Abstract
There is a paucity of prospective cohort studies investigating the impact of environmental factors on the development of cardiometabolic (CM) disorders like Type II diabetes (T2DM). The objective of the Air-Pollution and Cardiometabolic Diseases (AIRCMD) study is to investigate the impact of personal level air pollution measures [personal black carbon (BC)/sulfate measures] and ambient fine particulate matter [(PM2.5)/NO2] levels on propensity to Type II diabetes in Beijing, China. Subjects with metabolic syndrome will undergo 4 repeated study visits within each season over a 1-year period following an initial screening visit. At each study visit, subjects will be monitored for sub-acute exposure to personal and ambient measures of air-pollution exposure and will undergo a series of functional CM outcomes. The primary endpoints include independent associations between integrated 5-day mean exposure to PM2.5 and BC and homeostasis model assessment of insulin resistance (HOMA-IR) measures, 24-hour mean diastolic and mean arterial pressure and endothelial-dependent vasodilatation. The secondary endpoints will explore the mechanistic explanation for a causal relationship between exposures and propensity for Type II diabetes and will include additional functional outcomes such as arterial compliance, heart rate variability and plasma adipokines. The novel aspects of the study include the launch of infrastructure for future translational investigations in highly polluted urbanized environments and the creation of novel methodologies for linking personalized exposure measurements with functional CM outcomes. We believe that AIRCMD will allow for unprecedented new investigations into the association between environmental risk factors and CM disorders.
INTRODUCTION
Cardiometabolic (CM) diseases such as Type II diabetes mellitus (T2DM) and obesity are the leading causes of morbidity and mortality throughout the world. According to the International Diabetes Federation, over 366 million people had diabetes in 2011 with 80% of these cases occurring in low- and middle-income countries. Epidemiologic studies that have attempted to investigate environmental factors that accentuate risk for development of CM disorders have uncovered a number of components other than diet and exercise. These factors include ambient noise, stress (mental and emotional), lack of sleep and alterations in light cycle, and environmental pollutants. In many instances these factors are strongly correlated, rendering isolation of cause and effect quite difficult. For instance, ambient noise is nearly ubiquitous in areas associated with high levels of air pollution and the same areas are often perceived as stressful living environments1. The plausibility that environmental exposures are linked to metabolic disease is exemplified by persistent organic pollutants, toxins that have consistently shown to associate with insulin resistance (IR) and DM. Prospective cohort studies of subjects exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin or other POPs in occupational settings have reported increased risk of DM and IR2. Air pollution in Asia, Latin America and Africa is a significant public health burden, especially given its pervasive nature, extraordinarily high concentrations of pollutants (e.g., particulate matter), and the high population densities in these areas. Even modest cause-association may result in rather severe public health consequences3, 4.
In 2009 we demonstrated in an experimental animal model that PM2.5 exposure results in the development of Type II diabetes, potently interacting with factors like diet and hyperlipidemia in predisposing to T2DM5. Air-pollution appears to modulate multiple components of the metabolic syndrome. For instance, PM2.5 and diesel exhaust have been noted to be associated with elevated blood pressure6, impaired vascular endothelial function7, 8, inflammation3 and alterations in autonomic tone3, 9–11. There are now at least 6 published epidemiologic studies showing some degree of association between particulate matter or traffic-related air pollutants and DM12–16.
There are very few studies on the association between air-pollution and DM (or metrics of insulin sensitivity) in populations that suffer from exposure to very high levels of air pollutants. In a study of children in multiple Iranian cities, associations between recent 7-day exposure to particulate air pollution and markers of inflammation, oxidative stress and insulin resistance were noted that remained significant after adjustment for age, gender, body mass index, waist circumference, healthy eating index and physical activity level17. PM10 levels were extremely high in these cities, averaging ~150 μg/m3. In another study conducted in Taiwan, year-long fine particle exposures (mean level ≅ 35 μg/m3) were associated with elevations in HbA1c among 1023 elderly individuals. Though these studies do not report the prevalence or incidence of overt DM, they do suggest that insulin sensitivity (the pathological hallmark underlying cardiometabolic disease) is worsened by exposure to particulate air pollution at very high concentrations18. Despite these emerging data there is a paucity of studies examining the prospective impact of continual exposure to air-pollution over long durations in urban environments where exposure levels are high and where there is a high likelihood of interaction with other factors common in the urban environment that collectively could predispose to the development of CM diseases. The dose-response relationship and potential threshold concentrations for health responses (e.g., level above which the CM effects are saturated and no longer worsened [high end] or below which they are no longer apparent [low end]) requires clarification in future studies.
