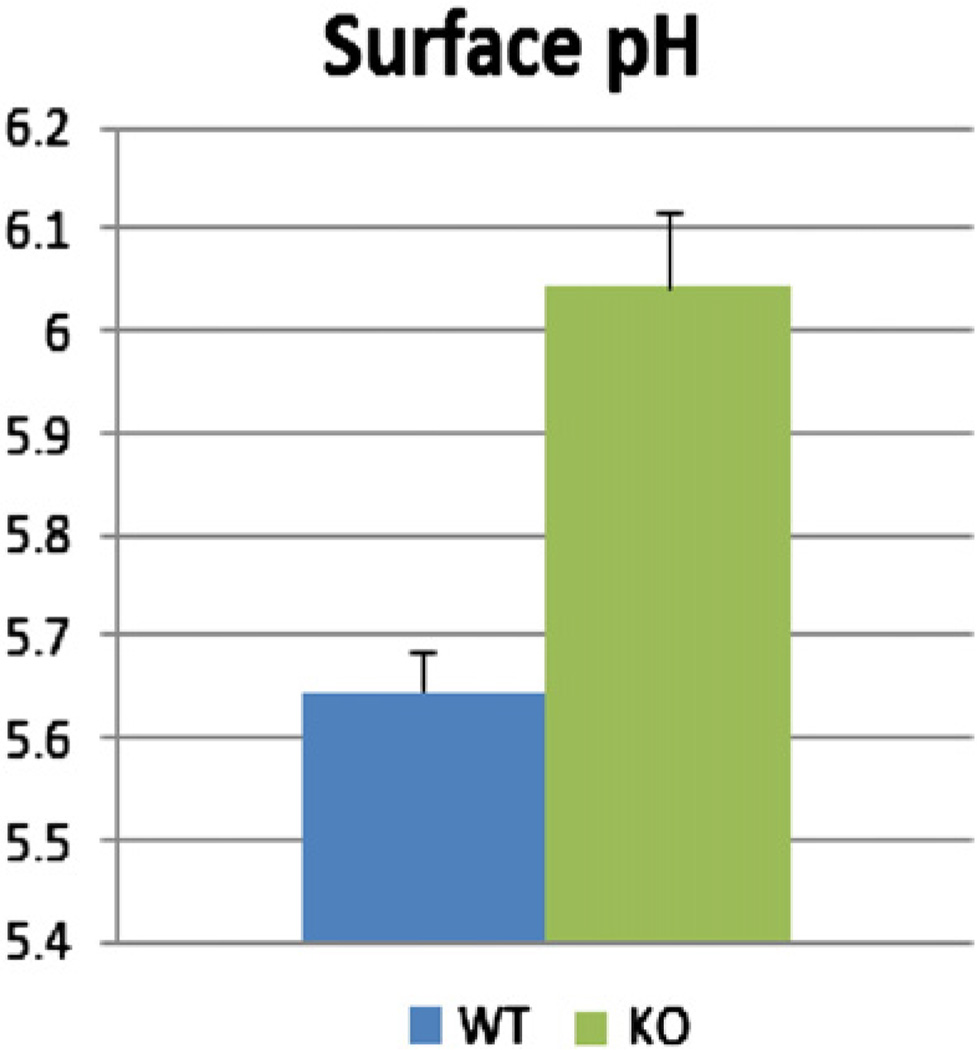
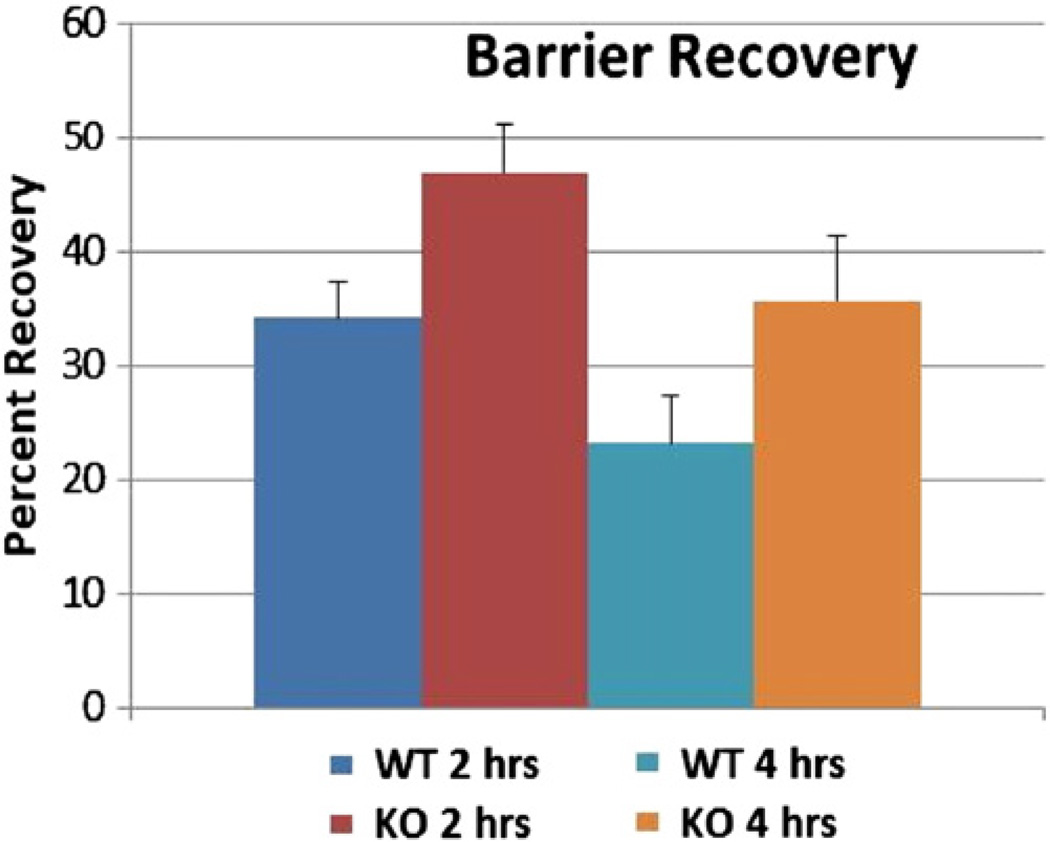
Because SPLA2 1B was expressed in very low abundance, and because SPLA IIA expression did not change in response to LXR or PPAR agonist application, our initial studies focused on SPLA2 IIF, which is abundant in both neonatal and adult skin; upregulated by barrier perturbation; and upregulated by PPAR or LXR agonists. To assess whether sPLA2 IIF was important for SC acidification, we measured SC acidity in transgenic mice in which sPLA2 IIF had been deleted. We found that the SC pH is significantly less acidic in mice lacking sPLA2 IIF, compared with their normal littermates (Fig 2). Furthermore, initial barrier recovery was delayed in sPLA2−/− mice (Fig. 3).
Fig. 2.
Surface pH is Less Acidic in Mice Lacking Epidermal sPLA2 IIF. Surface pH was measured using in 3 month old sPLA2 IIF−/− mice vs. their normal littermates. SC surface pH was measured with a flat, glass surface-electrode from Mettler-Toledo (Giessen, FRG), attached to a pH meter (pH 900; Courage & Khazaka, Cologne, FRG). Surface pH was measured from flanks in all mice. sPLA2 IIF−/− mice were a gift from Michael Gelb, PhD. The sPLA2 IIF−/− genotype was verified by PCR. Normal mice displayed an acidic pH. In contrast, sPLA2 IIF−/− mice were unable to acidify their SC normally.
Fig. 3.
Barrier Recovery is delayed at 2 but not 4 h in sPLA2 IIF−/− Mice. 3 month old sPLA2−/− mice and their normal littermates underwent barrier perturbation using tape-stripping. Basal epidermal permeability barrier function was assessed by measuring transepidermal water loss (TEWL) using TM300 connected to MPA5 (C&K, Cologne, Germany). For barrier recovery, TEWL was measured using an electrolytic water analyzer (Meeco, Warrington, PA) at 2 and 4 h after tape stripping. A 10-fold increase in TEWL was used as an indicator that the barrier had been perturbed. Percent barrier recovery at 2 and 4 h was calculated as described earlier (Man et al 2011). sPLA2−/− mice and 8 wild-type control mice. Barrier recovery was significantly slower in the sPLA2−/− mice at 2 h (p = 0.04). While barrier recovery still lagged at 4 h for sPLA2−/− mice, this difference was not significant (p = 0.12). N = 6 mice.
Specific clinical conditions with defects in SC acidity are discussed below (Section 4.2).
