Formation of inhibitory antibodies is a major complication in the clinical treatment of hemophilia A using factor-replacement therapy. Approximately 25% of hemophilia A patients develops antibodies after repeated infusion of factor VIII (FVIII) protein. Development of inhibitory antibodies significantly increases morbidity and lowers the quality of life of hemophilia A patients. The study of patient inhibitors revealed that anti-FVIII antibodies comprise mostly IgG isotypes and its subclasses IgG1 and IgG4 [1], with IgG4 predominantly present in patients with high titer inhibitors. The factors influencing antibody formation are complex. Recent evidence [2] has identified several of these factors, including (i) amounts of circulating FVIII determined by the type of mutation within the FVIII gene, (ii) polymorphic sites within the promoters of IL-10 (positive factor), TNFα (positive factor) and CTLA4 (negative factor), (iii) the formulation and intensity of FVIII infusions, and (iv) ‘danger’ signals such as inflammation associated with major bleeds and/or surgery. Interestingly, no strong correlation has been identified between MHC class II profiles and inhibitor formation.
Immune tolerance induction (ITI) protocols have been utilized since the 1970s in efforts to tolerize hemophilia patients to infused FVIII. The strategy can not only eliminate anti-FVIII antibodies, but also induce FVIII-specific tolerance in patients. However, the protocols require long-term and repetitive infusions of FVIII, which are costly and practically challenging [3]. Furthermore, one-third of the patients who underwent ITI failed to generate tolerance to FVIII. The success rate depends on the pretreatment and peak inhibitor titers of the patient and possibly other factors such as the type of FVIII infused. Formation of inhibitory antibodies in hemophilia patients increases the risks of morbidity and mortality, and management of bleeding episodes in these patients becomes very complicated. Recently, new approaches have been developed (see reviews [4–6]) to prevent or modulate the formation of anti-FVIII antibodies in either protein replacement or gene therapy-treated hemophilia A mice, including methods to manipulate antigen presentation [7,8], development of less immunogenic FVIII proteins or formulations [9], gene therapy protocols to evade immune responses [10–12], and immunomodulation strategies to target T and/or B-cell responses [13–19]. Interestingly, most of the successful protocols involve increases in either or both of the percentages and total numbers of CD4+Foxp3+ regulatory T (Treg) cells. It is also important that these induced Treg cells are activated in order to exert their regulatory function to suppress FVIII-specific responses. It was demonstrated that a shift from an immune-activating environment to a regulatory environment by induction of activated Treg cells to suppress T-helper cell function is not only important in blocking the initial activation of antibody responses, but also in facilitating the induction and maintenance of antigen-specific tolerance. This is similar to the findings in transplantation models, where induction of tolerance to grafts is usually associated with increased percentages or cell numbers of Treg cells.
Rapamycin is an immunosuppressant drug that was commonly used to prevent rejection in organ transplantation. Rapamycin binds the cytosolic protein FK-binding protein 12 (FKBP12) and the resulting complex inhibits the mammalian target of rapamycin (mTOR) pathway. In this issue of the Journal of Thrombosis and Haemostasis, Moghimi et al. [20] report that an immunomodulation strategy using transient oral delivery of rapamycin combined with repeated injections of low dosages of FVIII prevented induction of inhibitory antibody responses in hemophilia A mice. In tolerized mice, Th2 responses were suppressed, as shown by inhibition of IL-2, IL-4 and IL-10 expression and nearly complete elimination of IL-6 responses to FVIII. On the other hand, Foxp3, CD25 and TGF-b1 transcripts indicative of Treg cells were significantly increased. Furthermore, adoptive transfer of CD4+CD25+ Treg cells from tolerized mice protected the recipient mice from generation of high-titer inhibitory antibodies following immunization with FVIII. These results demonstrated that transient treatment of rapamycin prevented inhibitory antibody production to FVIII by suppressing the Th2 responses and inducing Treg cell expansion.