In this prospective cohort study, we will: 1) investigate the association between exposure to personal black carbon (BC), ambient PM2.5 and functional intermediate outcomes of relevance to the development of T2DM in an urban environment typified by high concentrations of inhaled pollutants; 2) Explore plausible biologic mechanisms mediating changes in functional variables. Our motivation in embarking on such an investigation initially in Beijing, China is simple. Being a typical megacity, Beijing has an extraordinarily high level of air pollution, the magnitude of which can only be attained in the West within laboratory environments. A study assessing adverse changes in the CM profile under such an extreme level of exposures may allow an opportunity to examine the linear exposure-response relationship across a wide range of exposure levels and evaluate the potential for threshold effects. Finally, such an initial endeavor will allow the establishment of infrastructure that will allow future replication of similar prospective studies that will inform us on the association between exposure to environmental factors and susceptibility to CM disease.
MATERIALS AND METHODS
AIRCMD is a collaborative study between The Ohio State University, University of Michigan and Peking Union Medical College. The Institutional Review Board (IRB) at each participating institution approved the protocol and all patients enrolled in the study will be required to provide written informed consent. Subjects with metabolic syndrome (n=50), will undergo 4 repeated assessments of functional outcomes of relevance with assessment of exposure variation in ambient PM2.5 and personal BC levels.
Endpoints
The primary endpoint for the trial is the independent association between previously integrated mean 5-day PM2.5 and 5-day personal BC levels and homeostasis model assessment of insulin resistance (HOMA-IR), blood pressure (BP, 24-hour mean systolic, diastolic and mean arterial pressure), and endothelial-dependent vasodilatation using reactive hyperemia peripheral arterial tonometry (RH-PAT). BC measurements will be obtained using personal BC-microaethelometers. Additional time periods of exposure will also be explored (1-day, 2-day and 7-day lag periods for PM2.5 and 1-day, 2-day lag periods for BC). Both dependent and independent variables will be performed cross-sectionally and longitudinally across multiple repeated time points. Secondary endpoints will include exploration of association between adipocytokines (adiponectin, resistin and leptin), HRV measures (SDNN), pulse wave velocity (PWV), central aortic BP, 24-hour ambulatory blood pressure end-points and exposure to PM2.5 and BC (integrated previous mean 5-day PM2.5/nitrate levels and personal BC levels and sulfate levels). This will be done cross-sectionally at each time point and longitudinally at multiple time-points.
Exposure Variables and Assessment of Functional End-points
Exposure Assessment
Subjects will be equipped with a personal aethalometer (AethLabs MicroAeth AE51) and a global positioning device (USGlobalSat DG-100 Datalogger). Subjects will be required to change filter tickets twice in a 24-hour period and will be provided with vials in which to store these filters. They will be instructed on the use of the device, retrieval of the filters and storage of the tickets within the special vials. The tickets are used in situ for 5 minute non-destructive BC measurements using the MicroAethelometer software. Analysis of sulfates will be performed on the filters at the end of the study to obtain daily average concentrations for the 5 day exposure period. Ambient PM2.5 (hourly from TEOM operated at 50°C) and 24 hour filter-based PM2.5 mass and chemical species (sulfate and organic and elemental carbon) are measured at a fixed site in NW Beijing at Tsinghua University. Official Beijing monitoring network data of hourly PM2.5, PM10 and NO2 measurements will be obtained from multiple sites around Beijing. Subject residential addresses will be matched to the closest location for PM2.5 measurements and integrated over multiple time points obtained as outlined above in the study. A central site stationary time series of 5 minute BC will be obtained from a dual wavelength aethelometer situated at PUMC to compare with the personal BC measurements.