4.1. Other sources of SC acidity
SC acidity was originally attributed to exogenous sources such as sebaceous gland secretion, lactic acid from keratinocyte metabolism, eccrine glands, or colonizing bacteria [33,52,53]. However, a normal acidic SC pH is found in mice lacking sebaceous glands (asebia) mice [10]. Further, acidification begins at the deeper SC levels, not at the surface, as would be expected, were these exogenous primary mechanisms [15]. Besides sPLA2, other endogenous sources, such as the sodium proton pump (NHE1) or urocanic acid, also appear to contribute to SC acidity.
NHE1 is a ubiquitous transporter that widely regulates intracellular pH [54,55]. NHE1 has been shown to acidify microdomains at the SG/SC interface [37]. These microdomains are the site of initial lipid processing by SM and GCS [37]. An additional mechanism that has been proposed for SC acidification is the generation of urocanic acid from filaggrin via histidine [56]. However, the histidase enzyme responsible for this conversion does not increase with neonatal SC acidification, and histidase deficient mice do not display a defect in SC acidity, possibly due to compensatory NHE1 and sPLA2 upregulation [21]. In contrast, when either the NHE1 or sPLA2 mechanisms are disabled, SC acidity dissipates, indicating that these pathways cannot completely compensate for each other.
4.2. sPLA2 acidification is central to neonatal SC acidity, while NHE1 may be more important in adult/aged skin
Both neonatal and elderly skin, suffer from suboptimal acidification. sPLA2 activity appears to be required to develop an acidic pH after birth, while declining NHE1 activity appears responsible for the impaired SC acidification seen in elderly skin. Thus, each acidification mechanism is developmentally regulated.
SC pH is neutral at birth [38,46,57,58], leading to impaired barrier recovery, even though SC morphology, lipid secretion, and baseline barrier function are normal [59]. While NHE1 expression decreases [15] in the first week after birth, sPLA2 activity increases substantially, and its distribution broadens to include all SC layers by five days after birth [15]. Pharmacologic inhibition of sPLA2 delayed development of SC acidity after birth, and suggests that sPLA2 activity is responsible for a full pH unit of acidity [16]. Nuclear hormone receptor agonists such as LXR and PPARα activate sPLA2. PPARα agonists enhance SC acidity in neonates [18–20] and adult SC, leading to improved barrier recovery after acute disruption [60]. LXR agonists also enhance neonatal SC acidity via sPLA2 activation leading to improved barrier homeostasis and SC integrity [47].
In contrast, little is known about changes in sPLA2 activity in aging skin. In ‘aged aged’ (i.e. over 80 years of age), declining barrier function appears to be due to a global decrease in lipid synthesis and secretion [48,61]. In ‘middle age’ (50–80 years), a more specific defect in NHE1 expression is found [62]. In this age group, normal barrier function can be restored by exogenously acidifying the SC [62] suggesting that the decline in NHE1 leads to a defect in SC acidification, and thus causing the decline in barrier function.
sPLA2 enzymes catalyze essential processes in both the viable cells of the epidermis and in the SC. sPLA2 activity is required for formation of the lamellar membranes, and acidification of the SC, especially in the post-natal period. Several sPLA2 isoforms are expressed in the epidermis. Of these, sPLA2 II F seems to control SC acidity.
Acknowledgments
We gratefully acknowledge the superb editorial assistance of Ms. Joan Wakefield and Ms. Jerelyn Magnusson. This work was supported by NIH grants R37HL036235 (MG), R21 ARO61583 and R01AG028492 (TM) and the Research Service, Department of Veterans Affairs, San Francisco, CA.
Contributor Information
Dusko Ilic, Email: dusko.ilic@kcl.ac.uk.
Michael Gelb, Email: gelb@chem.washington.edu.
Theodora M. Mauro, Email: maurot@derm.ucsf.edu.
