Abstract
Background
Scaling up diagnostic testing and treatment is a key strategy to reduce the burden of malaria. Delays in accessing treatment can have fatal consequences; however, few studies have systematically assessed these delays among children under five years of age in malaria-endemic countries of sub-Saharan Africa. This study identifies predictors of prompt treatment with first-line artemisinin combination therapy (ACT) and describes profiles of children who received this recommended treatment.
Methods
This study uses data from the most recent Demographic and Health Survey, Malaria Indicator Survey, or Anaemia and Parasite Prevalence Survey conducted in 13 countries. A Chi square automatic interaction detector (CHAID) model was used to identify factors associated with prompt and effective treatment among children under five years of age.
Results
The percentage of children with fever who received any anti-malarial treatment varies from 3.6 % (95 % CI 2.8–4.4 %) in Ethiopia to 64.5 % (95 % CI 62.7–66.2 %) in Uganda. Among those who received prompt treatment with any anti-malarial medicine, the percentage who received ACT ranged from 32.2 % (95 % CI 26.1–38.4 %) in Zambia to nearly 100 % in Tanzania mainland and Zanzibar. The CHAID analysis revealed that country of residence is the best predictor of prompt and effective treatment (p < 0.001). Depending on the country, the second best predictor was maternal education (p = 0.004), place of residence (p = 0.008), or household wealth index (p < 0.001).
Conclusions
This study reveals that country of residence, maternal education, place of residence, and socio-economic status are key predictors of prompt access to malaria treatment. Achieving universal coverage and the elimination agenda will require effective monitoring to detect disparities early and sustained investments in routine data collection and policy formulation.
Keywords: Malaria treatment, Prompt treatment, Under-five children, SSA, Malaria-endemic country, Anti-malarial, ACT
Background
Malaria burden remains high in sub-Saharan Africa (SSA) and affects mostly children under five years of age [1]. Several strategies have been proposed to reduce this burden, including improved vector control, intermittent preventive treatment during pregnancy, and standardized case management. For these interventions to be most effective, however, they must reach national and global coverage targets [2].
The World Health Organization (WHO) recommends prompt treatment, usually within 24 h of the onset of fever, with recommended anti-malarial medicines after confirmation of malaria through appropriate diagnostic tests [1]. Timely access to effective treatment with first-line artemisinin combination therapy (ACT) is fundamental to preventing progression of Plasmodium falciparum malaria to severe malaria and death, particularly among children under five years of age. Across SSA, only a small proportion of malaria patients, including children, receive prompt and effective treatment [1]. In Kenya, a systematic review documented a delay in treatment for fever, with only 5 % of fever cases receiving prompt treatment with an anti-malarial drug [3]. Similarly, in Tanzania, Khatib and colleagues found that less than 50 % of fever cases received treatment with ACT within 24 h [4]. Potential reasons for low access to prompt and effective treatment among children revolve around affordability, acceptability, availability, and adequacy to meet expectations in quality of care [5–9].
Several studies have looked into a specific context, dimension, or population related to prompt and effective treatment for malaria; however, few studies systematically look across all children in SSA [10, 11]. This study aims to fill this gap by examining the extent to which children under five years of age in selected countries in SSA received prompt and effective treatment and describing profiles of children who received this recommended treatment. The study also identifies subgroups that, when successfully targeted, may optimize benefits of interventions within the entire population. Although no longer recommended as a key malaria control indicator due to challenges in its interpretation [12], ‘promptness’ was assessed by whether treatment commenced the same day or day after onset of fever. The work involves the following US President’s Malaria Initiative (PMI) focus countries that have relevant national survey data and that were selected for impact evaluations: Angola, Benin, Ethiopia, Ghana, Kenya, Liberia, Madagascar, Malawi, Mali, Mozambique, Rwanda, Senegal, United Republic of Tanzania (mainland and Zanzibar), Uganda, and Zambia.
Methods
Data used for the analysis
This study uses data from the most recent Demographic and Health Survey (DHS), Malaria Indicator Survey (MIS), or Anaemia and Parasite Prevalence Survey (A&PS) conducted in each PMI priority country. Each of these surveys included a malaria module, in addition to the standard household and women’s questionnaires. All surveys were conducted between 2006 (Benin) and 2012 (Malawi), with most carried out in 2010 and 2011. The countries belong to three geographical regions of SSA: (1) west, (2) central, and (3) east (Fig. 1).
Fig. 1.
Countries and household surveys included in the study
Anti-malarial drug policies
All countries had adopted ACT as first-line treatment for malaria (‘effective treatment’) by the time the surveys were conducted; however, the duration between policy adoption and the survey varies from 2 years (Benin) to 7 years (Liberia, Ethiopia, and Mozambique), with an average of 5.2 years (Table 1).
Table 1.
Duration between year of ACT policy adoption and implementation of surveys
| Country | Type of ACT | Year adopted | Year of survey | Time in years between policy adoption and survey |
|---|---|---|---|---|
| Angola | ALu | 2006 | 2011 | 5 |
| Benin | ALu | 2004 | 2006 | 2 |
| Ethiopia | ALu | 2004 | 2011 | 7 |
| Ghana | ASAQ, ALu | 2004 | 2008 | 4 |
| Kenya | ALu | 2004 | 2009 | 5 |
| Liberia | ALu | 2004 | 2011 | 7 |
| Madagascar | ASAQ | 2006 | 2011 | 5 |
| Malawi | ASAQ, ALu | 2007 | 2012 | 5 |
| Mali | ASAQ, ALu | 2007 | 2010 | 3 |
| Mozambique | ALu | 2004 | 2011 | 7 |
| Rwanda | ALu | 2005 | 2010 | 5 |
| Senegal | ASAQ | 2005 | 2011 | 5 |
| Tanzania mainland | ALu, ASAQ | 2004 | 2010 | 6 |
| Uganda | ALu | 2004 | 2011 | 6 |
| Zambia | ALu | 2002 | 2007 | 5 |
| Zanzibar | ASAQ | 2004 | 2010 | 6 |
ALu artemether–lumefantrine, ASAQ arthesunate–amodiaquine
Data quality checks
Data quality checks included assessing response rates, evaluating completeness of data for key variables, and assessing reliability of birth history data. Response rates refer to the percentage of the number of people or households in the sample that completed an interview. In each country, more than 90 % of identified households and women were successfully interviewed, confirming adequate response rates. Misreporting of ages or dates of birth can affect malaria estimates because malaria infection varies significantly by a child’s age [13, 14]. In each country included in the study, at least 93 % of children had complete month and year of birth data, so no country was eliminated due to incomplete information. The reliability of maternal reporting was assessed by analysing the number of live births per year. Overall, the number of births was high for the 4 years preceding the survey but lower in the fifth year preceding the survey. This distribution did not, however, affect the overall reliability of the birth history data.
