Introduction
Angiotensin-II (Ang-II) activates two major types of receptors, angiotensin II type one (AT1R) and type two (AT2R) receptors. While AT1R is widely expressed and mediates most inflammatory Ang-II effects, AT2R, is less expressed and has opposite effects, promoting vasodilation and anti-inflammatory effects [1]. Physiologically, AT2R actions are usually masked by the more abundant AT1R. It has been suggested that ARBs can mediate their action through increasing angiotensin II (Ang II) availability to bind to the beneficial angiotensin type 2 receptor (AT2R), thus leading to unopposed AT2R stimulation. AT1R blockers (ARBs) represent a major class of antihypertensive medications. They are considered first line treatment for essential hypertension. Moreover, ARBs are the cornerstone treatment for other cardiovascular diseases especially in patients with diabetic and renal comorbidities. Clinical and experimental evidence have documented the beneficial actions of ARBs beyond the blood pressure lowering effect. Ischemic diseases such as stroke and proliferative retinopathy are characterized by hypoxia-driven release of angiogenic growth factors [2]. However, revascularization of the ischemic areas is inadequate, resulting in impaired neuro-vascular function. ARBs have been shown to exhibit vascular protective and pro- or anti-angiogenic effects depending on the tissue/cell type and disease condition under study [3]. Our group has demonstrated the vascular protective effects of ARBs and candesartan, in particular, in models of ischemic stroke and retinopathy. The positive impact of candesartan was mainly via enhancing the proangiogenic state and stimulation of reparative angiogenesis. This commentary aims to highlight the recently identified pathways engaged as a result of directly blocking the AT1 receptor or indirectly by possible activation of AT2 receptor, in the context of the published literature.
ARBs contribute to initiation and stabilization of new blood vessels
Angiogenesis, the formation of new blood vessels from existing vessels, is a complex process involving extracellular matrix degradation, endothelial migration, proliferation, differentiation, and eventually tube formation [2, 4]. Sprouting of new blood vessels is a guided process, where front-runner endothelial tip cells initiate the vascular sprouting, followed by stalk endothelial cells, which mediate vessel elongation and lumen formation [5]. Among the angiogenic factors, vascular endothelial growth factor (VEGF-A) via activation of its receptor VEGFR2 (KDR) is the major regulator of blood vessel formation and function. Tip cells have been shown to express VEGFR2 and endothelial cell-specific molecule-1 known also as endocan and exhibit long filopodial extensions [5]. In our recent study using an ischemic retinopathy mouse model, treatment with candesartan (10 mg/kg/day) significantly reduced capillary dropout area by 45% compared to untreated hypoxic controls, suggesting enhanced reparative retinal angiogenesis. Results showed that candesartan stimulated the number of tip cells at the border of capillary dropout toward the central retina compared to the untreated group [6]. One of the possible molecular pathways to enhance tip cell formation is the activation of endothelial nitric oxide synthase (eNOS) and release of nitric oxide (NO). In fact, dissection of NO downstream signaling using pharmacological inhibitors and inducers indicated that NO uses the soluble guanylate cyclase and cyclic GMP pathway in tip cells to drive angiogenesis [4]. In support, previous studies demonstrated that ARBs stimulate phosphorylation of eNOS and NO release [7-10]. Together, these observations support the notion that ARB-stimulated NO production could be involved in tip cell formation and thus enhanced reparative angiogenic response.
Following an initial phase of endothelial sprouting, the angiopoietin system in pericytes plays a crucial role in vascular stabilization via activation of the Tie-2 receptor [11]. While angiopoietin-1 increases pericyte survival, angiopoietin-2 can increase apoptosis [11]. ARBs are known to upregulate angiopoietin-1 [12], which in turn could activate and recruit pericytes through Tie-2 receptor expression [11]. In the ischemic stroke model, the pro-angiogenic response of candesartan was associated with vascular protection and reduced blood brain barrier permeability in the brain and suggested that candesartan mediates reparative angiogenesis resulting in improved functional outcome [13]. This preservation of barrier function despite the increase of the vascular permeability factor, VEGF-A, was attributed to the simultaneous upregulation of angiopoietin-1, which mediates new blood vessel stabilization and maturation through pericyte recruitment [14]. We and others have also shown increased vascular density and neuroprotection in vivo with losartan and valsartan pretreatment suggesting a class effect of ARBs [15, 16]. Other studies showed protective effects of candesartan and losartan on preventing pericyte cell death and hence, a possible role for ARBs to regulate pericyte loss in the ischemic retina [17, 18].
