Abstract
Chinese herbal medicine (CHM) has been shown to have beneficial effects for both skin disorders with barrier abnormality and as skin care ingredients. Yet, how CHM exerts their benefits is unclear. As most, if not all, inflammatory dermatoses are accompanied by abnormal permeability barrier function, we assessed the effects of topical CHM extracts on epidermal permeability barrier function and their potential mechanisms. Topical CHM accelerated barrier recovery following acute barrier disruption. Epidermal lipid content and mRNA expression of fatty acid and ceramide synthetic enzymes increased following topical CHM treatment in addition to mRNA levels for the epidermal glucosylceramide transport protein, ATP-binding cassette A12. Likewise, CHM extract increased mRNA expression of antimicrobial peptides both in vivo and in vitro. These results demonstrate that the topical CHM extract enhances epidermal permeability barrier function, suggesting that topical CHM could provide an alternative regimen for the prevention/treatment of inflammatory dermatoses accompanied by barrier abnormalities.
Keywords: barrier function, cathelicidin antimicrobial peptide, dermatitis, herbal medicine, β defensin
Background
Epidermal permeability barrier function regulates several metabolic responses in the underlying epidermis, including epidermal DNA and lipid synthesis (1,2). Recent studies have demonstrated the clinical relevance of barrier function, because inherited barrier defects increase the risk of developing inflammatory dermatoses, including atopic dermatitis (AD) (3). Therefore, the enhancement of permeability barrier function could represent a valuable preventive and/or therapeutic approach for the treatment not only of AD but also of other dermatoses accompanied by barrier abnormalities.
Several controlled studies have shown that Chinese herbal medicine (CHM) is effective for both the treatment and prevention of AD (4,5). Systemic CHM also prevents acute allergic contact dermatitis (6–9) and both systemic and topical CHM benefit normal skin (10,11). Commercially available skin care products that contain CHM display benefits that include anti-aging and improved skin hydration (11–15).
Questions addressed
Because CHM improves/prevents dermatoses with abnormal barrier function, we hypothesized that the responsible mechanism could be improved epidermal permeability barrier function, which could prevent xenobiotic penetration. Indeed, we showed that topical CHM prevents cutaneous inflammation in normal murine skin (16). Hence, we evaluated the effects of a topical CHM extract on barrier function in normal murine skin and the mechanisms by which CHM improves barrier function.
Experimental design
Materials
Six- to 8-week-old female hairless mice (hr/hr) were purchased from Charles River Laboratories (Wilmington, MA, USA) and fed mouse standard diet and water ad libitum. Herbal extract was made by soaking Radix Paeonlae rubra, Cat Nut, Phelloden Dron, Rhizoma Alismatis, Angelica sinensis and Glabrous Greenbrier in 100% ethanol for 3 weeks. The concentration for topical treatments was adjusted to 1%.
Experimental protocols and functional studies
All procedures were approved by the Animal Studies Subcommittee (IACUC) of the SF-VAMC and performed in accordance with their guidelines. Sixty microliters of 1% CHM or ethanol alone was applied to both flanks of mice twice daily for 7 days. Basal barrier function, skin surface pH, and stratum corneum (SC) hydration were assessed as described in figure legend (see also ref. 17).
Immunohistochemistry
Changes in overall morphology were visualized after H&E staining of 5-μm paraffin-embedded sections. Immunohistochemical assessment of changes in two antimicrobial peptides, mBD3 and cathelicidin antimicrobial peptide (CAMP), was performed in 5-μm frozen and paraffin sections, respectively [primary antibodies to mBD3 from Alpha Diagnostics (San Antonio, TX, USA); mouse cathelicidin (CAMP) antibody was a gift from Dr. Richard Gallo (UCSD)].
Nile red staining
Fresh skin samples from both CHM- and vehicle-treated animals twice daily for 7 days, as well as from animals treated once with CHM extract 15 min prior biopsy were taken. Five micrometers frozen sections were incubated with 0.0001% nile red in glycerin/water (75/25, v/v), as described previously (18).
Electron microscopy
Biopsies were postfixed with either 0.25% ruthenium tetroxide or 1% aqueous osmium tetroxide (3). Ultrathin sections were examined in a Zeiss electron microscope (Jena, Germany) (3). Images were captured using Digital Micrograph 3.10.0 software (Gatan, Inc., Pleasanton, CA, USA). Density of lamellar bodies in the first outmost layer of stratum granulosum was quantitated per 4-cm2 area from electron micrographs.
mRNA expression
mRNA expression was measured by real-time quantitative PCR (QPCR). Lipid synthetic enzymes [serine–palmitoyl transferase (SPT) and fatty acid 2 hydroxlase (FA2H)] and lipid transporters [ATP-binding cassette A12 (ABCA12)], CAMP, and mBD3 expression were assessed as described earlier (19,20). Primer sequences are in Table S1. Expression of mRNAs was normalized to GAPDH. Results for in vivo studies are presented as the percentage of vehicle-treated control (vehicle as 100%) while results for in vitro studies are presented as the ratio to untreated controls. Data are expressed as the means ± SEM. Unpaired two-tailed Student t-test with Welch’s correction was used to determine significant differences when two groups were compared. One-way ANOVA with post-Tukey test was used when more than three groups were compared.