Of 16 countries, 14 had information on whether anti-malarial treatment was received on the same or next day as the onset of fever (‘prompt treatment’). Two countries, Ethiopia and Mali, were excluded from the bivariate and multivariate analyses because they do not include this information. In addition, the Benin database did not have time-to-treatment for ACT and was excluded from some analyses.
Statistical analysis
The analysis included two steps. The first step involved computing proportions and conducting Chi square tests for each country to identify associations between prompt treatment and selected background characteristics of children, their primary caretakers, and their households. The data was weighted to account for oversampling, undersampling, and varying response in different regions included in national household surveys. The second step consisted of pooling data from all countries and running a Chi square automatic interaction detector (CHAID) model [15, 16]. This method is a sequential fitting algorithm; at each step, the model chooses the predictor variable that has the strongest interaction with an outcome of interest: here, prompt and effective treatment. The variable with the strongest association becomes the first branch of the tree, with a leaf for each category that is significantly different. CHAID then assesses the category groupings to pick the most significant combination of variables. It is particularly useful for identifying sub-groups that are more or less likely to experience the outcome of interest and for determining the relative contributions of these groups to overall coverage in the general population.
The analysis was restricted to children with fever who received any anti-malarial medicine. The covariates were the child’s sex, age group in months (<6, 6–23, 24–59), relationship to the head of household (child or stepchild, grandchild, other), maternal age in years (15–19, 20–29, 30–49), maternal education (none, primary, secondary and above), place of residence (urban, rural), household wealth quintiles, and country of residence.
The output of the CHAID model is presented in a hierarchical tree structure and consists of several levels of branches: root node, parent nodes, child nodes, and terminal nodes. The root node, ‘Node 0’, comprises children who received any anti-malarial medicine. Parent nodes are upper nodes compared to lower-level child nodes. Terminal nodes are any node that does not have child nodes. For each terminal node, the CHAID model provides the following indicators: (1) demographic weight in the sample; (2) gain, the number of children who received prompt and effective treatment in the terminal node, divided by the total number of children who received any anti-malarial treatment; (3) response, the proportion of children who received prompt and effective treatment among all those within the terminal node; and, (4) gain index percentage, which represents the increased probability of prompt and effective treatment in the terminal node compared to the overall study population.
Results
Treatment of fever among children under five
Among children with fever, the proportion that received any anti-malarial treatment varied from 3.6 % (95 % CI 2.8–4.4 %) in Ethiopia to 64.5 % (95 % CI 62.7–66.2 %) in Uganda. Coverage was above 50 % in four countries: Benin, Liberia, Tanzania mainland, and Uganda. The percentage of children with fever who received an ACT was below 10 % in nearly half of the countries included in the analysis. Higher percentages of children received an ACT in Uganda (44.2 %, 95 % CI 42.4–46.1 %), Liberia (39.7 %, 95 % CI 37.3–42.1 %), and Tanzania mainland (37.9 %, 95 % CI 35.1–40.6 %). Coverage was particularly low in Benin (0.9 %, 95 % CI 0.6–1.1 %), where ACT was rolled out in 2004, just 2 years before the survey [17]. The percentage of children who received ACT, among those who received any anti-malarial treatment, varied from 1.6 % (95 % CI 1.1–2.1 %) in Benin to 95.7 % (95 % CI 92.3–99.1 %) in Rwanda (Table 2).
Table 2.
Percentage of children under five with fever in the 2 weeks before the survey who received treatment with anti-malarial drug by country
| Country | Children with fever in the 2 weeks before the survey | Children who received ACT among those who received any anti-malarial treatment | ||||
|---|---|---|---|---|---|---|
| Received any anti-malarial | Received ACT | % (95% CI) | N | Year | ||
| % (95% CI) | % (95% CI) | N | ||||
| Angola | 28.3 (26.6–30.0) | 21.7 (20.1–23.2) | 2645 | 76.6 (73.5–79.6) | 738 | 2011 |
| Benina | 54.0 (52.5–55.5) | 0.9 (0.6–1.1) | 4204 | 1.6 (1.1–2.1) | 2243 | 2006 |
| Ethiopia | 3.6 (2.8–4.4) | 1.3 (0.8–1.8) | 2082 | 36.3 (27.8–44.9) | 124 | 2011 |
| Ghana | 43.0 (38.8–47.1) | 21.5 (18.1–24.9) | 551 | 50.0 (44.5–56.6) | 225 | 2008 |
| Kenya | 23.2 (20.9–25.4) | 7.8 (6.3–9.2) | 1385 | 33.5 (28.2–38.7) | 311 | 2009 |
| Liberia | 57.1 (54.7–59.5) | 39.7 (37.3–42.1) | 1617 | 69.6 (66.5–72.6) | 895 | 2011 |
| Madagascar | 19.8 (17.3–22.4) | 3.8 (2.6–5.0) | 959 | 19.3 (13.4–25.1) | 177 | 2011 |
| Malawi | 32.5 (29.0–36.0) | 29.6 (26.1–33.0) | 676 | 91.0 (87.1–94.8) | 216 | 2012 |
| Mali | 34.7 (31.2–38.2) | 7.8 (5.8–9.7) | 705 | 22.4 (17.1–27.6) | 243 | 2010 |
| Mozambique | 29.9 (27.4–32.4) | 17.9 (15.8–20.0) | 1313 | 59.9 (54.9–64.9) | 366 | 2011 |
| Rwanda | 10.8 (9.2–12.5) | 10.4 (8.7–12.0) | 1332 | 95.7 (92.3–99.1) | 140 | 2010 |
| Senegal | 8.2 (7.1–9.3) | 3.4 (2.6–4.1) | 2314 | 41.0 (33.7–48.3) | 176 | 2010 |
| Tanzania mainland | 60.1 (57.4–62.7) | 37.9 (35.1–40.6) | 1320 | 63.1 (59.7–66.5) | 785 | 2010 |
| Uganda | 64.5 (62.7–66.2) | 44.2 (42.4–46.1) | 2860 | 68.6 (66.5–70.7) | 1849 | 2010 |
| Zambia | 38.4 (35.4–41.3) | 11.1 (9.2–13.0) | 1034 | 29.0 (24.6–33.4) | 417 | 2007 |
| Zanzibar | 16.9 (12.5–21.3) | 5.6 (2.9–8.3) | 282 | 33.1 (18.3–48.0) | 42 | 2010 |
95 % CI 95 % confidence interval, N number of children
aArtemisinin combination therapy [ACT] was rolled out in Benin only 2 years before the survey
Prompt treatment of fever among children under five
The percentage of children who received prompt treatment, among children who received any anti-malarial drug, varied from 42.1 % (95 % CI 34.8–49.4 %) in Madagascar to 87.2 % (95 % CI 76.7–97.8 %) in Zanzibar. Among those who received prompt treatment with any anti-malarial medicine, the percentage who received ACT ranged from 32.2 % (95 % CI 26.1–38.4 %) in Zambia to nearly 100 % in Tanzania mainland and Zanzibar (Table 3).
