Abstract
Addictive drugs have in common that they are voluntarily self-administered by laboratory animals (usually avidly) and that they enhance the functioning of the reward circuitry of the brain (producing the “high” that the drug-user seeks). The core reward circuitry consists of an “in series” circuit linking the ventral tegmental area, nucleus accumbens, and ventral pallidum - via the medial forebrain bundle. Although originally believed to encode simply the set-point of hedonic tone, these circuits are now believed to be functionally far more complex - also encoding attention, expectancy of reward, disconfirmation of reward expectancy, and incentive motivation. “Hedonic dysregulation” within these circuits may lead to addiction. The “second-stage” dopaminergic component in this reward circuitry is the crucial addictive-drug-sensitive component. All addictive drugs have in common that they enhance (directly or indirectly or even transsynaptically) dopaminergic reward synaptic function in the nucleus accumbens. Drug self-administration is regulated by nucleus accumbens dopamine levels, and is done to keep nucleus accumbens dopamine within a specific elevated range (to maintain a desired hedonic level). For some classes of addictive drugs (e.g., opiates), tolerance to the euphoric effects develops with chronic use. Post-use dysphoria then comes to dominate reward circuit hedonic tone, and addicts no longer use drugs to get “high,” but simply to get back to normal (“get straight”). The brain circuits mediating the pleasurable effects of addictive drugs are anatomically, neurophysiologically, and neurochemically different from those mediating physical dependence, and from those mediating craving and relapse. There are important genetic variations in vulnerability to drug addiction, yet environmental factors such as stress and social defeat also alter brain-reward mechanisms in such a manner as to impart vulnerability to addiction. In short, the “bio-psycho-social” model of etiology holds very well for addiction. Addiction appears to correlate with a hypo-dopaminergic dysfunctional state within the reward circuitry of the brain. Neuroimaging studies in humans add credence to this hypothesis. Credible evidence also implicates serotonergic, opioid, endocannabinoid, GABAergic, and glutamatergic mechanisms in addiction. Critically, drug addiction progresses from occasional recreational use to impulsive use to habitual compulsive use. This correlates with a progression from reward-driven to habit-driven drug-seeking behavior. This behavioral progression correlates with a neuroanatomical progression from ventral striatal (nucleus accumbens) to dorsal striatal control over drug-seeking behavior. The three classical sets of craving and relapse triggers are a) re-exposure to addictive drugs, b) stress, and c) re-exposure to environmental cues (“people, places, things”) previously associated with drug-taking behavior. Drug-triggered relapse involves the nucleus accumbens and the neurotransmitter dopamine. Stress-triggered relapse involves a) the central nucleus of the amygdala, the bed nucleus of the stria terminalis, and the neurotransmitter CRF; and b) the lateral tegmental noradrenergic nuclei of the brain stem and the neurotransmitter norepinephrine. Cue-triggered relapse involves the basolateral nucleus of the amygdala, the hippocampus, and the neurotransmitter glutamate. Knowledge of the neuroanatomy, neurophysiology, neurochemistry, and neuropharmacology of addictive drug action in the brain is currently producing a variety of strategies for pharmacotherapeutic treatment of drug addiction, some of which appear promising.
Addiction - An Ages-Old Medical and Societal Problem
The abusive use of addictive drugs is a medical and societal problem as old as recorded human history. One particularly ancient reference to it may be found in the Hebrew/Christian Bible - where, in Genesis chapter 9 verses 20–23, the Semite Patriarch Noah is described as becoming drunken, disheveled, naked, and filthy from overindulgence in wine. Similarly ancient references to drug abuse may be found in the oral and written traditions of virtually all ethnic and cultural groups on the planet.
One of the most striking features of drug addiction is how few chemicals are subject to abuse. If one takes all congeners of all known chemicals, approximately 30,000,000 chemical substances are known [1].
Yet, only approximately 100 (including nicotine, ethanol, psychostimulants, opiates, barbiturates, benzodiazepines, and cannabinoids) are addictive. In truth, 100 is a stunningly small subset of 30,000,000. It poses the question - what makes those 100 chemicals addictive, while the remaining 30,000,000 chemicals lack this property? After all, upon cursory examination, there seem few pharmacological similarities among addictive drugs. Some - including barbiturates, ethanol, opiates, and benzodiazepines - are sedatives; while others - including nicotine, cocaine, and the amphetamines - are stimulants. Some - including opiates and cannabinoids - are anti-nociceptive, while others (under the proper laboratory or clinical situations) are pro-nociceptive. Some - such as ethanol and opiates - produce striking degrees of physical dependence, while others - such as cocaine - produce little if any physical dependence. However, a few commonalities are both apparent and instructive. All addictive drugs are subjectively rewarding, reinforcing, and pleasurable [1]. Laboratory animals volitionally self-administer them [2], just as humans do. Furthermore, the rank order of appetitiveness in animals parallels the rank order of appetitiveness in humans [2,3]. Most tellingly, perhaps, all addictive drugs (with the exception of the LSD-like and mescaline-like hallucinogens) activate the reward circuitry of the brain [1,4,5], thereby producing the subjective “high” that the drug abuser seeks. Furthermore, the degree of such activation of the brain’s reward circuitry correlatives well with the degree of subjective “high.”
The Brain’s Reward Circuitry
The brain’s reward circuitry was first discovered by Olds and Milner at McGill University in the early 1950s [6]. They found that animals would repeatedly return to an area of the laboratory in which they had received mild electrical stimulation of subcortical structures anatomically associated with the medial forebrain bundle. Subsequently, they found that animals would avidly perform tasks (e.g., depressing wall-mounted levers in their test chambers) in order to receive such brain stimulation. In the aftermath of this discovery of the phenomenon of brain-stimulation reward, Olds and Olds carried out extensive mapping studies of the rodent brain, confirming that a large majority of the brain sites supporting brain-stimulation reward are associated with the nuclei of origin, tracts, and terminal loci of the medial forebrain bundle [7–10]. Other workers [11] studied electrical brain-stimulation reward in non-human primates and confirmed that the anatomic brain substrates in primates were homologous to those in rodents. Using sophisticated electrophysiological techniques, Gallistel, Shizgal, and Yeomans determined that the primary neural substrate supporting electrical brain-stimulation reward is the moderately fast-conducting myelinated descending neural fiber system of the medial forebrain bundle [12]. This system originates in the anterior bed nuclei of the medial forebrain bundle (an array of deep subcortical limbic loci anterior to the hypothalamus and preoptic area), descends to the ventral tegmental area of the midbrain via the medial forebrain bundle, and then ascends via the medial forebrain bundle to a select group of forebrain limbic loci - including the nucleus accumbens, olfactory tubercle, and frontal cortex. Wise and Bozarth [13] were the first to realize that this assortment of brain loci and tracts constituted a neural circuit containing 3 synaptically-connected in-series neuronal elements - a descending link running from the anterior bed nuclei of the medial forebrain bundle to the ventral tegmental area, an ascending link running from the ventral tegmental area to the nucleus accumbens, and a further ascending link running from the nucleus accumbens to the ventral pallidum. The first link is the descending myelinated fiber tract first identified by Gallistel and colleagues [12], of unknown neurotransmitter type – although very recent evidence raises, by inference, the possibility that glutamate in the ventral tegmental area might play a role (see, e.g. [14]). The second link is the ascending fiber tract from ventral tegmental area to nucleus accumbens, with dopamine as its neurotransmitter (see below). The third link is the projection from nucleus accumbens to ventral pallidum, using gamma-aminobutyric acid (GABA), Substance P, and enkephalin as conjoint neurotransmitters [15–22]. This 3-neuron in-series circuit receives synaptic inputs from, and is functionally modulated by, a wide variety of other neural circuits - including cholinergic, endorphinergic, serotonergic, GABAergic, glutamatergic, enkephalinergic, dynorphinergic, and Substance P-containing neural elements [1].