Results
Topical CHM improves epidermal permeability barrier function in normal murine skin
We first assessed whether the topical CHM improves epidermal permeability barrier function in normal murine skin after topical applications of CHM twice daily for 7 days. No changes in gross or histological appearance were apparent (not shown). Although basal transepidermal water loss (TEWL), surface pH, and SC hydration remained unchanged in CHM- versus vehicle-treated groups (Fig. S1a–c), barrier recovery significantly accelerated in CHM-treated mice after repeated tape stripping (Fig. S1d).
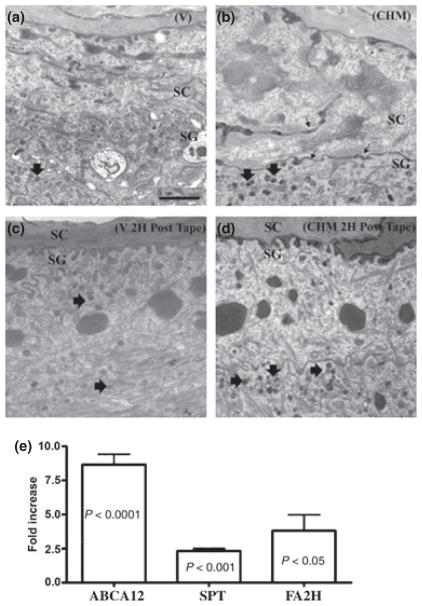
Because both epidermal lamellar body (LB) formation and secretion are crucial for barrier function (18), we next assessed whether these parameters change following topical CHM treatment. Premature secretion of LB was evident, and LB density increased before and 2 h after acute barrier disruption in CHM-treated skin (Fig. 1a–d). Quantitative studies in coded, randomized micrographs confirmed that a significant increase in LB density occurred in the cytosol of stratum granulosum cells following CHM treatment (CHM-treated 7.88 ± 1.03 versus vehicle-treated 4.58 ± 0.87, P < 0.03). Thus, topical CHM treatment stimulates LB formation and secretion, together likely accounting for improved barrier function.
Figure 1.
Topical Chinese herbal medicine (CHM) accelerate lamellar body (LB) formation and secretion in normal mouse skin. Hairless mice were topically treated with 60 μl of 1%herbal extract in ethanol or ethanol alone twice daily for 7 days. Biopsies were postfixed with either 0.25% ruthenium tetroxide or 1% aqueous osmium tetroxide. Ultrathin sections were examined in a Zeiss electron microscope. (a, b) Basal condition following vehicle (a) or CHM (b) treatment. (c, d) Vehicle- and CHM-treated 2 h after barrier disruption, respectively. Small arrows on (b) demonstrate LB presecretion at the SG–SC interface in CHM-treated skin. Arrows on (d) demonstrate LB in CHM-treated skin. Scale bars represent 1 μm (a–d). (e) ABCA12, SPT, and FA2H mRNA expression (n = 5 for Vehicle-treated and n = 3 for CHM-treated). Data are expressed as means ± SEM. Unpaired two-tailed student t-test with Welch’s correction was used to determine statistical differences. P < 0.05 was considered as significant difference. SPT, serine–palmitoyl transferase; SG, stratum granulosum; SC, stratum corneum; FA2H, fatty acid 2 hydroxlase; ABCA12, ATP-binding cassette A12.
Because the ultrastructural studies suggested that CHM treatment increases the LB production, we next assessed the expression of ABCA12, a transmembrane glycosylceramide transporter, required for LB formation (21,22). Topical CHM induced an eight-fold increase in ABCA12 mRNA expression (Fig. 1e, P < 0.0001), suggesting that accelerated delivery of newly synthesized lipid into LB could account for increased LB density in CHM-treated skin.
Because lipid production is required for the formation of LB (23), we next assessed epidermal lipid content by nile red fluorescence. A dramatic increase in nile red staining was observed in the SC following 7 days of CHM treatment (Fig. S2a, d vs. b, e). Because a single application of CHM did not alter staining intensity (Fig. S2c, f), enhanced staining in CHM-treated epidermis represents increased lipid content, rather than nonspecific staining from the CHM extract.