Table 3.
Percentage of children under five with fever in the 2 weeks before the survey who received prompt treatment with anti-malarial drug by country
| Country | Prompt treatment with any anti-malarial drug among those who received any anti-malarial drug | Prompt treatment with ACT among those who received prompt treatment with any anti-malarial drug | ||
|---|---|---|---|---|
| % (95% CI) | Na | % (95% CI) | Na | |
| Angola | 57.8 (51.2–61.4) | 738 | 72.4 (68.0–76.8) | 398 |
| Beninb | 77.8 (76.0–79.5) | 2243 | n/a | n/a |
| Ethiopiac | n/a | n/a | n/a | n/a |
| Ghana | 55.2 (48.6–61.7) | 225 | 51.0 (42.0–59.9) | 124 |
| Kenya | 50.6 (50.0–56.1) | 311 | 36.1 (28.1–44.0) | 145 |
| Liberia | 61.3 (58.1–64.5) | 895 | 69.9 (66.0–73.8) | 539 |
| Madagascar | 42.1 (34.8–49.4) | 177 | 36.6 (25.9–4.3) | 81 |
| Malawi | 73.3 (67.4–79.3) | 216 | 88.6 (83.6–93.7) | 156 |
| Malic | n/a | n/a | n/a | n/a |
| Mozambique | 74.4 (69.9–78.9) | 366 | 69.2 (63.9–74.5) | 292 |
| Rwanda | 70.8 (63.2–78.4) | 140 | 98.0 (95.1–100.0) | 101 |
| Senegal | 73.0 (67.0–80.3) | 176 | 47.5 (38.8–56.2) | 131 |
| Tanzania mainland | 68.9 (66.7–72.1) | 785 | 99.6 (99.0–100.0) | 548 |
| Uganda | 65.4 (63.2–67.5) | 1849 | 71.8 (69.3–74.4) | 1240 |
| Zambia | 53.4 (48.6–58.2) | 417 | 32.2 (26.1–38.4) | 225 |
| Zanzibar | 87.2 (76.7–97.8) | 42 | 100.0 | 36 |
95 % CI 95 % confidence interval, N number of children
aAlthough weighted proportions are presented, the N values are unweighted and therefore, may not match exactly
bChildren who received only ACT are excluded because the database does not contain information on when they started the treatment
cNo information on time to treatment
Region of residence or type of place of residence were significantly associated with access to prompt treatment with any anti-malarial medicine in ten countries, making geography the most frequent association. In Angola, Benin, and Uganda, urban residence was significantly associated with prompt treatment (p value = 0.042, p value <0.0001, and p value = 0.039, respectively), whereas in Liberia, more rural children received prompt treatment (p value = 0.025).
Wealth quintile was significantly associated with prompt treatment in six countries. In most of these countries, children in the two highest wealth quintiles were most likely to receive prompt treatment. In contrast, children in the lowest wealth quintile in Angola and Liberia were most likely to have received prompt treatment (p value = 0.030 and p value <0.0001, respectively).
Maternal characteristics were associated with prompt treatment with any anti-malarial medicine in some countries. In general, the percentage of children who received prompt treatment was high among children whose mothers had at least a secondary education. In Kenya and Mozambique, prompt access to treatment with any anti-malarial medicine was significantly lower among children with mothers aged 15–19 years and higher among those with mothers aged 20–29 years (p value = 0.043 and p value < 0.0001, respectively).
Children’s demographic characteristics were less likely to be significantly associated with prompt access to anti-malarial treatment. In Ghana, more male children received prompt treatment than female children (p value = 0.049). In contrast, more female children in Senegal received prompt treatment compared to males (p value = 0.046). In Tanzania mainland, the percentage of children who received prompt treatment was significantly higher among children ages 24–59 months compared to younger children (p value = 0.056) (Table 4).
Table 4.