Addictive drugs of different classes act on this 3-neuron in-series brain reward neural circuit at different points to activate the circuit and produce the drug-induced “high.” Barbiturates, benzodiazepines, cannabinoids, ethanol, nicotine, and opiates act on synapses associated with the ventral tegmental area. Amphetamines, cannabinoids, cocaine, opiates, and dissociative anesthetics such as ketamine and phencyclidine act on synapses associated with the nucleus accumbens [1–3,5].
Importantly, this brain reward circuitry evolved over the eons of evolution to subserve biologically essential normal rewarding behaviors such as feeding, drinking, sexual behavior, maternal and paternal behaviors, and social interactions. The reinforcement engendered by such normal reward is believed to underlie the consolidation of biologically essential memories (e.g., food and water location within an animal’s foraging or hunting range) [23]. After all, it is teleological thinking at its most tendentious and intellectually vacuous to imagine that these circuits emerged through hundreds of millions of years of vertebrate and mammalian evolution simply so that 21st century humans can imbibe, inhale, or inject themselves with addictive drugs [1]. From an appreciation of the natural and biologically-essential nature of the functioning of these reward circuits comes the notion that addictive drugs “hijack” the brain’s reward circuits - activating them more strongly than natural rewards, and diverting the drug addict’s life to pursuit of drug-induced pleasure at the expense of “getting off” on life’s normal pleasures and rewards [24,25]
The Intense Nature of Brain-Stimulation Reward
Electrical brain-stimulation reward is remarkable for the intensity of the reward and reinforcement produced [1]. When the stimulating electrode is properly on target within the ventral tegmental area, medial forebrain bundle, or nucleus accumbens, laboratory animals will volitionally self-stimulate those areas at maximal rates. They will - tellingly - ignore readily available food, water, toys, and sexually receptive animals of the opposite sex in order to self-deliver the brain-stimulation reward. They will also volitionally accept aversive and painful consequences in order to self-deliver the brain-stimulation reward. In awake humans, such electrical stimulation can evoke intense subjective feelings of pleasure [26–31], in some instances similar to descriptions of intense Medieval religious ecstasies (Arthur A. Ward Jr., personal communication, 1967). As the most addictive drugs (e.g., cocaine, methamphetamine) evoke comparable levels of subjective reward, it is easy to understand their intense addictive nature.
Using Electrical Brain-Stimulation Reward to Assess the Degree of Reward Evoked by Addictive Drugs
In the half-century since the initial discovery of the brain-stimulation reward phenomenon, a number of techniques have been developed to quantify the degree of reward enhancement produced by addictive drugs. Using simple rate-based measures (e.g., how rapidly a test animal will lever-press or nose-poke to receive brain-stimulation reward) is unsatisfactory, as many addictive drugs have either motor stimulating or depressant properties. Rate-independent test paradigms are essential. One of the earliest rate-independent paradigms was the two-lever titrating threshold paradigm [32]. In this paradigm, the test animal depresses a wall-mounted primary lever in its test chamber to receive the brain-stimulation, which decreases by a pre-set amount (either in amperes or hertz) with each successive stimulation. At some point, the stimulation decreases to a level insufficient to activate the neurons at the tip of the implanted electrode in the brain - the consequence of which is that the animal ceases to experience the subjective reward or pleasure evoked by the stimulation. At this point, the animal presses a wall-mounted secondary lever - which delivers no brain stimulation, but merely resets the brain stimulator back to its original intensity. By assessing the mean reset level, one obtains a rate-independent (and, thus, motor impairment- or motor-stimulation-free) measure of brain reward function. As monkeys (and even some laboratory rats) are clever enough to adopt an alternating lever-pressing pattern of behavior to keep the brain-stimulation intensity at maximum, a variant of the technique was developed in which the resetting of stimulation intensity is controlled by withholding of response on the primary lever [33]. Since animals will volitionally self-stimulate at even marginally-rewarding levels of stimulation, this technique yields rate-independent threshold measures of brain reward intensity that appear to track the rewarding-enhancing properties of addictive drugs with accuracy and consistency.
Another rate-independent brain-stimulation reward measuring technique commonly used in recent decades is the rate-frequency (or, alternatively, rate-amperage) curve-shift method [34,35]. In this paradigm, the test animal has only a single manipulandum (e.g., lever, nose-poke detector) in its test chamber, activation of which delivers the rewarding brain stimulation (usually with a duration of 250 msec). A test session consists of a successive series of short “bins” of opportunity to self-stimulate (e.g., 20-seconds), each bin being at a lower intensity of stimulation. As the hertz (or amperage level) decreases, the stimulation decreases to a level insufficient to activate the neurons at the tip of the implanted electrode in the brain and the animal ceases to experience the subjective reward - leading to a fairly abrupt cessation of responding. By plotting stimulation frequency (or amperage) against rate of responding, one obtains a rate-independent measure of brain-reward function. A typical rate-frequency or rate-amperage plot is sigmoidal in shape, and can be conceptualized in the same way as a dose-response curve in classical pharmacology. As in classical pharmacology, a left-shift constitutes an enhancement of efficacy (in this case, an intensification of brain reward) and a right-shift constitutes a diminution of efficacy (an inhibition of brain reward).
