Abstract
Background
Inflammatory breast cancer (IBC) is the most aggressive form of locally advanced breast cancer (LABC). Patients with IBC commonly present with skin metastasis, which are observed microscopically as tumor emboli within dermal lymphatics. These metastatic tumor cells aberrantly over-express E-cadherin and exhibit the ability to undergo self-renewal and are highly invasive. There are no therapeutics yet identified that target the structure and functions of IBC tumor emboli. The present studies evaluated the effects of the pan-histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA) using IBC tumor spheroids derived from established IBC cell lines and tumor spheroids derived from pleural effusion (PE) aspirates of patients with IBC and LABC, designated as PE-IBC and PE-LABC.
Methods
Methods used include culture of IBC cells from clonal density single cells in low adherence culture conditions that promote formation of IBC tumor spheroids, clonogenic assays, cell fractionation and Western blotting, confocal microscropy and modified Boyden chamber invasion assays.
Results
SAHA inhibited self-renewal of IBC tumor spheroids from established IBC cell lines and PE-IBC and PE-LABC, as assessed by decreased clonogenic growth. SAHA blocked homotypic aggregation of the cells that comprised the IBC tumor spheroids leading to loss of their 3 dimensional (3D) structure, which was associated with a change in location of E-cadherin protein from the plasma membrane in untreated IBC tumor spheroids to the cytoplasm of cells within IBC tumor spheroids with SAHA treatment. In addition, SAHA blocked the robust invasion exhibited by IBC tumor spheroids of established cell lines as well as by tumor spheroids derived from PE-IBC and PE-LABC.
Conclusions
SAHA targets the integrity and biological activities of IBC tumor spheroids and may be a promising agent to evaluate for its effectiveness in treatment of IBC.
Keywords: inflammatory breast cancer, self renewal, E-cadherin, homotypic aggregation, histone deacetylase enzymes, suberoylanilide hydroxamic acid, SAHA, cancer stem cells
Introduction
Inflammatory breast cancer (IBC) is the most aggressive variant of this disease, with a 5 year overall survival rate for IBC patients that is significantly lower compared to the overall survival rate for patients with non-IBC locally advanced breast cancer (LABC) (1). Although multi-modality therapy, including neoadjuvant chemotherapy, followed by surgical resection and radiation has been used to treat IBC patients for over 30 years, there has been no change in overall survival of IBC patients during this time period (2). There is an urgent need to identify therapeutic agents that target specific characteristics that are associated with the highly aggressive phenotype of IBC.
One of the signatures of IBC is the presence of invasion of tumor cells into the lymphatics of the dermis (1). IBC tumor emboli within the dermal lymphatics aberrantly over-express the calcium-dependent adhesion molecule E-cadherin. This protein which is located at the adherens junctions on the plasma membrane mediates homotypic aggregation which confers the 3 dimensional structure of the tumor emboli (3). E-cadherin has been associated with a normal phenotype, with loss of E-cadherin reported to commonly occur during the epithelial-mesenchymal transition (EMT). However, a recent study suggests that tumors with a highly malignant phenotype expression of E-cadherin that occurs in conjunction with its binding partner p120 catenin and small GTPases, resulting in increased motility and invasion (4). These observations are consistent with E-cadherin over-expression on the surface of highly invasive IBC tumor emboli. In addition to E-cadherin expression, IBC tumor emboli have been shown to possess characteristics consistent with this tumor type being enriched for cells with a tumor initiating i.e., cancer stem cell phenotype, including the ability to undergo self-renewal (5).
To date, no therapeutic agents have been identified that target the characteristics of IBC such as homotypic aggregation of IBC tumor emboli and self-renewal. Recent evidence suggests that HDAC enzymes, in tandem with histone acetylases (HAT) enzymes, control reversible chromatin remodeling which is a key epigenetic mechanism that regulates transcription of genes associated with multiple activities of normal and malignant cells including self renewal, survival and resistance to apoptosis, cell cycle progression, aggregation, motility, invasion, angiogenesis and metastasis (6, 7).