Homeostasis Model Assessment of IR (HOMA-IR)
Fasting (9 hour) basal insulin sensitivity will be measured by the validated HOMA-IR index. Given its ease of measurement and repeatability, HOMA-IR is an excellent method to assess insulin sensitivity in epidemiological studies such as this proposal. The average of 3 fasting blood samples taken 5 minutes apart will be used due to the pulsatile nature of insulin release. HOMA-IR = fasting insulin (μU/mL) × fasting glucose [(mmol/L)/22.5].19
Peripheral Endothelial Function using Reactive Hyperemia Peripheral Arterial Tonometry (RH-PAT)
The principle of RH-PAT has been described previously20, 21. The EndoPat2000 (Itamar Medical; 38900, Caesarea, Israel; http://www.itamar-medical.com/) semi-automated FDA-approved system will be utilized per published protocols in order to measure reactivity index (RI) as the primary outcome. Per established protocol, a blood pressure cuff is briefly placed on 1 upper arm, while the contralateral arm serves as a control. PAT probes are placed on 1 finger of each hand. After a 5-minute equilibration period, the cuff is inflated to 60 mm Hg above the subject’s systolic blood pressure (or to greater than 200 mm Hg) for 5 minutes and then deflated to induce reactive hyperemia. The RH-PAT data is digitally analyzed online (Endo-PAT2000 software version 3.0.4). The RH-PAT index reflects the extent of reactive hyperemia and is calculated as the ratio of the average amplitude of PAT signal over 1 minute beginning 1.5 minutes after cuff deflation (control arm, A; occluded arm, C) divided by the average amplitude of PAT signal of a 2.5-minute time period before cuff inflation (baseline) (control arm, B; occluded arm, D). Thus, RH-PAT index = (C/D)/(A/B) × baseline correction.
24-Hour Ambulatory Blood Pressure (ABP)
ABP will be performed using the oscillometric technique, which involves a portable, lightweight, noninvasive monitor with a self-insufflating cuff (Spacelabs Healthcare Ambulatory Blood Pressure Monitors; 98029, Issaquah, WA, USA; http://www.spacelabshealthcare.com). This system has been previously validated and ABP readings will be obtained at 30-minute intervals. The following ABP parameters will be evaluated: average daytime systolic blood pressure (SBP), average daytime diastolic blood pressure (DBP) and daytime heart rate (HR) (when awake), average night time SBP, average night time DBP and night time HR (when asleep), and average 24-hour SBP, average 24-hour DBP and average 24-hour HR. In addition, the subjects will maintain activity diaries.
Pulse Wave-Velocity and Central Aortic Blood Pressure
Pulse wave velocity (PWV) and peripheral (PAIx) and central (CAIx) augmentation index will be estimated with the SphygmoCor CP non-invasive central blood pressure assessment device (AtCor Medical; NSW Australia; http://atcormedical.com/index.html). The SphygmoCor uses a sensor that is connected to a desktop device to estimate the aortic pulse wave via the radial artery. From the morphology of the aortic wave, central augmentation index will be estimated using the following formula: increase in central pressure × 100/PP. Radial augmentation index will be calculated from the radial wave pulse as follows: (second peak SBP DBP)/(first peak SBP DBP) × 100. With the patient in the supine position, the pulse wave of the carotid and femoral arteries will be analyzed, estimating the delay with respect to the electrocardiogram wave and calculating the pulse wave velocity. Distance measurements will be taken with a measuring tape from the sternal notch to the carotid and femoral arteries at the sensor location.