ARBs increase proangiogenic state via expression of angiogenic growth factors
Over the past decade, we have shown that post-stroke treatment with ARBs and in particular with candesartan, resulted in vascular protection and was positively associated with a proangiogenic state [12-14, 19-21]. Our work clearly identified that the ARB-enhanced proangiogenic state coincided with increased brain levels of the pro-angiogenic factors: brain derived neurotrophic factor (BDNF) and VEGF isoforms; VEGF-A and B. Acute treatment with a single dose of candesartan (1 mg/kg), at reperfusion after 3 h middle cerebral artery occlusion (MCAO) resulted in significant upregulation of VEGF-A and B within 8 to 72 h [13, 20]. Interestingly, cerebrospinal fluid from candesartan treated animals showed a VEGF mediated proangiogenic effect in vitro using brain endothelial cells [13, 14]. Although the 1 mg/kg dose resulted in substantial blood pressure reduction, this proangiogenic effect was independent of the blood pressure lowering as we have shown in other studies using a sub-hypotensive dose and also in vitro experiments. When given in a sub-hypotensive dose of 0.3 mg/kg/day, candesartan resulted in sustained increases in BDNF and VEGF-A at 14 days, an effect that was associated with increased vascular density at the ischemic border zone and behavioral recovery [12]. In vitro, we have shown that candesartan stimulates human brain endothelial cell proliferation, migration and tube formation under both normoxic and hypoxic conditions and also with or without angiotensin II co-treatment. This proangiogenic effect seems to be mediated through a synergistic effect of the angiogenic factors; VEGF-A, VEGF-B and BDNF in vitro [12, 21]. Similarly, another study showed that irbesartan treatment induced an increase in angiogenesis-related gene expression including VEGF, VEGFR2 and ROBO4 [22].
ARBs and Anti-oxidant effects
Several studies have demonstrated that massive production of oxidizing and nitrating species contributes to vascular damage following ischemic retinopathy and ischemic stroke [13, 14, 19, 23-25]. In our study using an ischemic retinopathy mouse model, candesartan treatment (10 mg/kg /day) blocked hypoxia-induced retinal oxidative and nitrative stress [6]. The same study showed that candesartan treatment ameliorated glial Muller cell activation, an index of oxidative and nutritional stress. The mechanism behind candesartan's anti-oxidative and anti-nitrative effects was attributed to its ability to inhibit the inducible nitric oxide synthase enzyme (iNOS) and stimulate heme oxygenase-1 (HO-1) expression in the retina [6]. Findings from several studies support our reasoning regarding ARBs ability to inhibit iNOS expression and enhanced phosphorylation of eNOS thereby restoring vascular eNOS/iNOS ratio and thus having vascular protective and anti-inflammatory effects [7-10]. Using the ischemic stroke model, we showed anti-oxidant and anti-nitrative effects of candesartan treatments both in vitro and in vivo [13, 14, 19]. In vitro, treatment of brain endothelial cells with candesartan decreased the 4-hydroxynonenal level, which is a marker of lipid peroxidation, after exposing to oxidizing conditions, thereby indicating an anti-oxidant effect of candesartan [14]. In the same study, we also showed that candesartan reduced nitrotyrosine levels in response to peroxynitrite treatment thus exhibiting its antinitrative effects[14] . In vivo, at 24 hours after ischemia/reperfusion in the brain, a condition characterized by massive production of oxidizing and nitrating species, we reported decreased protein nitration in the ipsilateral hemisphere after candesartan treatment [14]. Therefore, in this study, candesartan treatment in a concentration range of 0.1 to 10µg/ml enhanced endothelial cell production of VEGF-A and VEGF-B, induced an autocrine angiogenic response, as well as a paracrine neuroprotective effect. The up regulation of VEGF-A expression could be at least partly attributed to the enhanced stabilization of the upstream (hypoxia inducible factor-1 α (HIF-1 α) with candesartan treatment[14].