References
- 1.Gurrieri S, Furstenberger G, Schadow A, Haas U, Singer AG, Ghomashchi F, Pfeilschifter J, Lambeau G, Gelb MH, Kaszkin M. Differentiation-dependent regulation of secreted phospholipases A2 in murine epidermis. J. Invest. Dermatol. 2003;121:156–164. doi: 10.1046/j.1523-1747.2003.12315.x. [DOI] [PubMed] [Google Scholar]
- 2.Sato H, Taketomi Y, Isogai Y, Masuda S, Kobayashi T, Yamamoto K, Murakami M. Group III secreted phospholipase A2 transgenicmice spontaneously develop inflammation. Biochem. J. 2009;421:17–27. doi: 10.1042/BJ20082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas U, Podda M, Behne M, Gurrieri S, Alonso A, Furstenberger G, Pfeilschifter J, Lambeau G, Gelb MH, Kaszkin M. Characterization and differentiation-dependent regulation of secreted phospholipases A in human keratinocytes and in healthy and psoriatic human skin. J. Invest. Dermatol. 2005;124:204–211. doi: 10.1111/j.0022-202X.2004.23513.x. [DOI] [PubMed] [Google Scholar]
- 4.Murakami M, Kudo I. Secretory phospholipase A2. Biol. Pharm. Bull. 2004;27:1158–1164. doi: 10.1248/bpb.27.1158. [DOI] [PubMed] [Google Scholar]
- 5.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 6.Grass DS, Felkner RH, Chiang MY, Wallace RE, Nevalainen TJ, Bennett CF, Swanson ME. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J. Clin. Invest. 1996;97:2233–2241. doi: 10.1172/JCI118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murakami M, Kambe T, Shimbara S, Higashino K, Hanasaki K, Arita H, Horiguchi M, Arita M, Arai H, Inoue K, Kudo I. Different functional aspects of the group II subfamily (Types IIA and V) and type X secretory phospholipase A(2)s in regulating arachidonic acid release and prostaglandin generation. Implications of cyclooxygenase-2 induction and phospholipid scramblase-mediated cellular membrane perturbation. J. Biol. Chem. 1999;274:31435–31444. doi: 10.1074/jbc.274.44.31435. [DOI] [PubMed] [Google Scholar]
- 8.Thommesen L, Sjursen W, Gasvik K, Hanssen W, Brekke OL, Skattebol L, Holmeide AK, Espevik T, Johansen B, Laegreid A. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J. Immunol. 1998;161:3421–3430. [PubMed] [Google Scholar]
- 9.Li-Stiles B, Lo HH, Fischer SM. Identification and characterization of several forms of phospholipase A2 in mouse epidermal keratinocytes. J. Lipid Res. 1998;39:569–582. [PubMed] [Google Scholar]
- 10.Yamamoto K, Taketomi Y, Isogai Y, Miki Y, Sato H, Masuda S, Nishito Y, Morioka K, Ishimoto Y, Suzuki N, Yokota Y, Hanasaki K, Ishikawa Y, Ishii T, Kobayashi T, Fukami K, Ikeda K, Nakanishi H, Taguchi R, Murakami M. Hair follicular expression and function of group X secreted phospholipase A2 in mouse skin. J. Biol. Chem. 2011;286:11616–11631. doi: 10.1074/jbc.M110.206714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miki Y, Yamamoto K, Taketomi Y, Sato H, Shimo K, Kobayashi T, Ishikawa Y, Ishii T, Nakanishi H, Ikeda K, Taguchi R, Kabashima K, Arita M, Arai H, Lambeau G, Bollinger JM, Hara S, Gelb MH, Murakami M. Lymphoid tissue phospholipase A2 group IID resolves contact hypersensitivity by driving antiinflammatory lipid mediators. J. Exp. Med. 2013;210:1217–1234. doi: 10.1084/jem.20121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hachem JP, Man MQ, Crumrine D, Uchida Y, Brown BE, Rogiers V, Roseeuw D, Feingold KR, Elias PM. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J. Invest. Dermatol. 2005;125:510–520. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- 13.Hatano Y, Man MQ, Uchida Y, Crumrine D, Scharschmidt TC, Kim EG, Mauro TM, Feingold KR, Elias PM, Holleran WM. Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis. J. Invest. Dermatol. 2009;129:1824–1835. doi: 10.1038/jid.2008.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunathilake R, Schmuth M, Scharschmidt TC, Gruber R, Grabher D, Leslie KS, Maurer TA, Mauro TM, Elias PM. Epidermal barrier dysfunction in non-atopic HIV: evidence for an “inside-to-outside” pathogenesis. J. Invest. Dermatol. 