Heart Rate Variability Analysis (HRV)
HRV will be measured over a 5-minute duration using the SpaceLabs Evo Digital Holter recorder (Spacelabs Healthcare; 98029, Issaquah, WA, USA; http://www.spacelabshealthcare.com). The electrocardiographic (ECG) recordings are digitized at 500 samples per second. Analysis of HRV is classified into frequency and time domain analyses. The SpaceLabs Impresario software utilizes fast Fourier power spectral analysis to calculate the frequency domain. Fast Fourier transformations provide a mathematical representation of the spectrum of frequency, also called Hertz (Hz), or power. Frequency domain analysis provides estimates of the spectrum density of R-R intervals within specific frequency bandwidths. Frequency domain measures of total power (0.01–1.00 Hz), low frequency (LF) (0.04–0.15 Hz), and high frequency (HF) (0.15–0.40 Hz) are converted to log transformations by the computer software to correct for skew. Total Hertz is the entire area under the curve in a power spectrum plot and represents the variance of all R-R intervals in the entire Holter recording. Normally, LF activity (primarily sympathetic with some parasympathetic innervation) predominates during waking hours and HF activity (parasympathetic innervation) predominates during sleep. Time domain analysis uses differing computations of the standard deviation of the beat-to-beat change in heart rate based on sinus R-R intervals over time. Time domain analysis of HRV can be further divided into 2 categories. The first is derived from the R-R intervals, using means and standard deviations of the intervals measured in milliseconds. Measures in this category include the SDNN, SDANN, and SD. The standard deviation of all R-R intervals obtained over the measurement period is the SDNN. The standard deviation of the means of R-R intervals found in successive 5-min time periods is the SDANN. The second category of time domain variables is derived from differences between adjacent R-R intervals and includes indices that are independent of circadian rhythms. Measures in this category are the pNN50 and the rMSSD. The proportion of the total R-R intervals that have differences of successive R-R intervals greater than 50 milliseconds represents pNN50. The square root of the mean squared differences of successive R-R intervals is the rMSSD. pNN50 and rMSSD correlate highly with high-frequency power, reflecting parasympathetic modulation.
Study Stages and Protocol
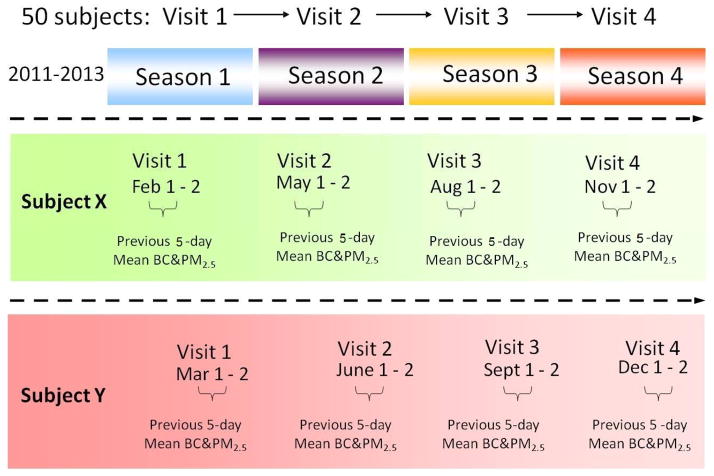
This study will contain three stages: eligibility assessment, screening visit, and study visits. In order to evaluate eligibility of patients for study entry, investigators will conduct phone screening or chart screening based upon inclusion/exclusion criteria as listed in Table 1. If subjects are found eligible, they will be scheduled for a further screening visit. The subjects appearing to preliminarily qualify by phone criteria will undergo a screening visit. Baseline anthropometrics (weight, height, body mass index, waist circumference, waist/hip ratio) and sitting clinic BP measurements will be recorded. Questionnaires for background information, self-reported air pollution awareness, dietary intake, and physical activity, family history of cardiovascular disease and socioeconomic status will be obtained. Cumulatively, 50 subjects will be recruited from clinics affiliated with the Fuwai Hospital, PUMC. Informed consent will be obtained prior to performing any procedures related to the study. Each subject will undergo 4 separate study visits. For each subject, the 4 visits will occur within a different season to allow assessment of the same end-point during multiple seasons as illustrated in Figure 1. On day 1, subjects will visit the clinic to be fit with the black carbon monitor (aethalometer) and the GPS device in order to have their locations and black carbon levels monitored while carrying out normal daily activities. On day 4, subjects will return to the clinic for the placement of ambulatory blood pressure (ABP) monitor as illustrated in Figure 2. After 24 hours (day 5), all three devices will be removed and returned to the investigators and the following tests will be performed on the subjects: (1) heart-rate variability measures over a 15-minute duration (the final 5 minutes of which will be analyzed for time and frequency domain endpoints) (2) resting BP measures (3) pulse-wave velocity and central aortic BP (4) EndoPat measurements for endothelial function and (5) Blood draws. All clinic visits will be in the morning between 8:00 and 11:00 a.m. Each subject will complete a total of 4 study visits over a 1-year period, resulting in 200 total measurements for each CM outcome.