A number of HDAC inhibitors have been developed that inhibit the activity of multiple HDAC enzymes. Suberoylanilide hydroxamic acid (SAHA); vorinostat; Zolinza ®, Merck & Co, Inc) is the first pan-HDAC inhibitor to be approved by the FDA (8,9). While the first indication for SAHA was for treatment of patients with cutaneous T cell lymphoma (CTCL), recent studies suggest that this pan-HDAC inhibitor may have activity in solid tumors, including metastatic breast cancer (10). Pre-clinical studies and clinical trials are underway to evaluate both pan-HDAC inhibitors and selective HDAC inhibitors for their utility in treatment of multiple types of solid tumors as well as hematological disorders.
Not only has there been a lack of progress in development of effective treatments for IBC, there are also few cell lines available with which to study IBC. Only 2 cell lines are widely available for study. The SUM149 IBC cell line was developed from pleural effusion cells of an IBC patient and is the most studied of all of the IBC cell lines (11,12). SUM190 IBC cells were developed from the primary tumor of an IBC patient. Both of these IBC cell lines are used in the present study. Due to the lack of cell lines to study IBC and LABC, the present study also used tumor cells isolated from pleural effusion of a patient with IBC and a patient with LABC as a comparison to the response of the established IBC cell lines to SAHA. Using a combination of established IBC cell lines and tumor cells isolated from patients with metastatic IBC and LABC, the present studies evaluated the effects of SAHA on specific characteristics associated with the aggressive phenotype of IBC.
Materials and Methods
Cell Lines and Conditions
The human MCF-7 breast cancer cell line was obtained from American Type Cell Culture (Manassas, VA). MCF-7 is an estrogen receptor positive (ER+) breast cancer cell line used as a control cell line in these studies. MCF-7 cells were cultured in Dulbecco's modified Eagles medium (DMEM/F12; Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen). The SUM149 and SUM190 cell lines were provided by Dr. Stephen Ethier (Asterand, Detroit MI) (11,12). SUM149 and SUM190 cells were maintained as 2 dimensional (2D) adherent cultures in Ham F12 Nutrient Mixture (Invitrogen) supplemented with 10% FBS, insulin (1 mg/ml; Sigma-Aldrich, St. Louis, MO), and hydrocortisone (1 mg/ml; Sigma-Aldrich). For low adherence culture conditions, which promote formation of tumor spheroids and self-renewal, SUM149 and SUM190 cells as well as PE-IBC and PE-LABC cells were maintained in serum free mammary epithelial growth medium (MEGM, BioWhittaker) supplemented with B27 (Invitrogen), 20 ng/ml EGF, 40 ng/ml bFGF (BD Biosciences), and 4 ug/ml heparin (Sigma) (13,14). All cell lines were maintained at 37°C under 5% CO2 in humidified incubators.
Assessment of self-renewal as measured by clonogenic growth
Single cell suspensions were plated into ultralow attachments plates or flasks (Corning) and maintained as described above. Tumor spheroids were treated with either DMSO as the solvent control or with SAHA at concentrations of 0.25 μM, 0.5, 2.5, 5.0, 10.0, 25.0, or 50.0 μM, with each concentration run in triplicate. After 10 days in culture, 0.5% thiazolyl blue tetrazolium bromide (MTT) was added to each well to allow visualization of the tumor spheroids and samples were returned to the incubator for 1 hr. Formation of tumor spheroids was measured with an Optronix Scanner (Oxford, UK) and data was analyzed using GelCount software. The IC50 for SAHA was calculated using nonlinear regression analysis of average tumor spheroid formation. The concentration of SAHA that inhibited 50% of spheroid formation, designated as the IC50 values, are represented from data obtained in three separate experiments for all cell lines and pleural effusion cells evaluated, with the Pearson's correlation co-efficients shown in Table 1.
Table 1. SAHA inhibits clonogenic growth of IBC cells.