2010;130:1185–1188. doi: 10.1038/jid.2009.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluhr J, Behne M, Brown BE, Moskowitz DG, Selden C, Mao-Qiang M, Mauro T, Elias PM, Feingold K. Stratum corneum acidification in neonatal skin: secretory phospholipase A2 and the sodium/hydrogen antiporter-1 acidify neonatal rat stratum corneum. J. Invest. Dermatol. 2004;122:320–329. doi: 10.1046/j.0022-202X.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 16.Fluhr JW, Kao J, Jain M, Ahn SK, Feingold KR, Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Invest. Dermatol. 2001;117:44–51. doi: 10.1046/j.0022-202x.2001.01399.x. [DOI] [PubMed] [Google Scholar]
- 17.Ilic D, Mao-Qiang M, Crumrine D, Dolganov G, Larocque N, Xu P, Demerjian M, Brown BE, Lim ST, Ossovskaya V, Schlaepfer DD, Fisher SJ, Feingold KR, Elias PM, Mauro TM. Focal adhesion kinase controls pH-dependent epidermal barrier homeostasis by regulating actin-directed Na+/H + exchanger 1 plasmamembrane localization. Am. J. Pathol. 2007;170:2055–2067. doi: 10.2353/ajpath.2007.061277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuenzli S, Saurat JH. Peroxisome proliferator-activated receptors in cutaneous biology. Br. J. Dermatol. 2003;149:229–236. doi: 10.1046/j.1365-2133.2003.05532.x. [DOI] [PubMed] [Google Scholar]
- 19.Komuves LG, Hanley K, Lefebvre AM, Man MQ, Ng DC, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR. Stimulation of PPAR alpha promotes epidermal keratinocyte differentiation in vivo. J. Invest. Dermatol. 2000;115:353–360. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 20.Fluhr JW, Man MQ, Hachem JP, Crumrine D, Mauro TM, Elias PM, Feingold KR. Topical peroxisome proliferator activated receptor activators accelerate postnatal stratum corneum acidification. J. Invest. Dermatol. 2009;129:365–374. doi: 10.1038/jid.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fluhr JW, Elias PM, Man MQ, Hupe M, Selden C, Sundberg JP, Tschachler E, Eckhart L, Mauro TM, Feingold KR. Is the filaggrin–histidine–urocanic acid pathway essential for stratum corneum acidification? J. Invest. Dermatol. 2010;130:2141–2144. doi: 10.1038/jid.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vergnano S, Menson E, Smith Z, Kennea N, Embleton N, Clarke P, Watts T P.T. Health. Characteristics of invasive Staphylococcus aureus in United Kingdom neonatal units. Pediatr. Infect. Dis. J. 2011;30:850–854. doi: 10.1097/INF.0b013e318224546d. [DOI] [PubMed] [Google Scholar]
- 23.Elias PM, Goerke J, Friend DS. Mammalian epidermal barrier layer lipids: composition and influence on structure. J. Invest. Dermatol. 1977;69:535–546. doi: 10.1111/1523-1747.ep12687968. [DOI] [PubMed] [Google Scholar]
- 24.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 25.Celli A, Mackenzie DS, Crumrine DS, Tu CL, Hupe M, Bikle DD, Elias PM, Mauro TM. Endoplasmic reticulum Ca2+ depletion activates XBP1 and controls terminal differentiation in keratinocytes and epidermis. Br. J. Dermatol. 2011;164:16–25. doi: 10.1111/j.1365-2133.2010.10046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias PM, Cullander C, Mauro T, Rassner U, Komuves L, Brown BE, Menon GK. The secretory granular cell: the outermost granular cell as a specialized secretory cell. J. Investig. Dermatol. Symp. Proc. 1998;3:87–100. doi: 10.1038/jidsymp.1998.20. [DOI] [PubMed] [Google Scholar]
- 27.Mao-Qiang M, Jain M, Feingold KR, Elias PM. Secretory phospholipase A2 activity is required for permeability barrier homeostasis. J. Invest. Dermatol. 1996;106:57–63. doi: 10.1111/1523-1747.ep12327246. [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Elias PM, Feingold KR, Mauro T. A role for ions in barrier recovery after acute perturbation. J. Invest. Dermatol. 1994;102:976–979. doi: 10.1111/1523-1747.ep12384225. [DOI] [PubMed] [Google Scholar]