Table 1.
Inclusion and exclusion criteria
| INCLUSION |
| Male or female, non-smoking, > 45 years old |
| Patients with metabolic syndrome defined by IDF criteria specific for Asians: waist circumference >90 cm in males and >80 cm in females |
| At least 3 of the 4 following criteria: |
| Triglyceride level: >150 mg/dL |
| High-density lipoprotein < 40 mg/dL in males and < 50 mg/dL in females |
| Controlled hypertension defined as 130 mmHg < SBP < 160mmHg, DBP < 95 mmHg at first visit |
| 100 mg/dL < fasting plasma glucose < 126 mg/dL |
| EXCLUSION |
| Self-reported daily secondhand smoke exposure > 1 hour long |
| History of any CV disease (previous myocardial infarction or stroke) |
| Type II diabetes on medications |
| Use of any anti-hypertensive, glucose-lowering, or lipid-lowering medications or drugs that can alter baseline insulin sensitivity, BP, or endothelial function (anti-oxidants, multi-vitamins, folic acid, fish oil supplementation, L-arginine) |
| Evidence of a secondary form of hypertension, such as coarctation of the aorta, hyperaldosteronism, unilateral renal disease, or pheochromocytoma. |
| Dyslipidemia secondary to other causes, including alcoholism, auto-immune disease, nephrotic syndrome, any viral or non viral hepatitis clinically active within 12 months prior to study entry, obstructive hepatic or biliary disease, dys- or macroglobulinemia, multiple myeloma, glycogen storage disease, uncontrolled hypothyroidism or hyperthyroidism, chronic pancreatitis and porphyria |
| History of drug abuse within the last 2 years |
Figure 1.
Hypothetical schemes of 4 study visits over a 1-year period.
Figure 2.

Personal BC and ABP monitoring on Day 4 at each study visit.
Sample Size and Power Calculations
The principal analysis in this study will be associations between the preceding integrated mean 5-day BC and PM2.5 level and the 4 primary CM outcomes: 24-hour mean systolic and diastolic BP, RI (endothelial function) and HOMA-IR. Based upon a sample size of 50 subjects, the minimum detectable effects of exposures on primary CM outcomes are presented in Table 2 in cross-sectional and longitudinal settings, with varying values of the within-subject correlation. Statistical power as functions of the effect size and covariance structure in a repeated measures design is graphically shown in Figure 3. Both table 2 and Figure 3 demonstrate adequate power to determine biologically meaningful and plausible effect sizes for the primary CM outcomes, the range of the minimum detectable effect sizes is consistent with what has been found in prior studies22, 23. For instance, assuming a within subject standard deviation of 1.27 in HOMA-IR, a two-sided Wald test at a significance level of 0.05 with a 80% statistical power in a cross-sectional setting should suffice. This will be able to detect an increase of 0.55 unit in HOMA-IR with a SD increase in exposure level such as sub-acute 5-day PM2.5. Therefore, a sample size of 50 subjects will provide adequate power to conduct future statistical analysis.
Table 2.
The minimum detectable effects for the primary CM outcomes in cross-sectional and longitudinal settings.
| Outcome | Outcome Mean ± SDa | Cross- Sectionalb | Longitudinalc
|
|||
|---|---|---|---|---|---|---|
| ρ=0.5 | ρ=0.6 | ρ=0.7 | ρ=0.8 | |||
| HOMA-IR | 2.81 ± 1.27 | 0.55d | 0.26 | 0.24 | 0.22 | 0.18 |
| RI | 1.76 ± 0.57 | 0.25 | 0.12 | 0.11 | 0.10 | 0.08 |
| SBP | 115.4 ± 7.0 | 3.06 | 1.44 | 1.34 | 1.21 | 0.86 |
| DBP | 68.2 ± 5.2 | 2.27 | 1.07 | 1.00 | 0.89 | 0.75 |
The mean and SD values for the 4 outcomes are taken from multiple publications and from a compilation of our own previous studies.