ARBs and Induction of HO-1
HO-1 is a cellular antioxidant enzyme that catalyzes the degradation of heme-containing molecules to biliverdin, free iron and carbon monoxide. The proposed mechanisms by which HO-1 exerts its cytoprotective effects include its ability to degrade the pro-oxidative heme, to release biliverdin and subsequently convert it to bilirubin, both of which have antioxidant properties, and to generate carbon monoxide, which has anti-proliferative and anti-inflammatory as well as vasodilatory properties [26-29] HO-1, HO-2 and HO-3 are the 3 isozymes of HO that have been identified. While HO-1 is an inducible enzyme, HO-2 is a constitutive one [26, 30]. HO-1 is induced by various molecular and environmental triggers, such as heme, radiation, cytokines, oxidative and heat stresses. HO-3 is believed to be a pseudo gene derived from HO-2 transcripts [31, 32]. In addition to antioxidant defensive mechanisms, HO-1 can contribute to angiogenesis and blood vessel formation [28]. The angiogenic properties of HO-1 are the result of both direct and indirect actions on endothelial cells. It directly enhances the angiogenic properties within endothelial cells probably through activation of genes responsible for cell proliferation [6, 28] and the indirect actions include enhanced VEGF expression and or signaling through VEGFR2 receptor to induce angiogenesis in vivo and in vitro [15]. In our study using the ischemic retinopathy mouse model, hypoxia reduced retinal HO-1 at the protein level [6]. Candesartan treatment restored retinal HO-1 expression [6]. We also found that silencing HO-1 completely blunted VEGFR2 activation and tube formation in retinal endothelial cells [15]. This observation was in agreement with previous studies that demonstrated the involvement of HO-1 in the angiogenic response in endothelial cells [33, 34]. Therefore, candesartan-restored HO-1 expression is required for VEGFR2-mediated angiogenic signal in ischemic retina [6]. Thus, activators of HO-1 can provide potential therapeutic targets to achieve ARB-mediated proangiogenic state without altering blood pressure.
ARBs and modulation of AT2 receptor
The above discussed observations of ARBs may be due to blocking of AT1 receptor, but the direct activation of the AT2 receptor remains a possibility. For this, we examined the proangiogenic effect of AT1R blockade in relation to AT2R. AT2R blockade with PD123319 abrogated the proangiogenic effect of candesartan while AT2R stimulation with agonists, CGP42112 and compound 21 (C21), achieved similar proangiogenic response in vitro which was mediated through BDNF upregulation [21, 35]. Similarly, in vivo AT2R stimulation with C21 provides increased vascular density, vascular protection and decreased BBB leakage after stroke without affecting BP [35, 36]. These findings demonstrate that the proangiogenic and vascular protective effects of ARBs in the brain can be achieved through AT2R stimulation even in the absence of AT1R blockade or blood pressure reduction. As depicted in the figure-1, ARBs exert pleotropic effects to enhance the proangiogenic state including production of angiogenic factors, activation of HO-1 mediated angiogenic response, reduction of oxidative and nitrative stress resulting in improved nitric oxide and stimulation of tip cells and pericytes and finally, possible activation of AT2 receptor. Understanding the various pathways by which ARBs stimulate reparative angiogenesis can help to devise new therapeutic targets of vascular protection beyond their known blood pressure control.
Figure 1.
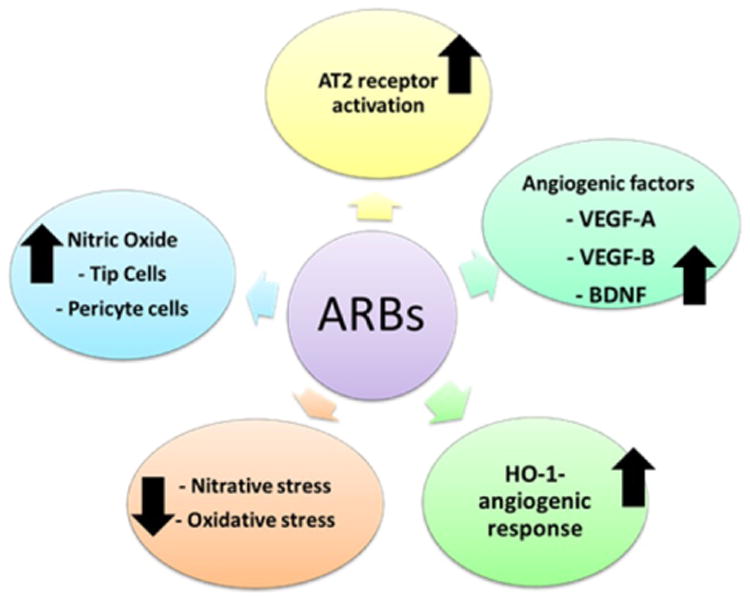
Schematic representation of the multiple pathways by which ARBs can regulate proangiogenic state including production of angiogenic factors, activation of HO-1 mediated angiogenic response, reduce oxidative and nitrative stress resulting in improved nitric oxide and stimulation of tip cells and pericyte and finally possible activation of AT2 receptor.
Acknowledgments
Source of Funding: This work was funded by Veterans Affairs Merit Review (BX000891), RO1-NS063965 and the Jowdy Professorship to SCF and RO-1-EY022408 to ABE.