- 29.Mauro T, Holleran WM, Grayson S, Gao WN, Man MQ, Kriehuber E, Behne M, Feingold KR, Elias PM. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch. Dermatol. Res. 1998;290:215–222. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- 30.Holleran WM, Takagi Y, Menon GK, Legler G, Feingold KR, Elias PM. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J. Clin. Invest. 1993;91:1656–1664. doi: 10.1172/JCI116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feingold KR. The importance of lipids in cutaneous function. J. Lipid Res. 2007;48:2529–2530. doi: 10.1194/jlr.E700004-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Hachem JP, Crumrine D, Fluhr J, Brown BE, Feingold KR, Elias PM. pH directly regulates epidermal permeability barrier homeostasis, and stratum corneum integrity/cohesion. J. Invest. Dermatol. 2003;121:345–353. doi: 10.1046/j.1523-1747.2003.12365.x. [DOI] [PubMed] [Google Scholar]
- 33.Puhvel SM, Reisner RM, Sakamoto M. Analysis of lipid composition of isolated human sebaceous gland homogenates after incubation with cutaneous bacteria. Thin-layer chromatography. J. Invest. Dermatol. 1975;64:406–411. doi: 10.1111/1523-1747.ep12512337. [DOI] [PubMed] [Google Scholar]
- 34.Korting HC, Lukacs A, Vogt N, Urban J, Ehret W, Ruckdeschel G. Influence of the pH-value on the growth of Staphylococcus epidermidis, Staphylococcus aureus and Propionibacterium acnes in continuous culture. Zentralbl. Hyg. Umweltmed. 1992;193:78–90. [PubMed] [Google Scholar]
- 35.Brook I. Microbiology of secondarily infected diaper dermatitis. Int. J. Dermatol. 1992;31:700–702. doi: 10.1111/j.1365-4362.1992.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 36.Heuss E. Die Reaktion des Schweisses beim gesunden Menschen. Monatsschr. Prakt. Dermatol. 1892;14:343. (400, 501). [Google Scholar]
- 37.Behne MJ, Meyer JW, Hanson KM, Barry NP, Murata S, Crumrine D, Clegg RW, Gratton E, Holleran WM, Elias PM, Mauro TM. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J. Biol. Chem. 2002;277:47399–47406. doi: 10.1074/jbc.M204759200. [DOI] [PubMed] [Google Scholar]
- 38.Behne MJ, Barry NP, Hanson KM, Aronchik I, Clegg RW, Gratton E, Feingold K, Holleran WM, Elias PM, Mauro TM. Neonatal development of the stratum corneum pH gradient: localization and mechanisms leading to emergence of optimal barrier function. J. Invest. Dermatol. 2003;120:998–1006. doi: 10.1046/j.1523-1747.2003.12262.x. [DOI] [PubMed] [Google Scholar]
- 39.Ohman H, Vahlquist A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Derm. Venereol. 1994;74:375–379. doi: 10.2340/0001555574375379. [DOI] [PubMed] [Google Scholar]
- 40.Schade H, Marchionini A. Zur physikalischen chemie der hautoberflache. Arch. f. Derm. u. Syph. 1928:290–293. [Google Scholar]
- 41.I.H. Blank. Measurement of pH of the skin surface. I. Technique. J. Invest. Dermatol. 1939:67–74. [Google Scholar]
- 42.Draize JH. The determination of the pH of the skin of man and common laboratory animals. J. Invest. Dermatol. 1942;5:77. [Google Scholar]
- 43.Schirren CG. Does the glass electrode determine the same pH-values on the skin surface as the quinhydrone electrode? J. Invest. Dermatol. 1955;24:485–488. doi: 10.1038/jid.1955.67. [DOI] [PubMed] [Google Scholar]
- 44.Beare JM, Cheeseman EA, Gailey AA, Neill DW. The pH of the skin surface of children with seborrhoeic dermatitis compared with unaffected children. Br. J. Dermatol. 1958;70:233–241. doi: 10.1111/j.1365-2133.1958.tb13330.x. [DOI] [PubMed] [Google Scholar]
- 45.Dikstein S, Zlotogorski A. Measurement of skin pH. Acta Derm. Venereol. Suppl. (Stockh) 1994;185:18–20. [PubMed] [Google Scholar]
- 46.Behrhrendt H, Green M. In: Pattern of Skin pH from Birth through Adolescence. Thomas, editor. Springfield, IL: 1971. [Google Scholar]
- 47.Fluhr JW, Crumrine D, Mao-Qiang M, Moskowitz DG, Elias PM, Feingold KR. Topical liver × receptor activators accelerate postnatal acidification of stratum corneum and improve function in the neonate. J. Invest. Dermatol. 2005;125:1206–1214. doi: 10.1111/j.0022-202X.2005.23964.x. [DOI] [PubMed] [Google Scholar]