Two-sided Wald test for slope at a significance level at 0.05 using simple linear regression with subject N=50.
Under longitudinal setting with varying within subject correlation coefficients, test the fixed effect of environmental exposure using two-sided Wald test in linear mixed models. Subject N=50, observation per subject m=4.
The minimum detectable effects are reported with SD increase in exposure level.
Figure 3.
Statistical power as functions of the effect and covariance structure for functional CM outcomes. Power curves are calculated by using a sample size of 50, within-subject correlation coefficients (as denoted by rho) at 0.5 and 0.8, and estimated SDs 1.27, 0.57, 7.0, and 5.2 for outcomes HOMA-IR, RI, SBP and DBP, respectively. Changes in outcomes are reported with SD increase in exposure factor.
Efficacy Analysis
The primary analyses will focus on a 5-day exposure window. Any outcome variables with an invalid normality assumption will be considered for the Box-Cox family of transformations prior to model building. The effect on important CM outcomes (HOMA-IR, RI, SBP and DBP) at each study visit will be first modeled by multiple linear regressions, with common covariates (such as age, gender, race, body mass index, waist/hip ratio, social economic status, season, duration of living in Beijing, and ambient temperature and relative humidity) adjusted and confounders selected by prior knowledge and separate univariate association with dependent variables. To explore non-linear dose-response association between exposure values and primary outcomes, generalized additive models (GAM) will be performed, in which flexibility will be introduced by applying smooth functions of exposures. To allow a better insight into possible changes in outcome-exposure relationships at the lower or upper tails of the outcome distribution, quantile regression technique will be adopted for the hypothesis of threshold effects. Based on the study design, primary analyses will be extended to longitudinal permitting improved control for subject-level factors and examination of within-subject variability of outcomes. Linear mixed models with a time effect on outcomes will be fitted, where fixed effects and covariance matrix of the random effects will be estimated by restricted maximum likelihood method. Generalized additive mixed models (GAMM) will also be used given a non-linear effect of exposure or time on the outcome in the longitudinal setting. Furthermore, the monitor of PM2.5 and BC in the study (and sulfur dioxide SO2 by aethalometer, needs to be validated) will provide us a great chance of assessing the complex health impact of air pollutants in a multi-pollutant instead of single-pollutant approach. The scheme of statistical inference for secondary endpoints will be similar to the primary analyses, with primary CM outcomes replaced by adipocytokines, HRV measures (SDNN), PWV, and mean 24-hour central aortic BP. Statistical models with different lags of exposure will be compared by certain criteria such as the Akaike Information Criterion (AIC) or Bayesian information criterion (BIC), and exposure periods with stronger effects will be evaluated.
DISCUSSION
AIRCMD is a multi-national collaborative project investigating the mechanistic link between environmental air pollutants and CM disease in a prototypical megacity in China. Several novel aspects of the design of this project are noteworthy: (1) Deliberate choice of undertaking an initial proof-of concept study in an urbanized environment with high exposure levels otherwise not encountered in North America; (2) Use of repeated measurements of functional measures across seasons to allow for expected seasonal variations in exposure variables and changes in functional outcome measures; (3) Application of personal BC measurements; (4) Incorporation of numerous functional outcome measurements of importance in the metabolic syndrome that together may help provide a mechanistic basis for the proclivity to T2DM; (5) Assessment of PM2.5, BC, sulfate and NO2 measurements (multi-pollutants) to enable the detection of interaction amongst these variables.