Socio-economic and demographic characteristics significantly associated with access to prompt treatment with any anti-malarial drug among children under five
| Country | Variable significantly associated with prompt treatment with any anti-malarial drug | Category with highest percentage of children who received prompt treatment with any anti-malarial drug | ||
|---|---|---|---|---|
| Variable | p value | Category | % (95% CI) | |
| A | B | C | D | E |
| Angola | Relationship to the head of household | 0.032 | Grandchild | 64.2 (55.3–73.2) |
| Education level of the mother | 0.029 | Secondary and above | 68.1 (60.7–75.6) | |
| Region of residence (endemicity) | 0.032 | Mesoendemica estavel | 67.4 (61.0–75.6) | |
| Place of residence | 0.042 | Urban | 62.6 (57.9–67.3) | |
| Wealth quintiles | 0.030 | Lowest | 67.5 (54.3–80.6) | |
| Benin | Education level of the mother | <0.0001 | Secondary and above | 85.2 (79.4–91.0) |
| Region of residence | <0.0001 | Littoral | 91.0 (85.6–96.1) | |
| Place of residence | <0.0001 | Urban | 84.5 (81.9–87.1) | |
| Wealth quintiles | <0.0001 | Highest | 84.1 (80.0–88.1) | |
| Ethiopiaa | n/a | n/a | n/a | n/a |
| Ghana | Child’s sex | 0.049 | Male | 59.4 (50.5–68.3) |
| Region of residence | <0.0001 | Upper east | 87.0 (72.9–100.0) | |
| Kenya | Age of the mother | 0.043 | 20–29 years | 53.0 (45.3–60.6) |
| Education level of the mother | 0.008 | Secondary and above | 66.5 (54.3–78.7) | |
| Region of residence | <0.0001 | Central province | 86.0 (63.0–100.0) | |
| Wealth quintiles | 0.023 | Fourth | 65.5 (51.4–79.6) | |
| Liberia | Place of residence | 0.025 | Rural | 65.6 (61.0–70.0) |
| Wealth quintiles | <0.0001 | Lowest | 69.8 (63.6–75.9) | |
| Madagascar | Region of residence | <0.0001 | n/ab | n/a |
| Malawi | Relationship to the head of household | 0.033 | Child | 75.5 (69.4–81.6) |
| Malia | n/a | n/a | n/a | n/a |
| Mozambique | Age of the mother | <0.0001 | 20–29 years | 79.1 (73.1–85.1) |
| Region of residence | <0.0001 | Tete | 99.7 (74.3–100.0) | |
| Rwanda | Wealth quintiles | 0.051c | Highest | 81.1 (64.6–97.6) |
| Senegal | Child’s sex | 0.046 | Female | 84.0 (75.1–92.9) |
| Tanzania mainland | Child’s age | 0.056c | 24–59 months | 73.0 (68.0–77.0) |
| Region of residence | <0.0001 | Mtwara | 96.4 (89.6–100.0) | |
| Uganda | Region of residence | <0.0001 | Karamoja | 81.1 (75.0–87.1) |
| Place of residence | 0.039 | Urban | 68.6 (63.6–73.6) | |
| Zambia | Education level of the mother | 0.007 | Secondary and above | 65.7 (56.2–75.2) |
| Region of residence | <0.0001 | Southern | 78.9 (66.5–91.3) | |
| Wealth quintiles | <0.0001 | Fourth | 66.8 (56.9–76.8) | |
| Zanzibara | n/a | n/a | n/a | n/a |
p values (column C) indicate that the corresponding variable (column B) is significantly associated with prompt treatment with any anti-malarial drug; categories in column D are those that have the highest proportion of children who received prompt treatment with any anti-malarial drug for each corresponding variable in column B
95 % CI 95 % confidence interval, N number of children
aData on time to treatment was not available
bRegion of residence is significantly associated with prompt treatment with any anti-malarial drug; however, the number of children for each of the 20 regions is fewer than 30, so percentages were not computed
cAlthough these values are p > 0.05, they were included because they show borderline significance
Profiling children under five who received prompt treatment with ACT
To identify the socio-economic profile of children who received prompt and effective treatment, data from all children who received any anti-malarial drug across the 13 countries were pooled. Eight independent variables were examined, but only five were included in the final model. The model comprised 20 nodes, of which 13 were terminal nodes. Parent nodes included at least 100 children, whereas child nodes included at least 50 children.
Country was the best predictor of access to prompt treatment with ACT (p value <0.0001) and therefore makes up the first level of the CHAID tree diagram. The analysis classified countries into six parent nodes that minimize variance of prompt and effective treatment within each node and maximize variance across nodes: (1) Angola, Ghana, and Zambia; (2) Kenya and Madagascar; (3) Liberia; (4) Malawi, Rwanda, Senegal, and Tanzania mainland; (5) Mozambique and Zanzibar; and, (6) Uganda. At the next levels of the CHAID tree diagram, these parent nodes were further split into child nodes by secondary and tertiary predictor variables, which were most commonly education of the mother or wealth quintile (Figs. 2, 3, 4, 5, 6, 7).
Fig. 2.
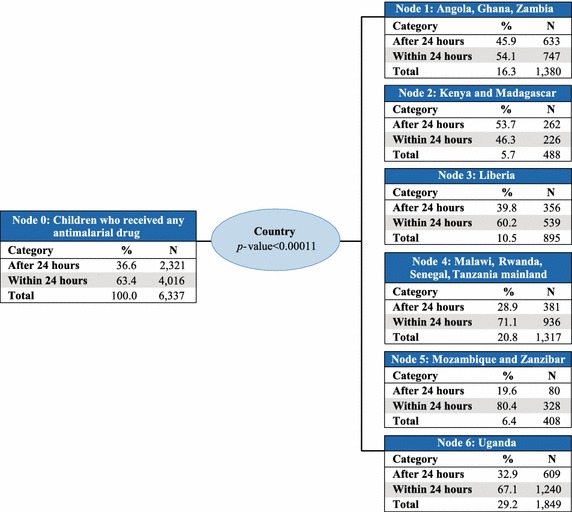
Prompt treatment with ACT among children under five treated with any anti-malarial medicine—Chi square automatic interaction detector tree diagram, level 1. N number of children. The total line of each box represents the share of the total number of children who received any anti-malarial treatment
Fig. 3.
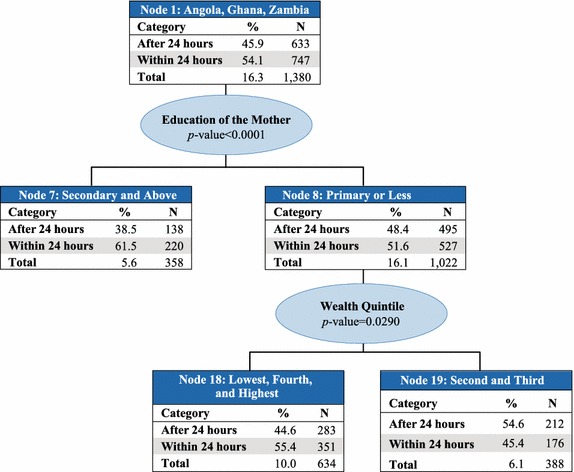
Node 1—Angola, Ghana, and Zambia. N number of children. The total line of each box represents the share of the total number of children who received any anti-malarial treatment
Fig. 4.

Node 2—Kenya and Madagascar. N number of children. The total line of each box represents the share of the total number of children who received any anti-malarial treatment
Fig. 5.
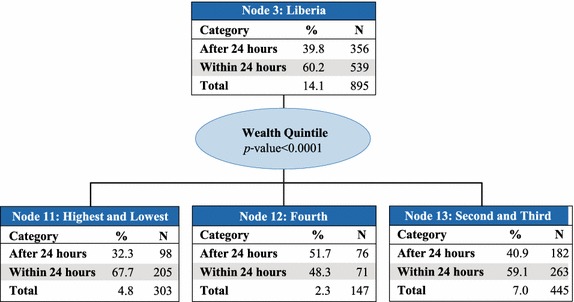
Node 3—Liberia. N number of children. The total line of each box represents the share of the total number of children who received any anti-malarial treatment
Fig. 6.