SAHA significantly inhibits clonogenic growth of IBC cell lines SUM190 and SUM149 mammospheres and of PE-IBC and PE-LABC mammospheres, used as a measure of self-renewal capacity. IC50 concentrations of SAHA range from 0.17 μM for SUM149 to 3.31 μM for SUM190.
| Sample | IC50 [μM] | R2 |
|---|---|---|
| MCF-7 | 0.37 | 0.94 |
| SUM149 | 0.17 | 0.91 |
| SUM190 | 3.31 | 0.91 |
| PE - IBC | 2.27 | 0.95 |
| PE - LABC | 1.52 | 0.94 |
E-cadherin antibody staining of IBC spheroids and photographic assessment of homotypic aggregation of IBC tumor cells
Confocal microscopy was used to define the location of E-cadherin on IBC tumor spheroids. Spheroids were treated with SAHA (5 μM, 6 hrs, 24 hrs), and slides were prepared by centrifuging spheroids onto slides at 500 rpm for 3 min using a Shandon cytospin. Slides were rinsed with PBS at room temperature, fixed in 4% paraformaldehyde in PBS for 15 min at room temperature followed by additional washes in PBS. Cells were permeabilized with 0.1% Triton-X 100 in PBS for 5 min followed by incubation in 5% goat serum in PBS for 1 hr to block non-specific antibody binding. Slides were then incubated with E-cadherin polyclonal antibody (Cell Signaling, Danvers, MA) in PBS for 18 hrs at 4°C. Nonspecific staining was determined by incubating slides with isotypic control antibody as the staining control. Slides were then washed in PBS three times for 10 min each, and incubated with goat-anti-rabbit secondary antibody conjugated with Alex-Fluor 568 (Invitrogen, Carlsbad, CA) in PBS at a 1:400 dilution for 2 hrs at room temperature. For nuclear staining, slides were then incubated with TO-PRO-3 (Invitrogen) in PBS at a dilution of 1:300 for 15 min. Slides were then washed in PBS three times for 10 min each followed by placing cover slips on slides using anti-fade mounting medium (Vector Laboratories, Burlingame, CA). Images were captured using a Zeiss LSM 510 confocal microscope. Representative confocal images are shown to allow visualization of the location of E-cadherin protein on SUM190 spheroids and to visualize the loss of homotypic aggregation induced by exposure of SUM190 spheroids to SAHA (Figures 2 A–F).
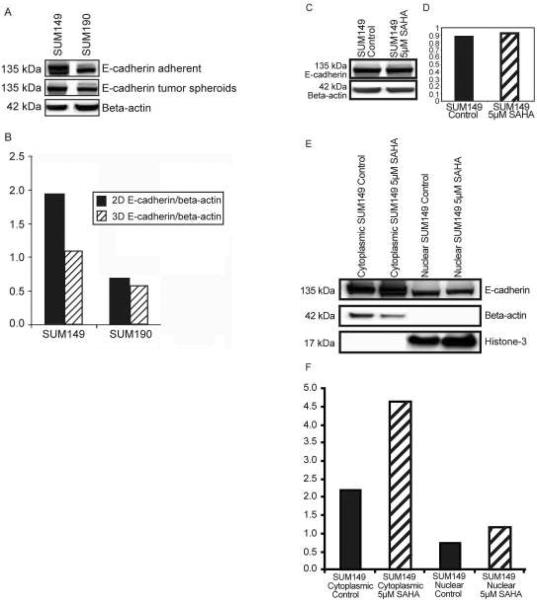
Figure 2. A–E. SAHA induced translocation of E-cadherin protein into the cytoplasm of IBC tumor spheroids.
Figure 2 A and B. Western blotting combined with densitometric analysis revealed that SUM149 cells produced approximately 2-fold greater amounts of E-cadherin protein when cultured as 2D adherent cells compared to levels of E-cadherin protein produced by SUM149 cells cultured as 3D tumor spheroids. This appears to be due to the presence of a glycosylated protein product in 2D adherent SUM149 cells. The amount of protein produced by SUM190 cells was equivalent in 2D adherent compared to 3D tumor spheroids.
Figure 2 C and D. SAHA treatment did not alter the total amount of E-cadherin protein produced by either SUM149 or SUM190 tumor spheroids.
Figure 2 E and F. Cell fractionation combined with Western blotting and densitrometry analysis revealed that SAHA altered the location of E-cadherin protein from the plasma membrane to the cytoplasm without altering the total amount of E-cadherin protein.