With rapid urbanization in developing countries, air pollution is a major environmental issue. For instance, two-thirds of cities in China have failed to meet the Grade II National Ambient Air Quality Standard24. The mean annual average in countries such as India and China is at least 5 fold higher than the U.S National Ambient Air Quality Standard (NAAQS) of 15μg/m3, and the WHO standard of 10μg/m3. While concern over environmental issues in countries such as China and India have mounted in the last few years, the awareness or knowledge that pervasive environmental toxins may pose a risk for chronic cardiovascular and CM diseases is rather limited. Our previous studies, along with others, have provided credible initial evidence in experimental animal models that inhaled particulates such as air-pollution may indeed interact with other risk factors in predisposing to T2DM via a variety of mechanisms including potentiation of inflammation, promotion of endoplasmic reticulum (ER) stress, alteration in brown adipose tissue function and change in autonomic tone5, 12, 14–17, 25–29. However, there is still inadequate prospective information from urban environments exposed to high levels of air-pollution over chronic durations that will allow discernment of potential threshold concentrations for health responses and interaction with other common factors such as diet, activity and exercise. In this study, HOMA-IR will be evaluated cross-sectionally and longitudinally. The incorporation of 24-ambulatory blood pressure measurements in conjunction with assessment of endothelial function, HRV measures and central aortic blood pressure data will provide an unprecedented opportunity to provide direct mechanistic information on multiple pathophysiologically relevant pathways. Changes in adipocytokines adiponectin, resistin and leptin over time may also help explain the association between air pollution and CM disease.
Subjects in this study will be equipped with microaethalometers to assess personal BC over 5 consecutive days at each visit. There is a growing appreciation of BC measurements as representing an integrative index of anthropogenic sources of air-pollution and is particularly germane in urbanized environments with a high degree of vehicular traffic and sources of power generation. The incorporation of GPS data with BC is to provide geographic context to the measurements and additionally would authenticate the veracity of measurements. PM2.5 assessment will be matched to subjects based on their location to the nearest PM2.5 monitor sites across Beijing by zip code. We will not include personal PM2.5 measurements that may provide better metrics of personal exposure and may avoid the well-known problems of exposure misclassification owing to the expense and the considerable effort that would have been required to integrate these measures in addition to BC and the rather comprehensive list of functional outcome measures.
The primary efficacy analysis in this study is to assess the functional impact of integrated mean exposures over durations as there may be a higher likelihood of observing such an effect, at least based on prior studies3, 6, 30. The rationale could be that functional CM outcomes are slowly altered by a sub-acute period of exposure, or that the average of 5 exposure measurements may represent a better estimate of true exposure. In the secondary efficacy analysis, associations between multiple lag structures of PM2.5 (from 1–30 days), moving average of personal BC exposure with outcomes will also be explored in order to investigate the temporal exposure-response relationships with strong effects.
Ambient air pollution comprises a complex mixture of pollutants. Traditionally, the health impact of air pollution is assessed for each pollutant independently, with PM2.5 being the most frequently studied pollutant. However, it is well recognized that PM2.5 mass measurements are an imperfect measure of toxicity31. Furthermore the limitations of single pollutant models in explaining health consequences are increasingly recognized32, 33. Expanding the number of pollutants measured in a study design is thus desirable34, 35. In this study, the placement of microaethalometers enables the exploration of the linkage between CM responses and temporal variations in personal BC and SO2 levels and ambient PM2.5 and NO2 measures. It will be of significant interest to examine the impact of the interaction between these various measures and functional outcomes in CM disease.
Since the link between air-pollution and cardiovascular disease is already established, demonstration of additional links between Type II diabetes will provide an additional rationale for limiting exposure to air-pollution.
Acknowledgments
This work was supported by NIEHS Grants R01ES017290, R01ES015146 and R01ES019616.