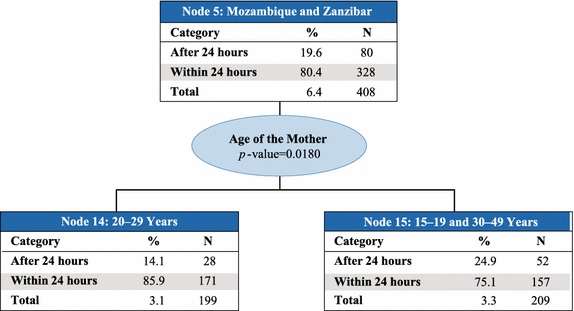
Node 5—Mozambique and Zanzibar. N number of children. The total line of each box represents the share of the total number of children who received any anti-malarial treatment
Fig. 7.
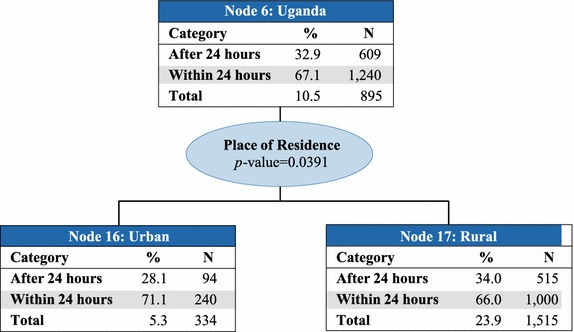
Node 6—Uganda. N number of children. The total line of each box represents the share of the total number of children who received any anti-malarial treatment
In Node 1–Angola, Ghana, and Zambia (Fig. 3), education level of the mother was the best predictor of prompt treatment with ACT (p value = 0.009). Among children whose mothers had secondary education or higher (Node 7), 61.5 % received prompt treatment with ACT. Among children whose mothers had primary education or less (Node 8), 51.6 % received prompt treatment with ACT. Household wealth quintile was also a predictor variable in Node 8 (p value = 0.029), with 55.4 % of children in the lowest, fourth, and highest wealth quintiles (Node 18) receiving prompt treatment with ACT, compared to only 45.4 % of their counterparts in the second and third quintiles (Node 19).
Education level of the mother was also the best predictor in Node 2–Kenya and Madagascar (Fig. 4) (p value = 0.036), with 56.8 % of children whose mothers had at least a secondary education (Node 9) receiving prompt treatment with ACT, compared to just 43.2 % of children whose mothers had primary education or less (Node 10). About 7.7 % of children who received any anti-malarial treatment live in these two countries, 46.3 % of whom received prompt treatment with ACT.
For children in Node 3–Liberia (Fig. 5), household wealth quintile was the best predictor of prompt treatment with ACT (p value <0.0001). About 67.7 % of children from the highest and lowest quintiles (Node 11), 48.3 % of children from the fourth quintile (Node 12), and 59.1 % of children from the second and third quintiles (Node 13) received prompt treatment with ACT. Children in Liberia represent 14.1 % of those who received any anti-malarial treatment.
Country was the only predictor for children in Node 4–Malawi, Rwanda, Senegal, and Tanzania mainland (Fig. 2); therefore, Node 4 is terminal. This group represents 20.8 % of children who received treatment with any anti-malarial medicine. Among these children, 71.1 % received prompt treatment with ACT.
In Node 5–Mozambique and Zanzibar (Fig. 6), age of the mother was the best predictor of prompt treatment with ACT (p value <0.0001). The proportion of children who received prompt treatment with ACT was significantly higher (85.9 %) among those with mothers ages 20–29 years (Node 14) compared to their younger and older counterparts in Node 15 (75.1 %, p value = 0.018). Children living in Mozambique and Zanzibar comprised just 6.4 % of children who received any anti-malarial treatment.
Place of residence was the best predictor in Node 6–Uganda (Fig. 7), with 71.1 % of children receiving prompt treatment with ACT in urban areas (Node 16) compared to 66.0 % in rural areas (Node 17) (p value = 0.039). Ugandans made up 10.5 % of all children who were treated with any anti-malarial medicine; 67.1 % of these children received prompt treatment with ACT.
Chi square automatic interaction detector gain index
The gain index from the CHAID analysis can help highlight whether it is more efficient to target specific groups or to invest in the overall population, with potential trickle-down effects to those groups. The 13 terminal nodes of the CHAID tree diagram represent 13 homogenous sub-groups of children who received any anti-malarial treatment, grouped into three major clusters (Table 5).
Table 5.
Chi square automatic interaction detector gain index
| Cluster node | Node description | Node | Gain | % prompte | Indexf | ||
|---|---|---|---|---|---|---|---|
| Na | %b | Nc | %d | ||||
| A | B | C | D | E | F | G | H |
| Cluster 1 | 2059 | 32.5 | 1504 | 37.5 | 73.0 | 115.3 | |
| 14 | Mozambique and Zanzibar: children from 20 to 29 years old mothers | 199 | 3.1 | 171 | 4.3 | 85.9 | 135.6 |
| 15 | Mozambique and Zanzibar: children from 15 to 19 years old mothers and 30–49 years old mothers | 209 | 3.3 | 157 | 3.9 | 75.1 | 118.5 |
| 16 | Uganda: children from urban areas | 334 | 5.3 | 240 | 6.0 | 71.9 | 113.4 |
| 4 | Malawi, Rwanda, Senegal, and Tanzania: all children | 1317 | 20.8 | 936 | 23.3 | 71.1 | 112.1 |
| Cluster 2 | 3366 | 53.1 | 2102 | 52.3 | 62.4 | 98.5 | |
| 11 | Liberia: children from lowest and highest wealth quintiles | 303 | 4.8 | 205 | 5.1 | 67.7 | 106.8 |
| 17 | Uganda: children from rural areas | 1515 | 23.9 | 1000 | 24.9 | 66.0 | 104.2 |
| 7 | Angola, Ghana, and Zambia: children from mothers with secondary education and above | 358 | 5.6 | 220 | 5.5 | 61.5 | 97.0 |
| 13 | Liberia: children from second and third quintiles | 445 | 7.0 | 263 | 6.5 | 59.1 | 93.3 |
| 9 | Kenya and Madagascar: children from mothers with secondary education and above | 111 | 1.8 | 63 | 1.6 | 56.8 | 89.6 |
| 18 | Angola, Ghana, and Zambia: children from mothers with primary or no education; children from lowest, fourth, and highest wealth quintiles | 634 | 10.0 | 351 | 8.7 | 55.4 | 87.4 |
| Cluster 3 | 912 | 14.4 | 410 | 10.2 | 45.0 | 70.9 | |
| 12 | Liberia: children from the fourth wealth quintile | 147 | 2.3 | 71 | 1.8 | 48.3 | 76.2 |
| 19 | Angola, Ghana, and Zambia: children from mothers with primary or no education; children from second and third wealth quintiles | 388 | 6.1 | 176 | 4.4 | 45.4 | 71.6 |
| 10 | Kenya and Madagascar: children from mothers with primary or no education | 377 | 5.9 | 163 | 4.1 | 43.2 | 68.2 |
| Total | 6337 | 100 | 4016 | 100 | 63.4 | n/a | |
N number of children
aNumber of children who received any anti-malarial treatment per node (demographic size in the sample)
bDemographic size in percentage = (0.1/Σ0.1) × 100
cNumber of children who received prompt treatment with ACT
dDemographic size among children who received prompt treatment with ACT in percentage = (0.3/Σ0.3) × 100
eProportion of children who received prompt treatment with ACT out of those who received any anti-malarial medicine = (0.3/Σ0.1) × 100
fNode index = [(0.3/Σ3)/(0.1/Σ0.1)] × 100
Cluster 1 comprises children from urban areas in Uganda plus all children in Mozambique, Zanzibar, Malawi, Rwanda, Senegal, and Tanzania. This cluster represents 32.5 % of children who received treatment with any anti-malarial medicine, among which 73.0 % of children received prompt treatment with ACT. This group accounts for 37.5 % of all children who received prompt treatment with ACT.