Using the rate-frequency curve-shift brain-stimulation reward paradigm, much work has been devoted to assessing the brain-reward enhancing properties of addictive drugs. With the exception of nicotine (which is inexplicably powerful at enhancing electrical brain reward functions), the degree of enhancement of brain reward by addictive drugs nicely parallels the degree of subjective “high” experienced by human users of such drugs [1]. Nicotine’s actions on rain reward are intriguing. According to most cigarette smokers, nicotine does not produce a powerful subjective “high.” Yet, nicotine is the most addictive chemical known [36]. Is it possible that nicotine’s actions in the electrical brain-stimulation rewards paradigm are more congruent with its addictive potency than with its potency on the subjective experience of “high?” That seems counterintuitive, yet it would appear that such a possibility must be entertained.
These methods can also be used to assess the degree to which potential anti-addiction pharmacotherapeutic medications attenuate addictive drug-enhanced brain reward - and thus, by inference, the degree to which such putatively therapeutic agents might attenuate addictive drug-induced “highs” in human drug abusers [37–41].
These methods can also be used to study the brain mechanisms underlying the “low-dose good-trip; high-dose bad-trip” subjective phenomenon often reported by drug addicts. Similar to human reports, animals also appear to experience enhancement of brain reward at low-to-moderate doses of addictive drugs, while experiencing inhibition of reward at high doses of the same drugs [42–43].
Activation of Brain Reward Substrates by Direct Intracerebral Microinjection of Addictive Drugs
Just as systemic injections of addictive drugs enhance brain reward substrates, so too does intracerebral microinjection. Compellingly, the brain sites that support intracerebral microinjections of addictive drugs are, by and large, the same brain sites that support electrical brain-stimulation reward [1,2,5,13]. Thus, it may be inferred that common neural substrates underlie reward-enhancement induced by addictive drugs, however administered. This is an important component of the conception that addictive drugs derive their addictive actions by enhancement of brain reward mechanisms.
Another technique for measuring the rewarding properties of addictive drugs is that of conditioned place preference/aversion [44–46]. In the most simple variant of this animal model, a two-compartment test chamber is used - each compartment having distinctly different environmental cues (e.g., one compartment with striped walls, smooth floor, and lemon odor; the other compartment with plain walls, rough floor, and pine scent). Between the two compartments is a vertical-sliding door which, when in place, prevents movement from one compartment to the other. The animal is initially placed in the chamber with the door absent - thus allowing free passage back and forth between both compartments. The environmental cues have been previously adjusted so that on initial exposure to the test chamber, animals show no inherent preference for one compartment over the other. On the next day, the door is inserted, blocking passage between the compartments. The animal is administered a drug, enough time is allowed to elapse so that peak drug effect is reached, and the animal is placed into one cuedistinct compartment for a modest period of time (e.g., 15 minutes). On the next test day, the animal is administered vehicle and placed into the other cue-distinct compartment for the same length of time. On successive days, animals alternate between the drug-paired and vehicle-paired compartments. This “training” goes on for approximately 10 days (i.e., 5 days being given drug and placed in the drug-paired cue-distinct compartment; 5 days being given vehicle and placed in the vehicle-paired cue-distinct compartment). The next day is the “test” day on which the door is once again removed, allowing free passage between compartments, and the test animal is not administered anything. If the animal volitionally spends a disproportionate amount of time in the formerly drug-paired compartment, it is inferred that the animal is displaying drug-seeking behavior and it is further inferred that the drug experience must have been rewarding. If the animal volitionally spends a disproportionate amount of time in the formerly vehicle-paired compartment, it is inferred that the animal is displaying drug-avoidance behavior and it is further inferred that the drug experience must have been aversive. Compellingly, the only drugs that consistently produce place preference rather than place neutrality or place aversion are addictive drugs [46]. Equally compellingly, place preference is evoked by intracerebral microinjections of addictive drugs - by and large into the same brain sites that support electrical brain-stimulation reward and support volitional intracerebral self-microinjection of addictive drugs [5]. This is yet another important component of the conception that addictive drugs derive their addictive actions by enhancement of brain reward mechanisms.
The Crucial Reward Neurotransmitter in the Brain is Dopamine
The crucial brain reward neurotransmitter activated by addictive drugs is dopamine, specifically in the “second-stage” ventral tegmental area to nucleus accumbens link in the brain’s reward circuitry. This has been learned over many decades of research, and is based upon many congruent findings.
First, virtually all addictive drugs are functional dopamine agonists - some direct, some indirect, some even transsynaptic [1,4,5]. In fact, with the exception of the LSD- and mescaline-like hallucinogens, functional dopamine agonism is the single pharmacological property that all addictive drugs share.
Second, intracerebral microinjections of dopamine agonists produce conditioned place preference (see discussion above) and support volitional intracerebral self-administration [5].
Third, dopamine antagonists are negative reinforcers in animals (animals will work to avoid or escape their administration), and produce subjectively aversive effects in humans [1,3].
Fourth, when dopamine antagonists are administered to animals volitionally self-administering addictive drugs, a compensatory increase in addictive drug intake occurs (to compensate for the decreased rewarding potency of the addictive drug) followed by extinction and cessation of the self-administration behavior (when the dopamine antagonism reaches a sufficient intensity so as to totally block the rewarding properties of the self-administered addictive drug) [3,47].
Fifth, measures of real-time synaptic neurochemistry in the nucleus accumbens of test animals volitionally engaged in intravenous self-administration of addictive drugs (the real-time neurochemical sampling being achieved by in vivo brain microdialysis [48]) show that: a) following the first volitional self-administration of the test session, extracellular dopamine overflow in the nucleus accumbens shows a tonic increase of approximately 200%; b) thereafter, extracellular dopamine levels in the nucleus accumbens fluctuate phasically between approximately 200% and 100% over baseline; and c) the low point of each phasic dip in extracellular nucleus accumbens dopamine accurately predicts the next volitional intake of addictive drug by the test animal [49–51].
Brain Anti-Reward Systems and Addiction - Proponent and Opponent Brain Reward Processes
Drawing from Solomon’s hypothesis on the existence of proponent and opponent motivational processes [52–54], Koob [55–57] has proposed that there are similar proponent and opponent processes at work in the brain substrates of reward [1]. The proponent brain reward processes are hypothesized to produce enhancement of brain reward, and to show development of tolerance over time. The opponent brain reward processes are hypothesized to produce inhibition of brain reward, and to show progressive enhancement of strength over time. The proponent (pro-reward) and opponent (anti-reward) processes are hypothesized to occur simultaneously and to functionally oppose each other in a mutually inhibitory fashion. Using electrical brain-stimulation reward in laboratory rodents, Nazzaro, Seeger, and Gardner [42,58] reported finding pro-reward and anti-reward processes that correspond remarkably well to Koob’s hypothesized proponent and opponent brain reward mechanisms.