References
- 1.Ross Z, Kheirbek I, Clougherty JE, Ito K, Matte T, Markowitz S, Eisl H. Noise, air pollutants and traffic: Continuous measurement and correlation at a high-traffic location in new york city. Environ Res. 2011;111:1054–1063. doi: 10.1016/j.envres.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Rignell-Hydbom A, Lidfeldt J, Kiviranta H, Rantakokko P, Samsioe G, Agardh CD, Rylander L. Exposure to p,p′-dde: A risk factor for type 2 diabetes. PLoS ONE. 2009;4:e7503. doi: 10.1371/journal.pone.0007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 4.Nawrot TS, Perez L, Kunzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: A comparative risk assessment. Lancet. 2011;377:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 8.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. American journal of respiratory and critical care medicine. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- 9.Gong H, Jr, Linn WS, Sioutas C, Terrell SL, Clark KW, Anderson KR, Terrell LL. Controlled exposures of healthy and asthmatic volunteers to concentrated ambient fine particles in los angeles. Inhal Toxicol. 2003;15:305–325. doi: 10.1080/08958370304455. [DOI] [PubMed] [Google Scholar]
- 10.de Paula Santos U, Braga ALF, Giorgi DMA, Pereira LAA, Grupi CJ, Lin CA, Bussacos MA, Zanetta DMT, do Nascimento Saldiva PH, Filho MT. Effects of air pollution on blood pressure and heart rate variability: A panel study of vehicular traffic controllers in the city of sao paulo, brazil. Eur Heart J. 2005;26:193–200. doi: 10.1093/eurheartj/ehi035. [DOI] [PubMed] [Google Scholar]
- 11.Hampel R, Breitner S, Schneider A, Zareba W, Kraus U, Cyrys J, Geruschkat U, Belcredi P, Muller M, Wichmann HE, Peters A. For the Cooperative Health Research in the Region of Augsburg Study G. Acute air pollution effects on heart rate variability are modified by snps involved in cardiac rhythm in individuals with diabetes or impaired glucose tolerance. Environ Res. 2011 doi: 10.1016/j.envres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50:32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- 13.Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the u.S. Diabetes Care. 2010;33:2196–2201. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, Tjonneland A, Overvad K, Sorensen M. Diabetes incidence and long-term exposure to air pollution: A cohort study. Diabetes Care. 2012;35:92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer U, Herder C, Sugiri D, Strassburger K, Schikowski T, Ranft U, Rathmann W. Traffic-related air pollution and incident type 2 diabetes: Results from the salia cohort study. Environ Health Perspect. 2010;118:1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes in african american women living in los angeles. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in taiwan. Occup Environ Med. 2011;68:64–68. doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- 19.Jayagopal V, Kilpatrick ES, Jennings PE, Hepburn DA, Atkin SL. Biological variation of homeostasis model assessment-derived insulin resistance in type 2 diabetes. Diabetes Care. 2002;25:2022–2025. doi: 10.2337/diacare.25.11.2022. [DOI] [PubMed] [Google Scholar]
- 20.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzawa Y, Sugiyama S, Sugamura K, Nozaki T, Ohba K, Konishi M, et al. Digital assessment of endothelial function and ischemic heart disease in women. Journal of the American College of Cardiology. 2010;55:1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 22.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, Benjamin A, Max P, Bard RL, Brook RD. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao J, Wang L. Improving urban air quality in china: Beijing case study. J Air Waste Manag Assoc. 2005;55:1298–1305. doi: 10.1080/10473289.2005.10464726. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR, Lippmann M, Rajagopalan S, Chen LC, Sun Q. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan YH, Chou CC, Lee CT, Liu JY, Cheng TJ. Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol. 2011;23:507–519. doi: 10.3109/08958378.2011.587472. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Z, Xu X, Zhang X, Wang A, Zhang C, Hüttemann M, Grossman LI, Chen LC, Rajagopalan S, Sun Q, Zhang K. Exposure to ambient particulate matter induces a non-alcoholic steatohepatitis-like phenotype and impairs hepatic glucose metabolism. Hepatology. 2012 doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 31.Bhatnagar A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- 32.Sahsuvaroglu T, Jerrett M. Sources of uncertainty in calculating mortality and morbidity attributable to air pollution. Journal of toxicology and environmental health. 2007;70:243–260. doi: 10.1080/15287390600884743. [DOI] [PubMed] [Google Scholar]
- 33.Mauderly JL, Samet JM. Is there evidence for synergy among air pollutants in causing health effects? Environmental health perspectives. 2009;117:1–6. doi: 10.1289/ehp.11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiolog. 2010;21:187–194. doi: 10.1097/EDE.0b013e3181cc86e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauderly JL, Burnett RT, Castillejos M, Ozkaynak H, Samet JM, Stieb DM, Vedal S, Wyzga RE. Is the air pollution health research community prepared to support a multipollutant air quality management framework? Inhal Toxicol. 2010;22 (Suppl 1):1–19. doi: 10.3109/08958371003793846. [DOI] [PubMed] [Google Scholar]