Western blot analysis of effects of SAHA on amount and location of E-cadherin protein in IBC tumor spheroids
SUM149 and SUM190 cells were cultured either under adherent conditions in Ham F12 medium with 10% FBS or under low adherence conditions in MEGM with growth factors and supplements. For analysis of total E-cadherin protein, cells were treated with 5 μM SAHA for 24 hrs. Following treatment, cells were isolated and spun down to isolate cell pellets, which were then lysed in 1% MPer lysis buffer (Thermo Fisher Rockford, IL). For analysis of E-cadherin protein in different cell fractions, cells were cultured under low adherence conditions, treated with SAHA at 5 μM for 24 hrs. Cells were isolated and centrifuged to obtain cell pellets, with removal of the supernatants to obtain pellet as dry as possible. Pellets were placed in buffers to isolate cytoplasmic or nuclear fractions using the nuclear and cytoplasmic extraction kit (Pierce Biochemicals, Rockford, IL) according to manufacturer's instructions. Equal amounts of protein (50–100 μg), as determined by total protein assay (Bio-Rad Laboratories, Hercules, CA), were then separated using 10% polyacrylamide gels (Bio-Rad). Proteins were transferred to nitrocellulose membranes (GE Healthcare, Piscataway, NJ), blocked for non-specific binding using a buffer containing 1X PBS, 0.1% Tween-20, and 5% milk and then probed with E-cadherin antibodies (Cell Signaling, Danvers, MA) followed by incubation with anti-rabbit IgG horseradish peroxidase-conjugated secondary antibodies (GE Healthcare). For analysis of total protein in whole cell lysates and cytoplasm fractions, E-cadherin protein was normalized to β-actin, used as a loading control. For analysis of E-cadherin protein in nuclear fractions, Histone 3 was used as a loading control. Protein bands were visualized by Chemiglow enhanced chemiluminescence system (Alpha Imager, San Leandro, CA). Images were scanned and quantified using an Alpha Innotech densitometer with the Alpha Imager application program. Experiments were repeated three times and representative Western blots and densitometric analysis are shown.
Assessment of invasion through an artificial Matrigel basement membrane
The effects of SAHA on in vitro invasion of SUM149 and SUM190 IBC spheroids and tumor spheroids from PE-IBC and PE-LABC through a Matrigel basement membrane was determined based on the number of cells that invaded through transwell inserts coated with the artificial basement membrane, Matrigel. Tumor spheroids were trypsinized, resuspended in media and counted. Plates (6-well) compatible with transwell inserts with 8 μm pore-size polycarbonate filters (Fisher Scientific) were coated with Matrigel in cold serum-free DMEM/F12 at a final concentration of 0.7 mg/ml and placed at room temperature for 40 min. Cells (in 500 μl serum-free medium) were added into the transwell inserts and incubated for 72 hrs in absence or presence of SAHA. As a control, 10% FBS was used to evaluate the baseline extent of invasion of tumor spheroids. The lower chambers were filled with 2 ml of media. After incubation, non-invading cells on the upper surface of the filter were removed with cotton swabs. Cells that had passed through the pores onto the lower side of the filter were fixed, stained with Hema-3 stain (Fisher Scientific), and quantitated. The experiments were performed in triplicate and repeated twice. Two-tailed Student's t-tests were performed to evaluate the effects of SAHA on invasion of IBC tumor spheroids.
Results
SAHA inhibited clonogenicity of IBC tumor spheroids
The effects of the pan-HDAC inhibitor SAHA on self-renewal of IBC cells cultured under low adherence conditions, which promote formation of tumor spheres, was assessed. For these studies, clonogenicity of tumor spheroids from established IBC cell lines as well as PE-IBC, and PE-LABC was evaluated. SAHA induced a dose dependent inhibition of self-renewal of SUM149 and SUM190 IBC tumor spheroids as well as inhibited self-renewal of freshly isolated PE-IBC and PE-LABC. As shown in Table 1, the concentrations of SAHA that inhibited 50% of clonogenic growth (IC50) of tumor spheroids ranged from 0.17 μM for SUM149 to 3.31 μM for SUM190, with the IC50 of PE-IBC and PE-LABC at doses between those observed for the SUM190 tumor spheroids. The IC50 doses of SAHA for all of the IBC cells was within the clinically achievable range of 2.5 μM SAHA.