Broderick, Gardner, and van Praag reported finding similar pro-reward and anti-reward processes measured neurochemically by in vivo brain electrochemistry (in vivo voltammetry) in laboratory rodents [42,59,60].
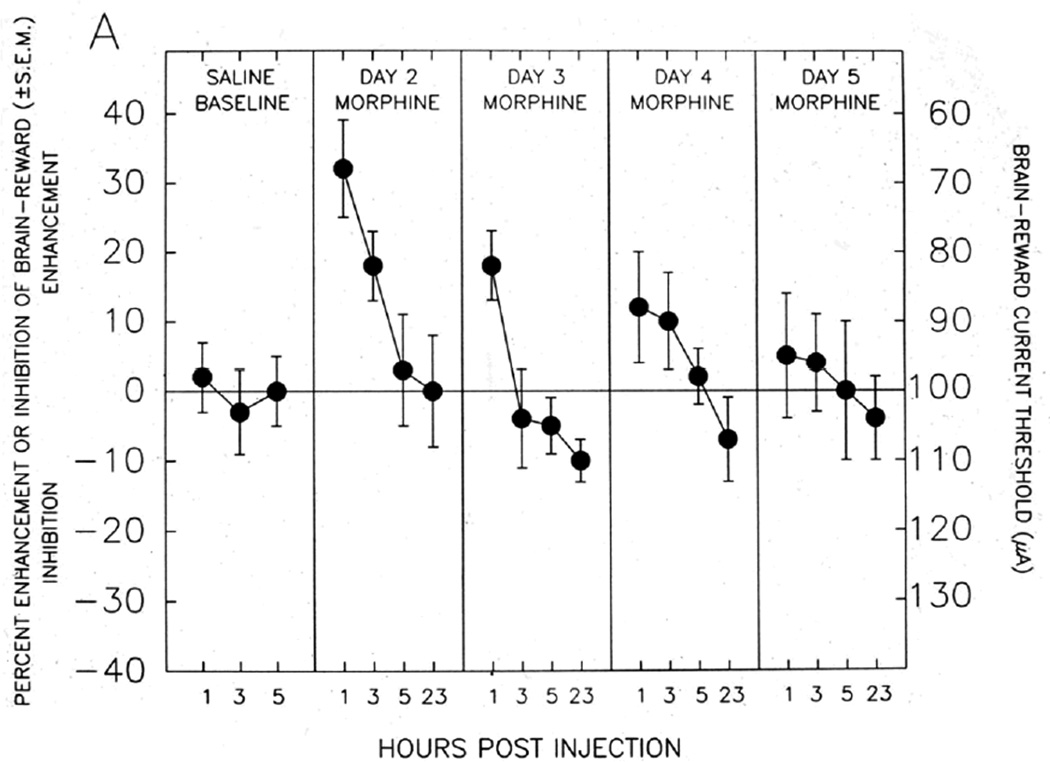
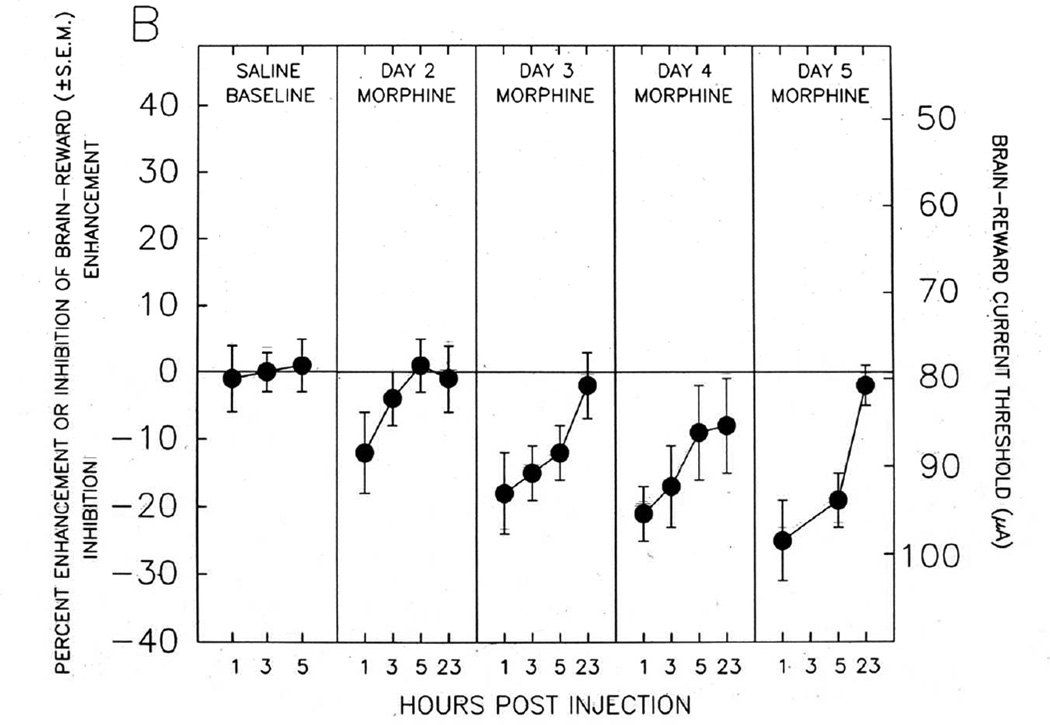
As can be readily seen from an inspection of Figure 4, these pro-reward and anti-reward mechanisms have important implications for understanding the nature of the overall shift in reward level or hedonic tone produced by addictive drugs. If the reward-enhancing effect shown in Figure 4A is combined with the reward-inhibiting effect shown in Figure 4B, it appears that administration of an opiate (in this case morphine) initially produces a strong enhancement of brain reward (the “high”) that is countered by only a weak simultaneous anti-reward process. The net effect on brain reward or subjective hedonic tone will be significant reward enhancement (“high”). However, with repeated opiate administration, the pro-reward mechanism depicted in Figure 4A progressively diminishes, while the anti-reward mechanism depicted in Figure 4B grows progressively stronger. Thus, with repeated opiate administration, the overall net effect on hedonic tone becomes more and more inhibitory - since the opponent processes grow stronger and are opposed by progressively weaker proponent processes. Gardner and David [1,4] proposed that this may constitute at least a partial mechanistic explanation for the frequent report by human heroin addicts that - after living with the disease of opiate addiction for some time - they no longer self-administer heroin to get “high,” but rather to simply get “straight” (i.e., to push their diminished subjective hedonic tone back towards normality).