Induction and activation of antigen-specific T cells were initiated by recognition of the antigen by the T-cell receptor (TCR) in the presence of costimulation signals, leading to production of IL2 and downstream activators of proliferation (Fig. 1A). Rapamycin, an inhibitor of the mTOR pathway, preferentially expands Treg cells compared with effector T (Teff) cells by several mechanisms [21,22] (Fig. 1B), firstly through the differential effect of IL-2 receptor (IL2R) signaling. IL2R stimulation promotes activation of JAK/STAT, MAPK and the P13K/Akt/mTOR pathways. Phosphatase and tensin homolog (PTEN) is an inhibitor of P13K. PTEN is constitutively expressed in Treg cells, leading to down-regulation of the P13K/Akt/mTOR pathway. In contrast, PTEN activity is low in Teff cells, resulting in significant activation via mTOR pathways in response to IL-2 receptor signaling. Thus, rapamycin treatment has little effect on expansion of Treg cells due to its insensitivity to the mTOR pathway compared with significant inhibition of the expansion of Teff cells. The second mechanism is differential expression of pro- and anti-apoptotic proteins. In the presence of rapamycin, high levels of anti-apoptotic proteins were expressed in Treg cells, whereas low levels of anti-apoptotic proteins and high levels of pro-apoptotic proteins were expressed in Teff cells. Treg cells become more resistant to apoptosis relative to Teff cells. Thirdly, alternative pathways independent of mTOR in Treg cells are activated via the PIM-2 pathway. The expression of PIM-2 is regulated by Foxp3 and it is constitutively expressed in Treg cells. Teff cells lacking PIM-2 are highly sensitive to the anti-proliferative effects of rapamycin, whereas Treg cells are resistant to these effects. However, recent evidence [23] also suggests that the mTOR pathway may be important in maintaining both homeostasis and alloantigen-driven proliferation of Treg cells. Upon withdrawal of rapamycin, an increase in mTOR activation augments Treg cell expansion in the presence of high levels of IL2 [24].
Fig. 1.
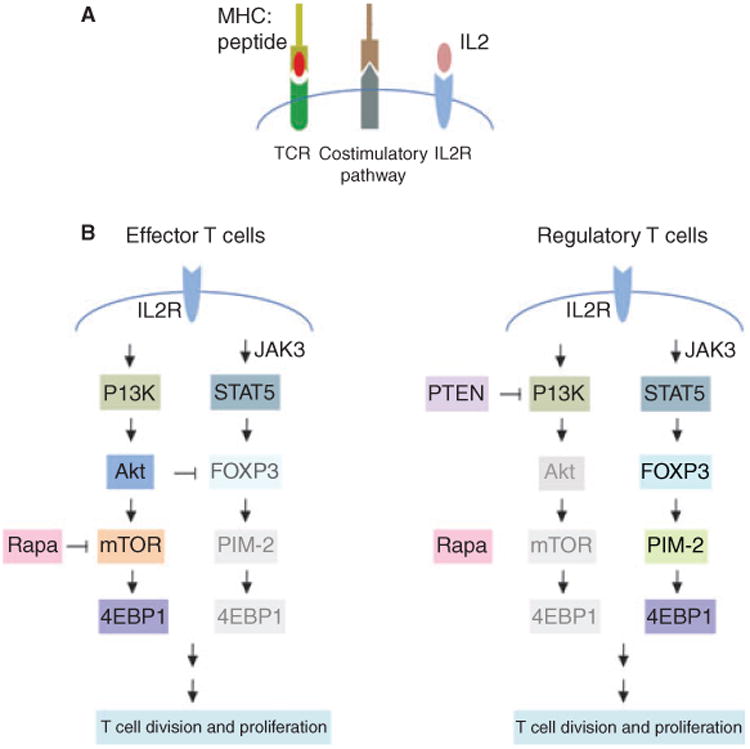
Activation signals for effector and regulatory T-cell division and proliferation. (A) Signals required for activation of antigen-specific T cells. Interaction of the T-cell receptor (TCR) with antigen presented in the context of MHC molecules sends the first signal for T-cell activation. MHCI/peptide complexes were recognized by the CD8 TCR and MHCII/peptide complexes by CD4 TCR. Costimulation provides the second signal to promote T-cell activation and IL2 production. IL2 in turn binds to IL2-receptor (IL2R) and induces T-cell proliferation. (B) Differential activation pathways used for proliferation of effector (Teff) and regulatory T (Treg) cells. For Teff cells, IL2R stimulation promotes preferentially the activation of the P13K/Akt/mTOR pathway, which is sensitive to the effects of rapamycin. In contrast, for Treg cells, IL2R stimulation induces preferentially the activation of the JAK/STAT signaling pathway due to high expression of PTEN, which blocks the P13K/Akt/mTOR pathway. The JAK/STAT/PIM-2 pathway is insensitive to rapamycin. Thus, administration of rapamycin suppresses the proliferation of Teff cells while allowing expansion of Treg cells.