Cluster 2 includes children in all but the fourth wealth quintile in Liberia; children from rural areas in Uganda; children from mothers with a secondary education and above in Angola, Ghana, Zambia, Kenya, and Madagascar; and children from the lowest, fourth, and highest wealth quintiles in Angola, Ghana, and Zambia. This cluster accounts for 53.1 % of children who received treatment with any anti-malarial medicine, among which 62.4 % of children received prompt treatment with ACT. This group accounts for 52.3 % of all children who received prompt treatment with ACT.
Cluster 3 includes children from the fourth wealth quintile in Liberia; children from mothers with primary or no education in Angola, Ghana, Zambia, Kenya, and Madagascar; and children from the second and third wealth quintiles in Angola, Ghana, and Zambia. This cluster represents 14.4 % of children who received treatment with any anti-malarial medicine. In this category, 45.0 % of children received prompt treatment with ACT, representing 10.2 % of all children who received prompt treatment with ACT (Table 5).
Discussion
This study presented coverage of prompt treatment with ACT among febrile children under five years of age and also profiled those children who received this recommended treatment in selected countries in SSA. Most countries with high coverage of treatment with any anti-malarial medicine also had high coverage of treatment with ACT, perhaps since one is dependent on the other. Mali and Benin both depart from this trend; treatment with ACT was much less likely than treatment with other anti-malarial drugs. This may be due to ACT holding a particularly small share of the market compared to other anti-malarial drugs [18] or the fact that ACT policy was implemented only shortly before the surveys were conducted [1]. These countries show how important policy and markets are to ensuring access to prompt and effective treatment for malaria.
The cross-country CHAID analysis revealed that the country of residence is the best predictor of prompt and effective treatment, followed by maternal education, household wealth quintile, maternal age, and place of residence. It is perhaps unsurprising that country is the best predictor of prompt and effective treatment since malaria treatment policies differ among countries and are in various stages of implementation (Table 1). National drug regulations also affect the availability of anti-malarial drugs, including ACT, and may therefore also affect the uptake of prompt and effective treatment. This analysis highlights the importance of ensuring that first-line drug policies are synchronized with latest epidemiological information and are accompanied by efficient rollout and awareness raising campaigns. Results related to country of residence may also be a reflection of malaria transmission and epidemiology. For example, Node 2 comprises Kenya and Madagascar, which are the only two countries in the study with a majority of the population living outside of high-transmission areas [1]. Uganda and Liberia, which have the highest parasite prevalence of the countries in the study, each made up their own node.
Children were more likely to have received prompt and effective treatment if their mothers had at least a secondary education (Angola, Ghana, Zambia, Kenya, and Madagascar). With the exception of Angola, these countries also have higher than average proportions of women with at least a secondary education. Similar findings were reported by a study in which febrile children of educated women were more likely to receive a malaria test than children of non-educated women [19]. Furthermore, there is substantial literature showing positive correlations between children’s uptake of health services and mother’s level of education, independent of other social and economic factors [20, 21]. Educated mothers may be more open to advances in public health and medicine, more aware of malaria symptoms and related health risks, more trusting of providers, or more aware and better able to negotiate first-line services and treatment [22]. These characteristics may facilitate quick decision-making and therefore increase likelihood of prompt treatment. In SSA, women’s educational attainment is ever increasing, which suggests that the trend of accelerating gains in children’s health is also likely to continue [23].
In Mozambique and Zanzibar, children whose mothers were in their twenties were more likely to receive prompt and effective treatment than children whose mothers were older or younger. This is consistent with literature that suggests that adolescents are less likely to access maternal and child health services [24, 25]. Older mothers may be less likely to seek prompt and effective treatment if they are caring for multiple children. If they draw upon prior experience with febrile children, they also may be less inclined to follow new health messaging, thereby inhibiting prompt treatment. Further research on the relationships among mother’s age and education and prompt and effective treatment may help ensure that the mothers least likely to seek health services can and will access timely malaria treatment for their children.
In Angola, Ghana, Zambia, and Liberia, children from both the lowest and highest wealth quintiles were among those most likely to receive prompt and effective treatment. Children from the lowest wealth quintile may be most likely to take advantage of free ACT offered in the public sector in Angola, Zambia, and Liberia [1]. On the other hand, children from the highest wealth quintile may be more likely to seek care outside the public sector or may be able to afford first-line ACT from private retailers. Having access to these additional options for malaria care may facilitate prompt treatment, particularly if the locations are closer to home. This may change as community case management interventions improve access to prompt treatment by reducing the distance between febrile children and trained prescribers of ACT. Increasing coverage of malaria interventions has improved equity [26–28], so it is possible that further gains in coverage may cancel inequities both within and across countries. Targeted research on equity will help ensure universal access to prompt and effective treatment.