Fig. 4. PRO-REWARD AND ANTI-REWARD BRAIN-STIMULATION REWARD SUBSTRATES ACTIVATED BY OPIATE ADMINISTRATION - FROM NAZZARO AND GARDNER.
Brain reward and anti-reward mechanisms recruited by systemic opiate administration. Enhancement or inhibition of brain-reward is measured using electrical brain-stimulation reward in laboratory rats. The stimulating electrode in Panel A is located in the medial portion of the mesotelencephalic dopaminergic reward fiber tract; the stimulating electrode in Panel B is located in the lateral portion of the mesotelencephalic dopaminergic reward fiber tract. Panel A: Acute systemic opiate administration enhances brain reward, which diminishes as opiate effects wear off during each test day, and which shows development of tolerance with successive opiate administrations. Panel B: Acute systemic opiate administration inhibits brain reward, which diminishes as opiate effects wear off during each test day, and which shows development of sensitization (enhancement, “reverse tolerance”) with successive opiate administrations. Overall hedonic tone is a combination of effects depicted in Panels A and B. Thus, with chronic opiate administration, the reward-enhancing effects diminish and the anti-reward effects intensify, producing and overall reward deficiency state. After [42,58].
Brain Anti-Reward Systems and Addiction - Brain Reward Processes During Withdrawal
As noted above, brain reward is enhanced by acute administration of addictive drugs. Conversely, in withdrawal, brain reward is inhibited - sometimes profoundly. This was first reported by Kokkinidis and McCarter in 1990 [61] using the electrical brain-stimulation reward paradigm in laboratory rodents, and has been amply confirmed since [62–65]. These robust inhibitory effects on brain reward would appear to constitute the mechanistic neural substrate for the inhibition of hedonic tone experienced by drug addicts during withdrawal. It should be well noted that this hedonic withdrawal state is unrelated to the physical withdrawal state experienced concomitantly by addicts experiencing acute withdrawal (e.g., cramps, diarrhea, and physical pain during opiate withdrawal).
Brain Anti-Reward Systems and Addiction - “Reward Deficiency” as a Driving Force in Addiction
In 1996, Blum and colleagues proposed that many aspects of addiction are driven by a chronic basal deficiency in brain reward which mechanistically underlies a chronic basal deficiency in subjective hedonic tone [66,67]. This hypothesis has been amplified and expanded, both by its original proponents and by others [68–71]. The fundamental notion is a simple one - that drug addicts are either born with or acquire a deficiency state in the dopaminergic brain substrates of reward and positive hedonic tone, and turn to addictive drug use to remedy this chronic reward deficiency. Thus, the reward deficiency hypothesis seeks to explain drug addiction as a type of self-medication, ultimately damaging and self-destructive though it may be.
The brain substrate of this reward deficiency syndrome may be mechanistically referable to more than a single deficiency in the functioning of the brain’s dopaminergic reward circuitry.
Blum and colleagues have hypothesized that it is referable to a deficiency of dopaminergic D2 receptors in the brain’s reward circuitry [70,72], a view congruent with reports - including neuroimaging of the brain - by Volkow [73–76], Nader [77], and Everitt and Robbins [78], among others.
Mash [79,80], Gardner [46,81–83], Heidbreder [84,85] and others have hypothesized that it may be referable to an aberration in dopaminergic D3 receptors in the brain’s reward circuitry.
Nestler and Kosten and colleagues have hypothesized that it may be referable to a deficiency in presynaptic dopamine levels in the reward-crucial nucleus accumbens [86,87]. They initially arrived at this hypothesis from studying laboratory rodents made vulnerable to drug addiction (i.e., exhibiting high preference for addictive drugs and high drug-seeking behavior) by two quite different means - one genetic and one experiential. By selective breeding, it is possible to genetically select for the behavioral phenotype of high addictive drug preference and high addictive drug-seeking behavior [88–93]. Conversely it is equally possible to genetically select for the behavioral phenotype of addictive drug avoidance and low addictive drug-seeking behavior. While some such strains have been deliberately bred, it has also been recognized that some rodent strains have acquired these converse behavioral phenotypes during natural evolution. In this regard, the Lewis and Fischer 344 rat strains are notable. Lewis rats are naturally highly addictive drug preferring and drug-seeking. Fischer 344 rats are naturally highly addictive drug avoidant. It is also possible to confer the behavioral phenotype of high addictive drug preference and high addictive drug-seeking behavior by experiential means - i.e., by exposing animals to repeated administration of addictive drugs. Nestler and Kosten and colleagues studied the dopaminergic brain reward circuits of laboratory rats made vulnerable (or resistant) to addictive drugs by both genetic and experiential means. They found that in animals displaying the behavioral phenotype of high addictive drug acceptance and high addictive drug-seeking - whether this behavioral phenotype was imparted genetically or experientially - there exists a pathological atrophy of the neurofillamentary transport system for the dopamine-synthesizing enzyme tyrosine hydroxylase in the dopamine axons of the second-stage medial forebrain bundle neurons of the brain’s reward circuitry. Congruently, they found a pathological deficiency in tyrosine hydroxylase in the dopaminergic axon terminals of the nucleus accumbens, a pathological deficiency of extracellular dopamine in the nucleus accumbens, and concomitant pathological aberrations in post-receptor transduction mechanisms (e.g., adenylate cyclase, cyclic AMP, PKA) in the next following postsynaptic neurons, all in comparison to control animals not displaying the addictive drug-accepting and drug-seeking behavioral phenotype [86,87].
As a result of such findings, Gardner [1] has hypothesized that some drug addicts may “have a defect in their ability to capture reward and pleasure from everyday experience” and has noted that this has been hypothesized by astute clinicians for more than 45 years [94,95]. Gardner [1] has further noted that “if this be so, then our goals are really two-fold: first, to rescue addicts from the clutches of their addictions, and second, to restore their reward systems to a level of functionality that will enable them to ‘get off’ on the real world.”