Thus, following short-term rapamycin treatment, a shift from an immune activating to an immune regulatory environment occurred, which created a regulatory milieu facilitating tolerance induction. Rapamycin is already used in the clinic and can be readily tested to treat hemophilia inhibitors. However, several questions still remain worthy of further investigation. As rapamycin induced ‘peripheral tolerance’ of a specific antigen, how long will the tolerance to FVIII persist? Is antigen presentation needed at all times for maintenance of antigen-specific tolerance? Will secondary challenge of antigen break the tolerance after withdrawal of antigen for a period of time? How can complete tolerance of full length FVIII (FL-FVIII) be achieved? Will tolerance of FVIII be achieved by using rapamycin combined with small peptides that encode CD4+ T-cell epitopes of FVIII? Can a lower dosage and shorter treatment of rapamycin be used to reduce the undesirable side effects and toxicity?
Prophylactic tolerance induction protocols involving a short immunosuppressive regimen with minimum side effects and toxicity are highly promising strategies for patients at high risk of inhibitor formation. Rapamycin, as used in Moghimi et al.'s study [20], and several other immunomodulation agents, including agents to block costimulatory pathways such as CTLA4-Ig combined with anti-CD40L [13,18] and anti-ICOS [14], agents to deplete T cells such as anti-CD3 [15,16], or agents to induce Treg cell expansion such as IL2-IL2mAb complexes [19], can be administered in combination with repeated injections of low doses of FVIII to induce long-term tolerance of FVIII. Transient immunosuppression did not hamper the immune system and prevent subsequent responses to other antigens or pathogens, as shown in several studies [14,15,19,20]. These evolving new strategies for tolerance induction can not only reduce the costs, but also shorten the treatment time and increase the success rate. Clinical testing of some of these regimens is highly anticipated.
Footnotes
Disclosure of Conflict of Interests: The author states that she has no conflict of interest.
References
- 1.Reding MT, Lei S, Lei H, Green D, Gill J, Conti-Fine BM. Distribution of Th1- and Th2-induced anti-factor VIII IgG subclasses in congenital and acquired hemophilia patients. Thromb Haemost. 2002;88:568–75. [PubMed] [Google Scholar]
- 2.van Helden PM, van Haren SD, Fijnvandraat K, van den Berg HM, Voorberg J. Factor VIII-specific B cell responses in haemophilia A patients with inhibitors. Haemophilia. 2010;16:35–43. doi: 10.1111/j.1365-2516.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 3.Darby SC, Keeling DM, Spooner RJ, Wan Kan S, Giangrande PL, Collins PW, Hill FG, Hay CR. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977-99. J Thromb Haemost. 2004;2:1047–54. doi: 10.1046/j.1538-7836.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 4.Waters B, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7:1446–56. doi: 10.1111/j.1538-7836.2009.03538.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang AH, Skupsky J, Scott DW. Factor VIII inhibitors: risk factors and methods for prevention and immune modulation. Clin Rev Allergy Immunol. 2009;37:114–24. doi: 10.1007/s12016-009-8122-5. [DOI] [PubMed] [Google Scholar]
- 6.Miao CH. Immunomodulation of inhibitors in hemophilia A: the important role of Treg cells. Expert Rev Hematol. 2010;3:469–83. doi: 10.1586/ehm.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawle FE, Pratt KP, Labelle A, Weiner HL, Hough C, Lillicrap D. Induction of partial immune tolerance to factor VIII through prior mucosal exposure to the factor VIII C2 domain. J Thromb Haemost. 2006;4:2172–9. doi: 10.1111/j.1538-7836.2006.02118.x. [DOI] [PubMed] [Google Scholar]