To further assess the mechanism underlying the CHM-induced increase in epidermal lipid content, we next assessed mRNA expression of two key synthetic enzymes of barrier-related key lipids; i.e., fatty acids and sphingolipids. Expression of SPT and FA2H mRNA increased more than one- and three-fold over vehicle-treated, respectively, following 7 days of CHM treatment (Fig. 1e). Thus, topical CHM treatment upregulates epidermal lipid synthetic enzyme mRNA expression, accounting for increased epidermal lipid content.
CHM extract increases CAMP and mBD3 expression
Two epidermal antimicrobial peptides, CAMP (LL-37) and mBD3 (hBD2), are packaged and secreted by LB and regulated in parallel with changes in permeability barrier function (20,24,25). Because topical CHM enhances LB production, we next determined whether CHM treatment also increases epidermal CAMP and/or mBD3 expression. Immunostaining for both CAMP and mBD3 markedly increased (Fig. 2a–d). mBD3, but not CAMP mRNA expression that increased significantly (Fig. 2e).
Figure 2.
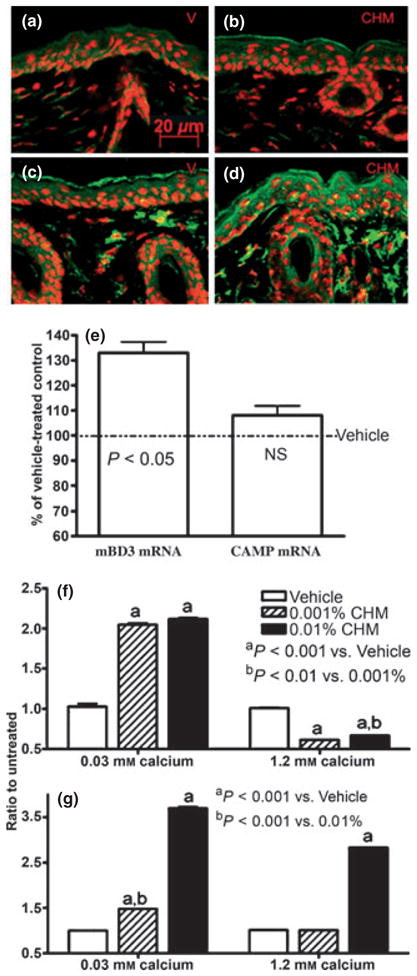
Topical Chinese herbal medicine (CHM) extract increases epidermal CAMP and mBD3 expressions in murine skin. Skin samples were from mice treated with either vehicle alone (a, c) or CHM extract (b, d) twice daily for 7 days. Five micrometers sections were incubated with the primary antibodies (1:500 dilutions) overnight at 4°C. After washing, sections were incubated with the secondary antibody for 30 min. Sections were examined with a Zeiss fluorescence confocal microscope, and digital images were captured with AxioVision software (Carl Zeiss Vision, Munich, Germany). Immunofluorescent staining (green color) for mouse b-defensin 3 (mBD3) (a, b), cathelicidin-related antimicrobial peptide (CAMP) (c, d). (a, c) Vehicle-treated and (b, d) CHM-treated. Propidium iodide was used for counterstaining. Scale bars represent 20 μm (a–d). (e) Changes in epidermal CAMP and mBD3 mRNA expressions over vehicle. Data are expressed as % of vehicle-treated control (n = 4 for both). (f, g) LL-37 and human β-defensin 2 mRNA expressions in keratinocytes (from foreskin), respectively. Second-passage human keratinocytes cultured in 0.3 or 1.2 mM calcium were treated with either 0.01%, 0.001% herbal extract or vehicle for 36 h. Total RNA was extracted with a commercial kit (RNeasy RNA isolation kit; Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s instructions. mRNA expressions were assessed as described in Experimental design section. Samples were run in triplicate, and results were normalized to untreated group (n = 3).
To further validate these results, LL-37 and hBD2, which are the human homolog of mouse CAMP and mBD3, expression also was assessed in vitro. Under low calcium conditions, CHM induced a dose-dependent increase in LL-37 (Fig. 2f) and hBD2 (Fig. 2g) mRNA expression in cultured human keratinocytes while at higher calcium conditions, hBD2 mRNA expression remained significantly elevated, but LL-37 declined. Notably, upregulation of hBD2 (but not LL-37) mRNA by CHM was dose-dependent under both lower and higher calcium conditions. Together, these results suggest that CHM increase cutaneous immunity.