SAHA inhibited homotypic aggregation and 3D integrity of IBC tumor spheroids
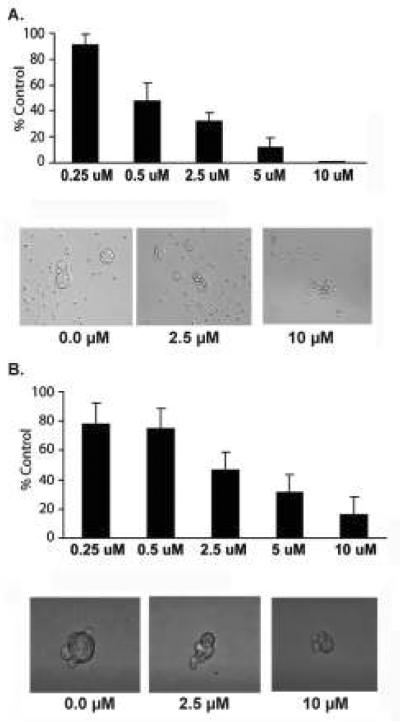
Both SUM149 and SUM190 cells form tumor spheroids when placed in low adherence conditions in defined medium. While SUM190 cells form very tight tumor spheres, SUM149 cells form tumor spheroids that are not as tightly packed as the SUM190 cells (Figure 1 A and B). Phase contrast microscopy was used to visualize the dose dependent effects of SAHA (2.5 μM-10 μM; 24 hrs) on homotypic aggregation of SUM149 and SUM190 IBC tumor spheroids, revealing that SAHA induced a loss of integrity of both SUM149 and SUM190 tumor spheroids (Figures 1 A and B). Similar dose and time dependent results were observed with treatment of PE-IBC with SAHA (data not shown).
Figure 1. A and B. SAHA inhibited the homotypic aggregation and 3D integrity of IBC tumor spheroids.
Phase contrast micrographs revealed that SAHA induced a dose dependent inhibition of the homotypic aggregation of cells within SUM149 (Figure 1 A) and SUM190 tumor spheroids (Figure 1 B) with a loss of their 3D integrity of both SUM149 and SUM190 tumor spheroids (Figure 1 A and B).
SAHA altered the location of E-cadherin protein in IBC tumor spheroids
SUM149 and SUM190 cells produce abundant E-cadherin protein when cultured under both 2D adherent conditions and in 3D low adherence culture conditions which promote formation of tumor spheres (Figure 2 A and B). When cultured as adherent cells, SUM149 cells produce approximately 2-fold greater amount of E-cadherin protein, which included a higher molecular weight glycosylation product, which was not detected in SUM149 tumor spheroids cultured under low adherence conditions (Figure 2 A and B). SUM190 cells produced equivalent amounts of E-cadherin protein regardless of the culture conditions. When cultured as tumor spheroids, both SUM149 and SUM190 cells produced E-cadherin protein, with no change in total E-cadherin protein when treated with SAHA (Figure 2 C). Cell fractionation studies combined with Western blotting and densitometry revealed that while SAHA did not alter the total E-cadherin protein produced by IBC tumor spheroids, SAHA treatment resulted in a change in the location of E-cadherin protein on IBC tumor spheroids. In untreated IBC tumor spheroids, E-cadherin was located on the plasma membrane, which was translocated into the cytoplasm by SAHA treatment (Figure 2 E and F).