List of abbreviations
- AT1R
Angiotensin type 1 receptor
- AT2R
Angiotensin type 2 receptor
- ARBs
AT1R blockers
- VEGF
vascular endothelial growth factor
- BDNF
brain derived neurotrophic factor
- Ang II
angiotensin II
- Ang-1
angiopoietin 1
- BBB
blood brain barrier
- BP
blood pressure
- MCAO
middle cerebral artery occlusion
- NO
nitric oxide
- HO
hemeoxygenase
- eNOS
endothelial nitric oxide synthetase
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Guimond MO, Gallo-Payet N. How does angiotensin AT(2) receptor activation help neuronal differentiation and improve neuronal pathological situations? Front Endocrinol (Lausanne) 2012;3:164. doi: 10.3389/fendo.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sapieha P. Eyeing central neurons in vascular growth and reparative angiogenesis. Blood. 2012;120(11):2182–94. doi: 10.1182/blood-2012-04-396846. [DOI] [PubMed] [Google Scholar]
- 3.Willis LM, El-Remessy AB, Somanath PR, Deremer DL, Fagan SC. Angiotensin receptor blockers and angiogenesis: clinical and experimental evidence. Clin Sci (Lond) 2011;120(8):307–19. doi: 10.1042/cs20100389. [DOI] [PubMed] [Google Scholar]
- 4.Priya MK, Sahu G, Soto-Pantoja DR, Goldy N, Sundaresan AM, Jadhav V, et al. Tipping off endothelial tubes: nitric oxide drives tip cells. Angiogenesis. 2014 doi: 10.1007/s10456-014-9455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanab AY, Elshaer SL, El-Azab MF, Soliman S, Sabbineni H, Matragoon S, et al. Candesartan stimulates reparative angiogenesis in ischemic retinopathy model: role of hemeoxygenase-1 (HO-1) Angiogenesis. 2015;18(2):137–50. doi: 10.1007/s10456-014-9451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Liu X, Wei X, Chen L, Xiang Y, Yi F, et al. Losartan, an angiotensin II type 1 receptor blocker, ameliorates cerebral ischemia-reperfusion injury via PI3K/Akt-mediated eNOS phosphorylation. Brain Res Bull. 2012;89(1-2):65–70. doi: 10.1016/j.brainresbull.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S, Shimabukuro M, Fukuda D, Soeki T, Yamakawa K, Masuzaki H, et al. Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser(1177)/Thr(497) of endothelial nitric oxide synthase in diabetic mice. Cardiovasc Diabetol. 2014;13:30. doi: 10.1186/1475-2840-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barauna VG, Mantuan PR, Magalhaes FC, Campos LC, Krieger JE. AT1 receptor blocker potentiates shear-stress induced nitric oxide production via modulation of eNOS phosphorylation of residues Thr(495) and Ser(1177) Biochem Biophys Res Commun. 2013;441(4):713–9. doi: 10.1016/j.bbrc.2013.10.108. [DOI] [PubMed] [Google Scholar]
- 10.Saavedra JM, Benicky J, Zhou J. Mechanisms of the Anti-Ischemic Effect of Angiotensin II AT(1) Receptor Antagonists in the Brain. Cell Mol Neurobiol. 2006;26(7-8):1099–111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai J, Kehoe O, Smith GM, Hykin P, Boulton ME. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(5):2163–71. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 12.Ishrat T, Pillai B, Soliman S, Fouda AY, Kozak A, Johnson MH, et al. Low-Dose Candesartan Enhances Molecular Mediators of Neuroplasticity and Subsequent Functional Recovery After Ischemic Stroke in Rats. Mol Neurobiol. 2014 doi: 10.1007/s12035-014-8830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozak A, Ergul A, El-Remessy AB, Johnson MH, Machado LS, Elewa HF, et al. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke. 2009;40(5):1870–6. doi: 10.1161/STROKEAHA.108.537225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soliman S, Ishrat T, Pillai A, Somanath PR, Ergul A, El-Remessy AB, et al. Candesartan induces a prolonged proangiogenic effect and augments endothelium-mediated neuroprotection after oxygen and glucose deprivation: role of vascular endothelial growth factors A and B. J Pharmacol Exp Ther. 2014;349(3):444–57. doi: 10.1124/jpet.113.212613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forder JP, Munzenmaier DH, Greene AS. Angiogenic protection from focal ischemia with angiotensin II type 1 receptor blockade in the rat. Am J Physiol Heart Circ Physiol. 2005;288(4):H1989–96. doi: 10.1152/ajpheart.00839.2004. [DOI] [PubMed] [Google Scholar]
- 16.Li JM, Mogi M, Iwanami J, Min LJ, Tsukuda K, Sakata A, et al. Temporary pretreatment with the angiotensin II type 1 receptor blocker, valsartan, prevents ischemic brain damage through an increase in capillary density. Stroke. 2008;39(7):2029–36. doi: 10.1161/STROKEAHA.107.503458. [DOI] [PubMed] [Google Scholar]