The Functional Roles of the Crucial Nucleus Accumbens Reward Neurons
Based upon an extensive review of the literature regarding brain reward mechanisms, Wise suggested more than 30 years ago that “the dopamine junction [in the nucleus accumbens] represents a synaptic way station for messages signaling the rewarding impact of a variety of normally powerful rewarding events. It seems likely that this synapse lies at a critical junction between the branches of the sensory pathways which carry signals of the intensity, duration, and quality of the stimulus, and the motivational pathways where these sensory inputs are translated into the hedonic messages we experience as pleasure, euphoria, or ‘yumminess’” [96]. While this remains an appropriate description of the role of the reward circuitry in encoding hedonic tone, an extraordinary amount of electrophysiological, neurochemical, and behavioral neuroscience research over the last 20 years has made it apparent that the crucial nucleus accumbens reward neurons do more than encode receipt of reward, although encoding receipt of reward is certainly one of their functions [97]. They appear also to encode expectancy of reward [98,99], amount of reward [98], delay of reward [100], reward-delay discounting [101], and errors in reward prediction [102]; to regulate motivation for drug-seeking behavior [103]; and to contribute to the synaptic neuroplasticity that underlies the acquisition of addictive behavior patterns [104].
Genetic and Experiential Contributions to the Disease of Addiction
As noted above, it is relatively easy to selectively breed laboratory animals for the behavioral phenotype of drug-seeking behavior (the behavioral phenotype breeds true after about 15 generations in laboratory rodents). At the human level, family, adoption, and twin studies have consistently demonstrated a substantial genetic influence in the development of drug addiction - with inherited risk estimates ranging between 40% and 60% [105,106]. The exact role of individual genes in this complex disease is not yet fully characterized, but the contribution of genetics to addiction is generally thought to be polygenic, with multiple low-impact genes combining to create the genetic vulnerability [107]. Just as roughly 50% of the vulnerability to addiction is believed to be genetic, so too is roughly 50% believed to be experiential. This should not be conceptualized as an “either-or” situation. It is not an question of biology versus environment. Genetic and experiential factors operate together to produce the behavioral phenotype of addiction. Furthermore, substantial evidence exists that environmental and social factors can influence the neurobiological substrates of addiction, including those referable to the functioning of the brain’s reward circuitry (see below).
Contributions to Addiction Vulnerability at the Animal Level
Considerable research has been devoted to identifying individual characteristics that predict high vulnerability to drug-seeking behavior in animals, with considerable success. Notable work in this area has been published by Le Moal, Piazza, and Simon of the Université of Bordeaux II in France, and by Everitt and Robbins of the University of Cambridge in the UK. Among the individual characteristics that have been found to predict high vulnerability to drug-seeking behavior are high reactivity to stress, high novelty-induced locomotor activity, high novelty-seeking, and high trait impulsivity [78,108–110]. Provocatively, transgenerational effects of stress on drug-seeking behavior have been found - the adult offspring of stressed mothers showing increased locomotor responses to novelty and increased propensity to self-administer addictive drugs [109].
Contributions to Addiction Vulnerability at the Human Level
A wide variety of individual characteristics that predict high vulnerability to drug addiction at the human level have also been identified. Among these are sensation-seeking and novelty-seeking [111,112]; trait impulsivity [113,114]; antisocial conduct disorder, especially in adolescence [115]; depression, which confers a 6-fold increased risk for drug or alcohol addiction [116,117]; and attention deficit/hyperactivity disorder (ADHD) [118,119]. In addition (as noted above), Blum and colleagues [66,67,70] have proposed that a reward deficiency syndrome referable to functional hypoactivity of dopaminergic brain reward substrates contributes significantly to addiction vulnerability at the human level. Also, Koob and colleagues have proposed that a lack of homeostatic reward regulation, again referable to aberrant functionality of dopaminergic brain reward substrates, contributes significantly to addiction vulnerability in humans [120–122]. Some of these human vulnerability factors to addiction have been successfully modeled at the animal level [123].
Neurobiologically-Measured Aberrations in Brain Reward Function as Addiction Vulnerability Factors
Volkow and colleagues have carried out positron emission tomography studies of dopaminergic function in brains of awake human subjects, using displacement of [11C]raclopride as a measure of extracellular dopamine. They reported a noteworthy finding - that healthy adult male subjects with robust striatal dopamine levels experience the psychostimulant methylphenidate as displeasurable while subjects with deficits in striatal dopamine experience methylphenidate as pleasurable [73,75]. This is intensely interesting and provocative, as the nucleus accumbens reward-relevant brain locus is located in the ventral striatum. Such findings suggest that striatal dopamine deficiency may constitute a vulnerability factor for addiction, which is conceptually congruent with Blum’s [66,67,70] formulation of reward deficiency as an addiction risk factor.
Using positron emission tomography and displacement of [18F]fluoroclebopride as a measure of extracellular dopamine in monkeys, Nader and colleagues [77] have reported provocative findings linking striatal dopamine, social rank within monkey troops, addictive drug exposure, and vulnerability to addiction. This is extremely important work, as it explicates the biopsychosocial model of addiction with undeniably robust neurobiological measures of functionality within the brain’s reward circuitry. First, Nader and colleagues [77] found that individually-housed socially dominant monkeys show more robust levels of striatal dopamine than individually-housed socially submissive monkeys - raising the possibility that the submissive animals may have increased risk for drug-seeking behavior. Second, Nader and colleagues found that these differences in striatal dopamine levels between dominant and submissive monkeys are accentuated in socially-housed monkeys. Third, Nader and colleagues found that the submissive monkeys are, in fact, more drug-seeking and drug-taking - showing a robust elevation and right-shift in the dose-response curve for self-administered cocaine [77].
Thus, submissiveness in a social hierarchy is correlated with dopamine deficiency in the reward circuitry of the brain, and both are correlated with enhanced drug-seeking and drug-taking behavior. Somewhat to the same point, Robbins and Everitt and colleagues have shown that isolation rearing of rats impairs reinforcing efficacy of intravenous cocaine and intra-accumbens amphetamine [124]. Nader and colleagues have also shown pronounced striatal dopamine deficiencies in cocaine-experienced monkeys as compared to cocaine-naive monkeys [125]. Other workers, using positron emission tomography and displacement of [11C]raclopride as a measure of extracellular striatal dopamine in both cats and monkeys have similarly shown dopamine deficiencies in amphetamine-experienced animals as compared to amphetamine-naive animals [126,127].
Additionally, Everitt and colleagues have shown reduced dopamine D2/D3 receptor binding in nucleus accumbens of drug-naive trait-impulsive laboratory rats [78]. Relating this to addiction vulnerability, these workers have shown that the high impulsive rats are significantly more drug-seeking than the non-impulsive rats [78,128].
Furthermore, Everitt and Robbins and colleagues have shown that high reactivity to novelty predicts the propensity to initiate drug self-administration, while high impulsivity predicts the propensity to progress from simple drug-taking behavior to addiction-like drug-taking behavior - characterized by persistent and compulsive drug-taking in the face of aversive consequences [129].