SAHA altered location of E-cadherin protein and inhibits homotypic aggregation of IBC spheroids
Confocal microscopy was used to visualize the distinct pattern of E-cadherin protein localized to the adherens junctions of cells within SUM190 tumor spheroids. In untreated SUM190 spheroids, E-cadherin protein was located at the surface of the plasma membrane at the point of cell:cell contact between cells within tumor spheroids (Figure 3 A–C). Treatment of SUM190 tumor spheroids with 5 μM SAHA for 6 hrs induced a loss of E-cadherin protein at the adherens junctions, with E-cadherin protein visible within the cytoplasm of IBC tumor cells that had been detached from the spheroid structure (Figure 3 D–F). The SAHA-induced alteration in E-cadherin localization at the plasma membrane occurred simultaneously with the diminished homotypic aggregation of cells within the spheres, resulting in loss of integrity of the 3D tumor spheroid structures (Figures 3 D–F). The results of confocal imaging studies are consistent with results shown in Figures 1 and Figures 2 which demonstrated that SAHA did not alter the total amount of E-cadherin protein in IBC tumor spheroids but mediated a translocation of protein to the cytoplasm of IBC tumor spheroids which was associated with the loss of homotypic aggregation and integrity of IBC tumor spheres.
Figure 3. A–F. SAHA altered the location of E-cadherin protein and blocked homotypic aggregation of IBC tumor spheroids.
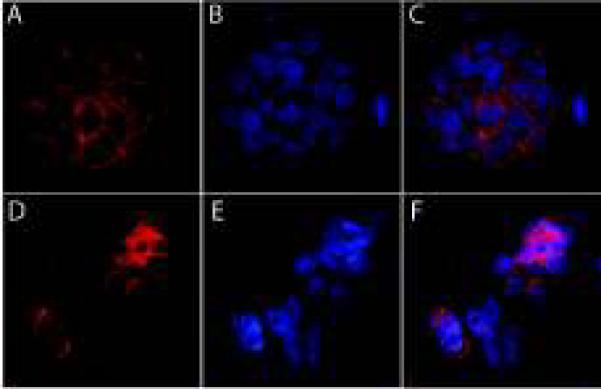
Figure 3 A–C. E-cadherin protein (red fluorescence) was visualized at the adherens junctions of SUM190 cells that comprise the IBC tumor spheroids. Blue fluorescence is associated with nuclear DNA stained with TO-PRO-3. Figure 3 C is the merged image showing E-cadherin protein localization at the plasma membrane with nuclear staining shown in blue.
Figure 3 D–F. Following exposure to 5 mM SAHA for 6 hrs, E-cadherin protein was diminished at the adherens junctions at the plasma membrane, with visible E-cadherin protein within the cytoplasm. The decreased E-cadherin protein staining at the cell surface was associated with the loss of homotypic aggregation of cells within the tumor spheroid, leading to disintegration of the tumor spheroid structure.
IBC tumor spheroids exhibit robust invasion across an artificial basement membrane which is blocked by SAHA
SUM149 cells cultured as either adherent cells or as tumor spheroids exhibit the ability to invade across an artificial basement membrane. In contrast, SUM190 cells are not invasive when cultured under 2D adherent conditions but acquire this characteristic when cultured under low adherence conditions. SAHA significantly (p<0.05) inhibited invasion of SUM149 and SUM190 tumor spheroids as well as invasion exhibited by PE-IBC and PE-LABC (Figure 4).
Figure 4. SAHA blocks invasion of IBC tumor spheroids from established IBC cells lines and from PE-IBC and PE-LABC.
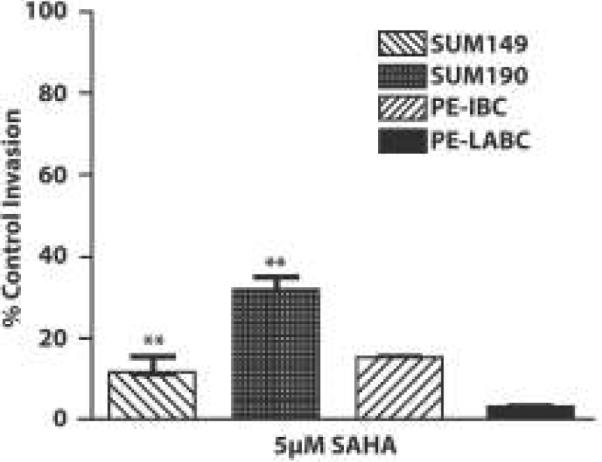
SAHA significantly inhibited invasion of IBC tumor spheroids derived from established cell lines and from pleural effusion cells isolated from a patient with IBC (PE-IBC) and patient with LABC (PE-LABC).