Conclusions
We demonstrate here that topical CHM treatment enhances both epidermal permeability barrier homeostasis and antimicrobial peptide expression in normal epidermis. These results offer a compelling rationale for utilizing this CHM preparation in diverse clinical settings that are characterized by abnormal barrier function and/or increased risk of infection. Moreover, as topical CHM improves permeability barrier function in normal skin, it could be useful in skincare products.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants AR 19098 and the Medical Research Service, Department of Veterans Affairs Medical Center.
Abbreviations
- CHM
Chinese herbal medicine
- BD
β defensin
- CAMP
cathelicidin antimicrobial peptide
- TEWL
transepidermal water loss
- LB
lamellar body
- SPT
serine–palmitoyl transferase
- FA2H
fatty acid 2 hydroxlase
- ABCA12
ATP-binding cassette A12
- SC
stratum corneum
- H&E
hematoxylin and eosin
Footnotes
Conflicts of interest
All authors have no conflicts of interest.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Topical CHM extract improves epidermal permeability barrier function in normal mouse skin.
Figure S2. Topical CHM extract increases epidermal lipid in murine model.
Table S1. Primer sequences.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Proksch E, Feingold KR, Man MQ, et al. J Clin Invest. 1991;87:1668–1673. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proksch E, Holleran WM, Menon GK, et al. Br J Dermatol. 1993;128:473–482. doi: 10.1111/j.1365-2133.1993.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 3.Scharschmidt TC, Man MQ, Hatano Y, et al. J Allergy Clin Immunol. 2009;124:496–506. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang XG, Chen DY, Lin J, et al. Chin J Dermatovenereol (in Chinese) 2005;19:307–308. [Google Scholar]
- 5.Zhang XM, Lv DH, Zhang XM. Chin J Dermatovenereol (in Chinese) 2006;20:220–221. [Google Scholar]
- 6.Lee HS, Kim SK, Han JB, et al. Br J Dermatol. 2006;155:33–38. doi: 10.1111/j.1365-2133.2006.07303.x. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi Y, Kohno K, Inoue S. Int Immunopharmacol. 2003;3:1313–1324. doi: 10.1016/s1567-5769(03)00132-2. [DOI] [PubMed] [Google Scholar]
- 8.Choi MS, Kim EC, Lee HS, et al. Biol Pharm Bull. 2008;31:51–56. doi: 10.1248/bpb.31.51. [DOI] [PubMed] [Google Scholar]
- 9.Kim DY, Jung JA, Kim TH, et al. J Ethnopharmacol. 2009;122:567–572. doi: 10.1016/j.jep.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Shu XX, Liu SB, Zhang L, et al. Flav Frag Cosmet (in Chinese) 2003;6:17–18. [Google Scholar]
- 11.Lu Q, Zhao H, Mu Y. Chin J Aesthetic Med (in Chinese) 2007;16:1574–1576. [Google Scholar]
- 12.Liu GB, Huang Z, Huang CG, et al. Detergent Cosmet (in Chinese) 2005;28:22–24. [Google Scholar]
- 13.Ahshawat MS, Saraf S, Saraf S. Int J Cosmet Sci. 2008;30:183–193. doi: 10.1111/j.1468-2494.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Liu HW, Cheng B. Chin J Aesthetic Med (in Chinese) 2008;17:138–141. [Google Scholar]
- 15.Wu Y, Du LX, Chen C, et al. J Clin Dermatol (in Chinese) 2009;38:349–351. [Google Scholar]
- 16.Man WY, Man M, Marin-Ezquerra G, et al. J Invest Dermatol. 2010;130:S7. (abstr) [Google Scholar]
- 17.Man MQ, Barish GD, Schmuth M, et al. J Invest Dermatol. 2008;128:370–377. doi: 10.1038/sj.jid.5701026. [DOI] [PubMed] [Google Scholar]
- 18.Mao-Qiang M, Brown BE, Wu-Pong S, et al. Arch Dermatol. 1995;131:809–816. doi: 10.1001/archderm.131.7.809. [DOI] [PubMed] [Google Scholar]
- 19.Oda Y, Uchida Y, Moradian S, et al. J Invest Dermatol. 2009;129:1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aberg KM, Man MQ, Gallo RL, et al. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefévre C, Audebert S, Jobard F, et al. Hum Mol Genet. 2003;12:2369–2378. doi: 10.1093/hmg/ddg235. [DOI] [PubMed] [Google Scholar]
- 22.Smyth I, Hacking DF, Hilton AA, et al. PLoS Genet. 2008;4:e1000192. doi: 10.1371/journal.pgen.1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias PM, Feingold KR. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 24.Oren A, Ganz T, Liu L, et al. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 25.Braff MH, Di Nardo A, Gallo RL. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.