This body of work is remarkable for tying together behavioral traits (impulsivity, high reactivity to novelty), social factors (dominance versus submission), prior drug experience (cocaine or amphetamine exposure versus non-exposure), dopamine functionality within the brain’s reward circuitry (measured by dopamine displacement studies), and drug-seeking behavior. The biopsychosocial model of addiction seems well supported by such work, using laboratory animal models.
The Natural Progression of the Disease of Addiction
The disease of addiction is characterized by progressive stages. It always starts with occasional reward-driven use, which then progresses to steady (albeit non-addictive) reward-driven use [130–132]. Reward-driven use progresses to habit-driven use, and habit-driven use progresses to compulsive use [24,133–136]. At the human level, there is often psychological denial of aberrant or self-damaging behavior [137] coupled with impaired insight [138] - often lasting for years. Addictive, abusive, self-damaging drug use coupled with psychological denial is then - for some addicts - followed by “bottoming out” (loss of spouse, family, employment, home, car, possessions). For a fortunate subset of addicts, this is followed by treatment and achievement of abstinence. However, in most cases there is persistent vulnerability to drug-craving and persistent vulnerability to relapse to drug-seeking behavior [134,139–141].
Although the neurobiological substrates of the various stages of disease progression are not fully understood, Robbins and Everitt [142] have proposed that the progression of addiction from reward-driven behavior to habit-driven behavior correlates with a progression of the controlling neurobiological locus of the disease from ventral striatum (nucleus accumbens) to dorsal striatum. They base their hypothesis on several factors - the involvement of the dorsal striatum in habit formation, the phenomenon of Pavlovian-to-Instrumental transfer, and the existence of an anatomic ascending spiral of striato-nigral-striato loop pathways from the nucleus accumbens shell to the dorsolateral striatum. The first factor - involvement of dorsal striatum in stimulus-response (S-R) associations or habits - has been long recognized [143]. The second factor - Pavlovian-to-Instrumental transfer - is a learning phenomenon that sheds light on the interrelationships between the reward circuitry of the brain, habit learning, and addictive behavior. In Pavlovian-to-Instrumental transfer, animals are first trained to associate a conditioned stimulus with a reward (Pavlovian learning). The animals are then trained to perform an instrumental task (e.g., pressing a wall-mounted lever in their test chambers) to receive the same reward (Instrumental learning). The animals are then exposed to extinction of the instrumental learning (i.e., the instrumental response is no longer rewarded). Presentation of the conditioned stimulus enhances instrumental responding during extinction - thus modeling an important component of addictive behavior (i.e., the ability of drug-associated environmental cues to drive drug-seeking behavior in the absence of drug-induced reward). Provocatively, lesions of the nucleus accumbens core and the central nucleus of the amygdala abolish Pavlovian-to-Instrumental transfer, while lesions of the nucleus accumbens shell and the basolateral amygdala have no effect [142]. Pharmacologically, dopamine D2/D3 receptor antagonism abolishes Pavlovian-to-Instrumental transfer, while dopamine agonists potentiate it [142]. The third factor - an ascending spiral of striato-nigral-striato loop pathways from the nucleus accumbens shell to the dorsolateral striatum - has been demonstrated anatomically by Haber and colleagues [144].
Putting all of these factors together, Robbins and Everitt [142] propose that “Compulsive drug-seeking behavior is inflexible, since it persists despite considerable cost to the addict, becomes dissociated from subjective measures of drug value, becomes elicited by specific environmental stimuli, and involves complex goal-directed behaviors for procurement and self-administration of drugs. Limbic cortical-ventral striatopallidal circuits that underlie goal-directed drug-seeking actions may eventually consolidate habitual, S-R drug seeking through engagement of corticostriatal loops operating through the dorsal striatum. This progression from action to habit may have its neural basis within the ‘spiraling’ loop circuitry of the striatum, by which each striatal domain regulates its own DA [dopaminergic] innervation and that of its adjacent domain in a ventral-to-dorsal progression [144]. Thus, the NAc [nucleus accumbens] shell regulates its own DA innervation via projections to the VTA [ventral tegmental area] and also that of the NAc core. The NAc core in turn regulates its own DA innervation via projections to the VTA and also that of the next, more dorsal tier of the dorsal striatum via projections to the substantia nigra pars compacta and so on. Chronically self-administered drugs, through their ability to increase striatal DA, may consolidate this ventral-to-dorsal striatal progression of control over drug-seeking as an habitual form of responding” [142,145]. This proposal is congruent both with neuroimaging studies [25,146–148] and with electrophysiological studies [149].
Addiction and Physical Dependence - Different Phenomena and Different Brain Substrates
It is very important to realize that addiction and physical dependence are different phenomena with different underlying brain substrates [3]. Physical dependence results from the development of pharmacological tolerance, and manifests itself upon abrupt discontinuation of drug administration (or administration of an antagonist drug). Addiction is a chronic progressively deteriorating disease characterized by compulsive drug use in the presence of harm to the addict and to the addict’s life. Addiction is commonly described as the “Disease of the 5 Cs” - Continued Compulsive drug use despite injurious Consequences, coupled with loss of Control and persistent drug Craving [3].
Many drugs (including, but not limited to, antihypertensives, cardiac medications, and asthma medications) produce pronounced physical dependence but are not addictive. Some drugs (e.g., cocaine) are highly addicting but produce little or no physical dependence.
Furthermore, laboratory animals will avidly self-administer addictive drugs in the absence of tolerance, physical dependence, or withdrawal discomfort [1,3]. Indeed, laboratory animals will avidly self-administer addictive drugs in the absence of any prior drug exposure whatever, rendering the issue of self-administration because of physical dependence moot. The importance of this fact can hardly be overstated, as it unambiguously shows that drug-taking behavior cannot be explained simply by the ability of addictive drugs to ameliorate the withdrawal discomfort associated with abstinence from prior administration of such drugs [1,3].
Critically, the brain sites mediating volitional drug self-administration are different from those mediating the development and expression of physical dependence. This dissociation of brain substrates and loci subserving addiction and physical dependence was first demonstrated by Bozarth and Wise [150] in a pioneering and now classic series of experiments using direct intracerebral microinjections of opiates into specific brain loci in awake behaving animals. They found that laboratory rats willingly (indeed, avidly) self-administer opiates into the brain’s reward loci, but that such self-administration (even if prolonged) does not evoke opiate physical dependence. Conversely, they found that opiate microinjection into more posterior brainstem loci (in the vicinity of the dorsal raphé nucleus) produces strong opiate physical dependence (and strong physical withdrawal symptoms upon abrupt discontinuation), but that animals do not self-administer opiates into those loci. This double dissociation of brain loci is definitive with respect to addiction and physical dependence being different phenomena.