Discussion
IBC has a propensity to invade into the lymphatic system of the dermis (1) and to form aggregates of cells, defined as tumor emboli, which exhibit homotypic aggregation which is mediated through the aberrant over-expression of E-cadherin (3,5). In addition, IBC tumor cells have been shown to express surface characteristics consistent with the enrichment for cells with a s “tumor initiating” phenotype, i.e. cancer stem cells, that are capable of self-renewal and multipotency (5). In addition to these characteristics. There are no therapeutic agents yet identified that effectively inhibit self-renewal and the tight homotypic aggregation that are characteristics of IBC tumor emboli.
The present studies used established IBC cell lines SUM190, SUM149 as well as used tumor cells isolated from pleural effusion aspirates from a patient with IBC and a patient with LABC, designated as PE-IBC and PE-LABC respectively, to evaluate the effects of the pan-HDAC inhibitor SAHA, on self renewal of IBC tumor spheroids and on their tight homotypic aggregation. SAHA significantly inhibited self-renewal of IBC tumor spheroids formed by established IBC cell lines SUM149 and SUM190 and by PE-IBC and PE-LABC. The inhibitory effects of SAHA on homotypic aggregation were apparent visually at a microscopic level using phase contrast microscopy and appeared as a loss of integrity of the 3D structure of IBC spheroids. Based on the known role of E-cadherin in mediating homotypic aggregation, further studies were performed to determine the effects of SAHA on E-cadherin protein production by IBC tumor spheroids.
The present results confirm previous reports that IBC cells express high levels of E-cadherin (3,5). Previous studies using anti-E-cadherin antibodies and small interfering (si) RNA approaches to knockdown E-cadherin have demonstrated that the result of effectively blocking the homeotypic aggregation of IBC tumor emboli is the inhibition of IBC metastasis (5). Interestingly, while SAHA did not alter the total amount of E-cadherin protein it altered its location, leading to a loss of homotypic aggregation of IBC tumor spheroids. SAHA is among the first therapeutic agent that has been shown to effectively alter E-cadherin function in IBC. While E-cadherin has been reported to commonly be lost during the process of EMT, which is associated with tumor progression and metastasis, IBC is a notable exception in that it is over-expressed during IBC invasion into the dermal lymphatics (3, 5). The specific role of E-cadherin in IBC metastasis and the mechanisms of its regulation are currently unclear and represents an important avenue for further study. Future studies will elucidate the associations between aberrant E-cadherin expression in IBC, and its association between its cytoplasmic binding partner p120 catenin, small GTPases, and beta catenin signaling pathway, which may together be critical to mediating the rapid invasion observed in IBC.
Taken together, the present studies demonstrate that cells derived from established SUM190 and SUM149 IBC cell lines as well as cells isolated from pleural effusion of patients with metastatic IBC and LABC exhibit self-renewal as assessed by clonogenicity, which is consistent with an enriched subpopulation with characteristics of cancer stem cells. IBC tumor spheroids serve as a good in vitro surrogate model with which the peculiar characteristics exhibited by IBC tumors can be studied, including the aberrant over-expression of E-cadherin and the homotypic aggregation of IBC cells which exhibit robust invasive activity. Using approaches such as those taken in the present experiments, it is possible to identify potential therapeutic targets which can be matched by the availability of FDA approved therapeutic agents that can be immediately evaluated for their effects on characteristics that define the aggressive phenotype of IBC. Additionally, the present results are the first to demonstrate the potential utility of agents that are capable of epigenetic modulation of IBC tumors as a strategy to target the molecular signatures to define IBC as the most aggressive form of locally advanced breast cancer.
Condensed Abstract.
The pan-histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA; vorinostat) blocked self-renewal and homotypic aggregation of inflammatory breast cancer (IBC) tumor spheroids, leading to loss of the integrity of these 3 dimensional structures. While SAHA did not alter the total amount of E-cadherin protein, it induced a change in location from the plasma membrane to the cytoplasm. SAHA is the first agent shown to be capable of altering the structure and functions of IBC tumor spheroids, which serve as in vitro surrogates of IBC tumor emboli that are the classic pathological signatures of IBC. These studies suggest that SAHA warrants further evaluation for its therapeutic effects in IBC, the most aggressive form of locally advanced breast cancer.