Availability of prompt and effective treatment or quality of care issues may also explain why place of residence was a secondary predictor of prompt and effective treatment in Uganda. Urban areas tend to have more options for health care (i.e., both public and private providers) and better access to preferred treatment compared to rural areas, which are mostly considered remote areas [29]. Affordable Medicines Facility-malaria evaluators reported higher availability of ACT in urban outlets in Uganda than rural areas, and even with an increase in availability at endline, rural areas remained behind [30]. This evidence supports the findings of the CHAID analysis, which showed that urban children in Uganda were more likely to receive prompt and effective treatment than rural children.
These are the predictors of which children are most likely to receive prompt and effective treatment, but a next step is strategizing for future interventions. The gain index from the CHAID analysis can help highlight whether it is more efficient to target specific groups or to invest in the overall population, with potential trickle-down effects to those groups. Increasing coverage in a particular node or cluster will yield corresponding returns at the population level, quantified by the gain index. For example, the gain index of Cluster 3 is just 70.9, showing that these children are much less likely to receive prompt and effective treatment than the overall population. Since Cluster 3 comprises only 14.4 % of children who received treatment with any anti-malarial medicine (i.e., this study’s target population), it is a relatively small population that may be easier to target with an intervention to increase access to prompt treatment. However, it may not be cost effective to invest significant resources into a small group like this because the benefits to national coverage goals may be limited. On the other hand, efforts to increase the proportion of children in Cluster 2 who receive prompt and effective treatment from 62.4 to 100 % may be more feasible since baseline coverage is higher. Investing in these children may also yield more visible public health impact since this cluster represents a majority (53.1 %) of the target population. These results may be useful in strategic planning for malaria control interventions at the national and subnational levels. Close monitoring can help malaria control programs determine if these children are being reached by future interventions in the quest for universal coverage.
Although this study presents informative findings, it does have limitations. It is difficult to track the extent to which patients with diagnosed malaria receive prompt and effective treatment using self-reported survey data because testing and treatment information are traditionally not linked in survey data. Self-reported survey data also require caretakers to identify specific drugs, which may be especially difficult in countries where many malaria drugs are on the market, compared to countries where only ACT is available [31]. Finally, timeliness data rely on the ability of caretakers to determine whether treatment commenced the same day or next day as the onset of fever, which can be difficult to ascertain.
In addition, data were collected from surveys during a 6-year span from 2006 to 2012. This time difference in survey implementation could affect comparability in pooled results, as could the type of survey conducted (DHS, MIS, or A&PS). Furthermore, varying amounts of time between malaria policy implementation and ACT rollout could differently affect coverage and access to prompt and effective treatment within countries and across countries. There is also considerable variation in the distribution of wealth quintiles and in equity of health interventions over time and among countries [32]. There is substantial subnational variation in malaria prevalence in several countries in SSA, which may affect expectations regarding ACT use and access to prompt treatment.
Finally, the data collected in these surveys do not examine additional factors related to access and social acceptability of malaria treatment and services. This information could help refine the profiles of children who received prompt and effective treatment. Future CHAID analyses could also include more complex covariates, such as source of treatment.
Conclusion
This study reveals that country of residence, maternal education, place of residence, and socio-economic status are key predictors of prompt access to malaria treatment. The prominence of country as a predictor variable highlights the roles ACT policy implementation and rollout may play in access to prompt and effective treatment. The other key variables were of differing importance across countries, reminding implementers that understanding the context of their programmes is critical to success. Achieving universal coverage and the elimination agenda will require effective monitoring and early detection of coverage disparities within populations. Although national surveys provide a sporadic opportunity for this work, investing in routine surveillance will improve real-time tracking of ACT coverage and corresponding action, in line with recent recommendations from WHO’s Global Technical Strategy for Malaria.
Authors’ contributions
JAS, JBOE, and YY were involved in the design, data interpretation, and writing of the manuscript. YY conceptualized the study. JBOE collated the data and undertook the data analysis. EE provided extensive input and review. All authors read and approved the final manuscript.
Acknowledgements
This research has been supported by the US President’s Malaria Initiative (PMI) through the US Agency for International Development (USAID) under the terms of MEASURE Evaluation cooperative agreement AIDOAA-L-14-00004. Views expressed are not necessarily those of PMI, USAID, or the US Government.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Contributor Information
Jui A. Shah, Email: jui.shah@icfi.com
Jacques B. O. Emina, Email: jacques.emina@gmail.com
Erin Eckert, Email: eeckert@usaid.gov.
Yazoume Ye, Email: yazoume.ye@icfi.com.
References
- 1.WHO . World malaria report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.UNICEF and Roll Back Malaria Partnership . Malaria and children: progress in intervention coverage. New York: UNICEF; 2007. [Google Scholar]
- 3.Chuma J, Abuya T, Memusi D, Juma E, Akhwale W, Ntwiga J, et al. Reviewing the literature on access to prompt and effective malaria treatment in Kenya: implications for meeting the Abuja targets. Malar J. 2009;8:243. doi: 10.1186/1475-2875-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatib R, Selemani M, Mrisho G, Masanja I, Amuri M, Njozi M, et al. Access to artemisinin-based anti-malarial treatment and its related factors in rural Tanzania. Malar J. 2013;12:155. doi: 10.1186/1475-2875-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuma J, Okungu V, Molyneux C. Barriers to prompt and effective malaria treatment among the poorest population in Kenya. Malar J. 2010;9:144. doi: 10.1186/1475-2875-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwelunmor J, Idris O, Adelakun A, Airhihenbuwa CO. Child malaria treatment decisions by mothers of children less than five years of age attending an outpatient clinic in south-west Nigeria: an application of the PEN-3 cultural model. Malar J. 2010;9:354. doi: 10.1186/1475-2875-9-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Getahun A, Deribe K, Deribew A. Determinants of delay in malaria treatment-seeking behaviour for under-five children in south-west Ethiopia: a case control study. Malar J. 2010;9:320. doi: 10.1186/1475-2875-9-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kizito J, Kayendeke M, Nabirye C, Staedke SG, Chandler CI. Improving access to health care for malaria in Africa: a review of literature on what attracts patients. Malar J. 2012;11:55. doi: 10.1186/PREACCEPT-2317562776368437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik EM, Hanafi K, Ali SH, Ahmed ES, Mohamed KA. Treatment-seeking behaviour for malaria in children under five years of age: implication for home management in rural areas with high seasonal transmission in Sudan. Malar J. 2006;5:60. doi: 10.1186/1475-2875-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamel MJ, Odhacha A, Roberts JM, Deming MS. Malaria control in Bungoma District, Kenya: a survey of home treatment of children with fever, bednet use and attendance at antenatal clinics. Bull World Health Organ. 2001;79:1014–1023. [PMC free article] [PubMed] [Google Scholar]
- 11.Kwabe TS, Abdulfatai O, Agbam EF, Usman A, Bashiru AM. Mothers management of malaria fever among under-five nomadic Fulani children of northeastern Nigeria. Am J Infect Dis Microbiol. 2013;1:26–33. [Google Scholar]
- 12.MEASURE Evaluation, MEASURE DHS, President’s Malaria Initiative, Roll Back Malaria Partnership, UNICEF, World Health Organization. Household survey indicators for malaria control. Roll Back Malaria Monitoring and Evaluation Reference Group Survey and Indicator Task Force. 2013.