- 17.Miller AG, Tan G, Binger KJ, Pickering RJ, Thomas MC, Nagaraj RH, et al. Candesartan attenuates diabetic retinal vascular pathology by restoring glyoxalase-I function. Diabetes. 2010;59(12):3208–15. doi: 10.2337/db10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Zhang M, Huang X, Fang F, Chen B, Wang S, et al. Protection of retinal vasculature by losartan against apoptosis and vasculopathy in rats with spontaneous hypertension. J Hypertens. 2010;28(3):510–9. doi: 10.1097/HJH.0b013e328333663f. [DOI] [PubMed] [Google Scholar]
- 19.Ishrat T, Kozak A, Alhusban A, Pillai B, Johnson MH, El-Remessy AB, et al. Role of matrix metalloproteinase activity in the neurovascular protective effects of Angiotensin antagonism. Stroke Res Treat. 2014;2014:560491. doi: 10.1155/2014/560491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, et al. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS One. 2011;6(9):e24551. doi: 10.1371/journal.pone.0024551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alhusban A, Kozak A, Ergul A, Fagan SC. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344(2):348–59. doi: 10.1124/jpet.112.197483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C, Lu XC, Luo Y, Cao J, Yang B, Gao Y, et al. Angiogenesis related gene expression profiles of EA.hy926 cells induced by irbesartan: a possible novel therapeutic approach. Chin Med J (Engl) 2012;125(8):1369–75. [PubMed] [Google Scholar]
- 23.Sennlaub F, Courtois Y, Goureau O. Inducible nitric oxide synthase mediates retinal apoptosis in ischemic proliferative retinopathy. J Neurosci. 2002;22(10):3987–93. doi: 10.1523/JNEUROSCI.22-10-03987.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010;332(1):125–34. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- 25.Abdelsaid MA, Matragoon S, Ergul A, El-Remessy AB. Deletion of Thioredoxin Interacting Protein (TXNIP) Augments Hyperoxia-Induced Vaso-Obliteration in a Mouse Model of Oxygen Induced-Retinopathy. PLoS One. 2014;9(10):e110388. doi: 10.1371/journal.pone.0110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koike A, Minamiguchi I, Fujimori K, Amano F. Nitric Oxide Is an Important Regulator of Heme Oxygenase-1 Expression in the Lipopolysaccharide and Interferon-gamma-Treated Murine Macrophage-Like Cell Line J774.1/JA-4. Biol Pharm Bull. 2015;38(1):7–16. doi: 10.1248/bpb.b14-00405. [DOI] [PubMed] [Google Scholar]
- 27.Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2(10):2557–68. [PubMed] [Google Scholar]
- 28.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68(1):121–7. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(9):1786–95. doi: 10.1161/01.ATV.0000178169.95781.49. [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57(4):585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 31.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41(2):385–94. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi S, Omata Y, Sakamoto H, Higashimoto Y, Hara T, Sagara Y, et al. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336(2):241–50. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Gong J, Maiti D, Vong L, Wu L, Schwarz JJ, et al. MEF2C ablation in endothelial cells reduces retinal vessel loss and suppresses pathologic retinal neovascularization in oxygen-induced retinopathy. Am J Pathol. 2012;180(6):2548–60. doi: 10.1016/j.ajpath.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Zhang X, Lu H, Matsukura M, Zhao J, Shinohara M. Silencing heme oxygenase-1 gene expression in retinal pigment epithelial cells inhibits proliferation, migration and tube formation of cocultured endothelial cells. Biochem Biophys Res Commun. 2013;434(3):492–7. doi: 10.1016/j.bbrc.2013.03.101. [DOI] [PubMed] [Google Scholar]
- 35.Alhusban A, Fouda AY, Bindu P, Ishrat T, Soliman S, Fagan SC. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J Hypertens. 2015;33(1):170–80. doi: 10.1097/HJH.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 36.Min LJ, Mogi M, Tsukuda K, Jing F, Ohshima K, Nakaoka H, et al. Direct Stimulation of Angiotensin II Type 2 Receptor Initiated After Stroke Ameliorates Ischemic Brain Damage. Am J Hypertens. 2014 doi: 10.1093/ajh/hpu015. [DOI] [PubMed] [Google Scholar]