Acknowledgements
These studies were supported in part by American Airlines-Komen Foundation Promise Grant KGO81287 (FMR, MC), NIH NCI R01 CA-138239-02 (WW, MC), and the State of Texas Grant for The Rare and Aggressive Breast Cancer Research Program (MC). The technical assistance of Kimberly Boley is gratefully acknowledged.
Abbreviations
- CTCL
cutaneous T cell lymphoma
- DMSO
dimethyl sulfoxide
- EGF
epidermal growth factor
- EMT
epithelial-mesenchymal transition
- FBS
fetal calf serum
- HAT
histone acetylases
- HDAC
histone deacetylase enzymes
- IBC
inflammatory breast cancer
- LABC
locally advanced breast cancer
- MTT
thiazolyl blue tetrazolium bromide
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PE
pleural effusion
- SAHA
suberoylanilide hydroxamic acid
- siRNA
small interfering ribonucleic acid
- 2D
2 dimensional
- 3D
three dimensional
References
- 1.Singletary SE, Cristofanilli M. Defining the clinical diagnosis of inflammatory breast cancer. Semin Oncol. 2008 Feb;35(1):7–10. doi: 10.1053/j.seminoncol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Valero V, Buzdar AU, Kau SW, Broglio KR, Gonzalez-Angulo AM, Sneige N, Islam R, Ueno NT, Buchholz TA, Singletary SE, Hortobagyi GN. Inflammatory breast cancer (IBC) and patterns of recurrence: understanding the biology of a unique disease. Cancer. 2007 Oct 1;110(7):1436–44. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 3.Alpaugh ML, Tomlinson JS, Ye Y, Barsky SH. Relationship of sialyl-Lewis(x/a) underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma. Am J Pathol. 2002 Aug;161(2):619–28. doi: 10.1016/S0002-9440(10)64217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, Yang LH, Li QC, Zhao C, Wang EH. Abnormal expression of p120-catenin, E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer. Lung Cancer. 2009 Mar;63(3):375–82. doi: 10.1016/j.lungcan.2008.12.012. Epub 2009 Jan 21. [DOI] [PubMed] [Google Scholar]
- 5.Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol. 2008 Aug;173(2):561–74. doi: 10.2353/ajpath.2008.071214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MJ, Kim YS, Kummar S, Giaccone G, Trepel JB. Histone deacetylase inhibitors in cancer therapy. Curr Opin Oncol. 2008 Nov;20(6):639–49. doi: 10.1097/CCO.0b013e3283127095. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007 Aug 13;26(37):5541–52. doi: 10.1038/sj.onc.1210620. Review. [DOI] [PubMed] [Google Scholar]
- 8.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007 Oct;12(10):1247–52. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 9.Duvic M, Vu J. Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs. 2007 Jul;16(7):1111–20. doi: 10.1517/13543784.16.7.1111. Review. [DOI] [PubMed] [Google Scholar]
- 10.Luu TH, Morgan RJ, Leong L, Lim D, McNamara M, Portnow J, Frankel P, Smith DD, Doroshow JH, Wong C, Aparicio A, Gandara DR, Somlo G. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res. 2008 Nov 1;14(21):7138–42. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ethier SP. Human breast cancer cell lines as models of growth regulation and disease progression. J Mammary Gland Biol Neoplasia. 1996 Jan;1(1):111–21. doi: 10.1007/BF02096306. Review. [DOI] [PubMed] [Google Scholar]
- 12.Forozan F, Mahlamäki EH, Monni O, Chen Y, Veldman R, Jiang Y, Gooden GC, Ethier SP, Kallioniemi A, Kallioniemi OP. Comparative genomic hybridization analysis of 38 breast cancer cell lines: a basis for interpreting complementary DNA microarray data. Cancer Res. 2000 Aug 15;60(16):4519–25. [PubMed] [Google Scholar]
- 13.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003 May 15;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005 Jan;10(1):75–86. doi: 10.1007/s10911-005-2542-5. Review. [DOI] [PubMed] [Google Scholar]