- 8.Skupsky J, Zhang AH, Su Y, Scott DW. B-cell-delivered gene therapy induces functional T regulatory cells and leads to a loss of antigen-specific effector cells. Mol Ther. 2010;18:1527–35. doi: 10.1038/mt.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenko EL, Pipe SW. Strategies towards a longer acting factor VIII. Haemophilia. 2006;12(Suppl. 3):42–51. doi: 10.1111/j.1365-2516.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsui H, Shibata M, Brown B, Labelle A, Hegadorn C, Andrews C, Chuah M, VandenDriessche T, Miao CH, Hough C, Lillicrap D. A murine model for induction of long-term immunologic tolerance to factor VIII does not require persistent detectable levels of plasma factor VIII and involves contributions from Foxp3+ T regulatory cells. Blood. 2009;114:677–85. doi: 10.1182/blood-2009-03-202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu L, Mei M, Ma X, Ponder KP. High expression reduces an antibody response after neonatal gene therapy with B domain-deleted human factor VIII in mice. J Thromb Haemost. 2007;5:1805–12. doi: 10.1111/j.1538-7836.2007.02629.x. [DOI] [PubMed] [Google Scholar]
- 12.Ide LM, Gangadharan B, Chiang KY, Doering CB, Spencer HT. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood. 2007;110:2855–63. doi: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao CH, Ye P, Thompson AR, Rawlings DJ, Ochs HD. Immunomodulation of transgene responses following naked DNA transfer of human factor VIII into hemophilia A mice. Blood. 2006;108:19–27. doi: 10.1182/blood-2005-11-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng B, Ye P, Blazar BR, Freeman GJ, Rawlings DJ, Ochs HD, Miao CH. Transient blockade of the inducible costimulator pathway generates long-term tolerance to factor VIII after nonviral gene transfer into hemophilia A mice. Blood. 2008;112:1662–72. doi: 10.1182/blood-2008-01-128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng B, Ye P, Rawlings DJ, Ochs HD, Miao CH. Anti-CD3 antibodies modulate anti-factor VIII immune responses in hemophilia A mice after factor VIII plasmid-mediated gene therapy. Blood. 2009;114:4373–82. doi: 10.1182/blood-2009-05-217315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters B, Qadura M, Burnett E, Chegeni R, Labelle A, Thompson P, Hough C, Lillicrap D. Anti-CD3 prevents factor VIII inhibitor development in hemophilia A mice by a regulatory CD4+CD25+-dependent mechanism and by shifting cytokine production to favor a Th1 response. Blood. 2009;113:193–203. doi: 10.1182/blood-2008-04-151597. [DOI] [PubMed] [Google Scholar]
- 17.Scott DW. Gene therapy for immunological tolerance: using ‘transgenic’ B cells to treat inhibitor formation. Haemophilia. 2010;16(102):89–94. doi: 10.1111/j.1365-2516.2010.02203.x. [DOI] [PubMed] [Google Scholar]
- 18.Reipert BM, Allacher P, Hausl C, Pordes AG, Ahmad RU, Lang I, Ilas J, Windyga J, Klukowska A, Muchitsch EM, Schwarz HP. Modulation of factor VIII-specific memory B cells. Haemophilia. 2010;16:25–34. doi: 10.1111/j.1365-2516.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu CL, Ye P, Yen BC, Miao CH. In Vivo Expansion of Regulatory T cells With IL-2/IL-2 mAb Complexes Prevents Anti-factor VIII Immune Responses in Hemophilia A Mice Treated With Factor VIII Plasmid-mediated Gene Therapy. Mol Ther. 2011 doi: 10.1038/mt.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghimi B, Sack B, Nayak S, Markusic D, Mah C, Herzog R. Tolerance induction to factor VIII by transient co-administration with rapamycin. J Thromb Haemost. 2011;9:1524–33. doi: 10.1111/j.1538-7836.2011.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMahon G, Weir MR, Li XC, Mandelbrot DA. The evolving role of mTOR inhibition in transplantation tolerance. J Am Soc Nephrol. 2011;22:408–15. doi: 10.1681/ASN.2010040351. [DOI] [PubMed] [Google Scholar]
- 22.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–11. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Camirand G, Lin Y, Froicu M, Deng S, Shlomchik WD, Lakkis FG, Rothstein DM. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and allo-antigen-driven proliferation in lymphocyte-replete mice. J Immunol. 2011;186:2809–18. doi: 10.4049/jimmunol.0903805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Procaccini C, De Rosa V, Galgani M, Abanni L, Cali G, Porcellini A, Carbone F, Fontana S, Horvath TL, La Cava A, Matarese G. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–41. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