- 13.Gahutu J-B, Steininger C, Shyirambere C, Zeile I, Cwinya-Ay N, Danquah I, et al. Prevalence and risk factors of malaria among children in southern highland Rwanda. Malar J. 2011;10:134. doi: 10.1186/1475-2875-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nwaorgu OC, Orajaka BN. Prevalence of malaria among children 1–10 years old in communities in Awka north local government area, Anambra state, south east Nigeria. Afr Res Rev. 2011;5:264–281. doi: 10.4314/afrrev.v5i5.21. [DOI] [Google Scholar]
- 15.Kass GV. An exploratory technique for investigating large quantities of categorical data. Appl Stat. 1980;29:119–127. doi: 10.2307/2986296. [DOI] [Google Scholar]
- 16.Kitsantas P, Hollander M, Li L. Using classification trees to assess low birth weight outcomes. Artif Intell Med. 2006;38:275–289. doi: 10.1016/j.artmed.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Ogouyèmi-Hounto A, Ndam NT, Kinde Gazard D, d’Almeida S, Koussihoude L, Ollo E. Prevalence of the molecular marker of Plasmodium falciparum resistance to chloroquine and sulphadoxine/pyrimethamine in Benin seven years after the change of malaria treatment policy. Malar J. 2013;12:147. doi: 10.1186/1475-2875-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palafox B, Tougher S, Patouillard E, Goodman C, Hanson K, Tassiba ME, et al. ACTwatch 2009 supply chain survey results, Benin. Nairobi: ACTwatch project, Population Services International; 2012. [Google Scholar]
- 19.Johansson EW, Gething PW, Hildenwall H, Mappin B, Petzold M, Peterson SS, et al. Diagnostic testing of pediatric fevers: meta-analysis of 13 national surveys assessing influences of malaria endemicity and source of care on test uptake for febrile children under five years. PLoS One. 2014;9:e95483. doi: 10.1371/journal.pone.0095483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai S, Alva S. Maternal education and child health: is there a strong causal relationship? Demography. 1998;35:1. doi: 10.2307/3004028. [DOI] [PubMed] [Google Scholar]
- 21.Sandiford P, Cassel J, Montenegro M, Sanchez G. The impact of women’s literacy on child health and its interaction with access to health services. Popul Stud. 1995;49:1. doi: 10.1080/0032472031000148216. [DOI] [Google Scholar]
- 22.Cleland JG, Van Ginneken JK. Maternal education and child survival in developing countries: the search for pathways of influence. Soc Sci Med. 1988;27:1357–1368. doi: 10.1016/0277-9536(88)90201-8. [DOI] [PubMed] [Google Scholar]
- 23.Gakidou E, Cowling K, Lozano R, Murray CJ. Increased educational attainment and its effect on child mortality in 175 countries between 1970 and 2009: a systematic analysis. Lancet. 2010;376:959–974. doi: 10.1016/S0140-6736(10)61257-3. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds HW, Wong EL, Tucker H. Adolescents’ use of maternal and child health services in developing countries. Int Fam Plan Perspect. 2006;32:6–16. doi: 10.1363/3200606. [DOI] [PubMed] [Google Scholar]
- 25.Magadi MA, Agwanda AO, Obare FO. A comparative analysis of the use of maternal health services between teenagers and older mothers in sub-Saharan Africa: evidence from Demographic and Health Surveys (DHS) Soc Sci Med. 2007;64:1311–1325. doi: 10.1016/j.socscimed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Shah JA, Franca-Koh AC, Ye Y, Herrera S. Increased equity in malaria control interventions in Malawi from 2000 to 2012. In: 63rd Annual Meeting of the American Society for Tropical Medicine and Hygiene, New Orleans, 2014.
- 27.Florey L, Taylor C, Winter R, Franca-Koh AC, Herrera S, Shah JA, et al. Improving socioeconomic equity in ITN access, ownership and use in Rwanda from 2000–2010. In: 63rd Annual Meeting of the American Society for Tropical Medicine and Hygiene, New Orleans, 2014.
- 28.Taylor C, Franca-Koh AC, Herrera S, Shah JA, Florey L, Ye Y. Reduction in disparity of insecticide-treated nets ownership and use among socioeconomic groups after scale up in Uganda. In: 63rd Annual Meeting of the American Society for Tropical Medicine and Hygiene, New Orleans, 2014.
- 29.Yadav P, Cohen JL, Alphs S, Arkedis J, Larson PS, Massaga J, et al. Trends in availability and prices of subsidized ACT over the first year of the AMFm: evidence from remote regions of Tanzania. Malar J. 2012;11:299. doi: 10.1186/1475-2875-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tougher S, Ye Y, Amuasi J, Kourgueni I, Thomson R, Goodman C. Effect of the Affordable Medicines Facility—malaria (AMFm) on the availability, price, and market share of quality-assured artemisinin-based combination therapies in seven countries: a before-and-after analysis of outlet survey data. Lancet. 2012;380:1916–1926. doi: 10.1016/S0140-6736(12)61732-2. [DOI] [PubMed] [Google Scholar]
- 31.Eisele TP, Silumbe K, Yukich J, Hamainza B, Keating J, Bennett A, et al. Measuring coverage in MNCH: accuracy of measuring diagnosis and treatment of childhood malaria from household surveys in Zambia. PLoS Med. 2013;10:e1001417. doi: 10.1371/journal.pmed.1001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barros AJD, Ronsmans C, Axelson H, Loaiza E, Bertoldi AD, França GVA, et al. Equity in maternal, newborn, and child health interventions in countdown to 2015: a retrospective review of survey data from 54 countries. Lancet. 2012;379:1225–1233. doi: 10.1016/S0140-6736(12)60113-5. [DOI] [PubMed] [Google Scholar]