- 48.Ghadially R, Brown BE, Sequeira-Martin SM, Feingold KR, Elias PM. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. Invest. 1995;95:2281–2290. doi: 10.1172/JCI117919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 50.Demerjian M, Man MQ, Choi EH, Brown BE, Crumrine D, Chang S, Mauro T, Elias PM, Feingold KR. topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalize epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15:154–160. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 51.Man M, Hupe M, Mackenzie D, Kim H, Oda Y, Crumrine D, Lee SH, Martin-Ezquerra G, Trullas C, Mauro TM, Feingold KR, Elias PM, Man MQ. A topical Chinese herbal mixture improves epidermal permeability barrier function in normal murine skin. Exp Dermatol. 2011;20:285–288. doi: 10.1111/j.1600-0625.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thueson DO, Chan EK, Oechsli LM, Hahn GS. The roles of pH and concentration in lactic acid-induced stimulation of epidermal turnover. Dermatol. Surg. 1998;24:641–645. doi: 10.1111/j.1524-4725.1998.tb04221.x. [DOI] [PubMed] [Google Scholar]
- 53.Di Marzio L, Cinque B, De Simone C, Cifone MG. Effect of the lactic acid bacterium Streptococcus thermophilus on ceramide levels in human keratinocytes in vitro and stratum corneum in vivo. J. Invest. Dermatol. 1999;113:98–106. doi: 10.1046/j.1523-1747.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 54.Sarangarajan R, Shumaker H, Soleimani M, Le Poole C, Boissy RE. Molecular and functional characterization of sodium–hydrogen exchanger in skin as well as cultured keratinocytes and melanocytes. Biochim. Biophys. Acta. 2001;1511:181–192. doi: 10.1016/s0005-2736(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 55.Noel J, Pouyssegur J. Hormonal regulation, pharmacology, and membrane sorting of vertebrate Na+/H+ exchanger isoforms. Am. J. Physiol. 1995;268:C283–C296. doi: 10.1152/ajpcell.1995.268.2.C283. [DOI] [PubMed] [Google Scholar]
- 56.Krien PM, Kermici M. Evidence for the existence of a self-regulated enzymatic process within the human stratum corneum — an unexpected role for urocanic acid. J. Invest. Dermatol. 2000;115:414–420. doi: 10.1046/j.1523-1747.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 57.Visscher MO, Chatterjee R, Munson KA, Pickens WL, Hoath SB. Changes in diapered and nondiapered infant skin over the first month of life. Pediatr. Dermatol. 2000;17:45–51. doi: 10.1046/j.1525-1470.2000.01711.x. [DOI] [PubMed] [Google Scholar]
- 58.Yosipovitch G, Maayan-Metzger A, Merlob P, Sirota L. Skin barrier properties in different body areas in neonates. Pediatrics. 2000;106:105–108. doi: 10.1542/peds.106.1.105. [DOI] [PubMed] [Google Scholar]
- 59.Fluhr J, Fowler AJ, Hachem JP, Crumrine D, Elias PM, Feingold KR. Stratum corneum acidification in neonates: impact on permeability barrier homeostasis and integrity/cohesion. J. Invest. Dermatol. 2002;119:249. [Google Scholar]
- 60.Man MQ, Choi EH, Schmuth M, Crumrine D, Uchida Y, Elias PM, Holleran WM, Feingold KR. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion, and post-secretory lipid processing. J. Invest. Dermatol. 2006;126:386–392. doi: 10.1038/sj.jid.5700046. [DOI] [PubMed] [Google Scholar]
- 61.Barland CO, Zettersten E, Brown BS, Ye J, Elias PM, Ghadially R. Imiquimodinduced interleukin-1 alpha stimulation improves barrier homeostasis in aged murine epidermis. J. Invest. Dermatol. 2004;122:330–336. doi: 10.1046/j.0022-202X.2004.22203.x. [DOI] [PubMed] [Google Scholar]
- 62.Choi EH, Man MQ, Xu P, Xin S, Liu Z, Crumrine DA, Jiang YJ, Fluhr JW, Feingold KR, Elias PM, Mauro TM. Stratum corneum acidification is impaired in moderately aged human and murine skin. J. Invest. Dermatol. 2007;127:2847–2856. doi: 10.1038/sj.jid.5700913. [DOI] [PubMed] [Google Scholar]