Equally importantly, the different brain sites mediating volitional drug self-administration and physical dependence are, in turn, different from those mediating the antinociceptive properties of opiates. This additional dissociation of brain substrates and loci was first demonstrated by Pert and Yaksh [151] in an equally pioneering and now classic series of experiments using direct intracerebral microinjections of opiates into specific brain loci in awake behaving monkeys. The monkeys were fitted with an electrical plate on one foot, through which painful electrical stimulation was given. Each monkey had a manipulandum (a lever mounted on the front of its primate test chair) that it could depress to lower the intensity of the painful stimulation. Each monkey also had many surgically-implanted intracerebral cannulae through which morphine could be microinjected by the researchers. Hundreds of brain loci were tested for their ability to induce analgesia to the painful foot-shock. Only two analgesic loci were found. One corresponded to the periaqueductal gray matter of the brain stem, and the second was in the vicinity of the lateral neospinothalamic pathway as it ascends through the lateral midbrain and into the periventricular and intralaminar nuclei of the thalamus [151]. From these brain loci, especially the periaqueductal gray area, descending neuronal tracts run down the spinal cord to synapse within the dorsal horns - to activate synaptic pain gates [152–154], blocking ascending nociceptive neural signals and producing analgesia.
Thus, a triple dissociation manifests itself. The brain loci mediating opiate-seeking behavior are distinct from those mediating opiate-induced physical dependence, which are in turn distinct from those mediating opiate-induced analgesia. Further discussion of brain mechanisms and analgesia - and the false notion that medically appropriate opiate analgesia can induce opiate addiction - will be addressed below.
Persistent Drug Craving and Relapse - The Real Clinical Problem in Addiction
Any cigarette smoker who has tried to quit the smoking habit knows that the real clinical problem in addiction is persistent drug craving and relapse. As Mark Twain (Samuel Clemens), the famous 19th century American author and humorist phrased it - “It’s easy to quit smoking. I’ve done it hundreds of times.” Behind this amusing aphorism is a terrible truth - it is extremely difficult to overcome the persistent drug cravings that the abstinent addict is left with after achieving (often with great difficulty) abstinence. This is why acute “detoxification” programs are almost invariably clinical failures in treating drug addiction. Indeed, the failure rate is so high that physicians running such programs may be reasonably said to be engaging in medical malpractice.
Since the founding of the Alcoholics Anonymous organization in the 1930s and the publication of its so-called “Big Book” [155] it has been recognized that there are three classical triggers to drug craving (and relapse to drug-seeking behavior) in recovering drug addicts - re-exposure to drug, exposure to stress, and re-exposure to environmental cues previously associated with drinking or drugging.
In recent decades, the development of animal models of relapse have permitted a remarkable amount of research to be carried out on the neural substrates of craving and relapse. The first such model is the so-called “reinstatement” model [46,156,157]. This model is built upon the intravenous drug self-administration animal model in which animals are intravenously catheterized and allowed to voluntarily self-administer addictive drugs. To model relapse, the animals are first allowed to self-administer drug until highly stable day-to-day drug-taking behavior is achieved. Then, the addictive drug is removed from the infusion pump and saline (or vehicle) is substituted. The drug-taking behavior extinguishes due to lack of reinforcement. The animal is then tested daily until highly stable day-to-day non-responding is achieved. Then the animal is exposed to relapse triggers (drug, stress, cues) and amount of drug-seeking responding (on the manipulandum which previously activated the infusion pump and delivered the addictive drug) is measured - such responses not being reinforced by drug. A second relapse model is the so-called “reactivation” model [45,46,158], which is built upon the conditioned place preference animal model of drug-seeking behavior (see above). To model relapse in this model, the animal is allowed to acquire a conditioned place preference to an addictive drug. Then the animal is exposed to the conditioned place preference test chamber (with open access to all compartments) repeatedly, day after day, until the drug-induced place preference is fully extinguished. Then, on test day, the animal is exposed to relapse triggers and amount of drug-seeking behavior is measured as amount of time spent in the previously drug-paired compartment. Both of these models yield remarkably robust relapse to drug-seeking behavior with seemingly high face, construct, and predictive validity to human relapse [159,160].
Neuroanatomy and Neurochemistry of Brain Circuits Mediating Relapse
By a variety of research strategems - including combining intracerebral microinjections or anatomically discrete intracerebral lesions with one of the animal relapse models outlined above - it has been possible to discover the brain circuits underlying relapse to drug-seeking behavior triggered by the three classical relapse triggers.
Relapse to drug-seeking behavior triggered by noncontingent re-exposure to drug is mediated by the dopaminergic medial forebrain bundle tract linking the ventral tegmental area with the nucleus accumbens [46,156,157,161].
Relapse to drug-seeking behavior triggered by stress appears to involve two separate neural circuits in the brain. The first such circuit originates in the lateral-tegmental noradrenergic cell groups (especially nucleus A2 as described by Moore and Bloom [162]) and projects anteriorly to synapse in the hypothalamus, nucleus accumbens, amygdala, and bed nucleus of the stria terminalis [156,157,161]. This relapse circuit uses norepinephrine as its major neurotransmitter. In view of the major role that the noradrenergic locus coeruleus plays in brain-mediated stress events [see, e.g., 163], it is somewhat counterintuitive that the A2 lateral-tegmental nucleus, rather than the locus coeruleus, should be the major noradrenergic brain locus mediating stress-triggered relapse to drug-seeking behavior, but this seems to be the case. The second neural circuit mediating stress-triggered relapse to drug-seeking behavior originates in the central nucleus of the amygdala and projects to the bed nucleus of the stria terminalis. This neural circuit uses corticotrophin-releasing factor (CRF) as its major neurotransmitter.
Relapse to drug-seeking behavior triggered by environmental cues previously paired with drinking or drugging also appears to involve two separate neural circuits in the brain - one originating in the ventral subiculum of the hipppocampus and one originating in the basolateral complex of the amygdala. Both such circuits use glutamate as their major neurotransmiiter. In a pioneering series of experiments, Gardner and colleagues showed that discrete low-level electrical stimulation of these circuits triggers relapse to drug-seeking behavior, and demonstrated that these relapse circuits are glutamaterically mediated [164,165].
Knowing the exact neural circuits involved in mediating relapse to drug-seeking behavior, and their respective neurotransmitters, opens up the distinct possibility of being able to develop anti-craving and anti-relapse medication using medication development strategies that are highly targeted on specific neurobiological substrates of relapse [166–168].
Incubation of Craving - An Animal Model of the Extreme Fragility of Control Over Vulnerability to Relapse in Human Addicts
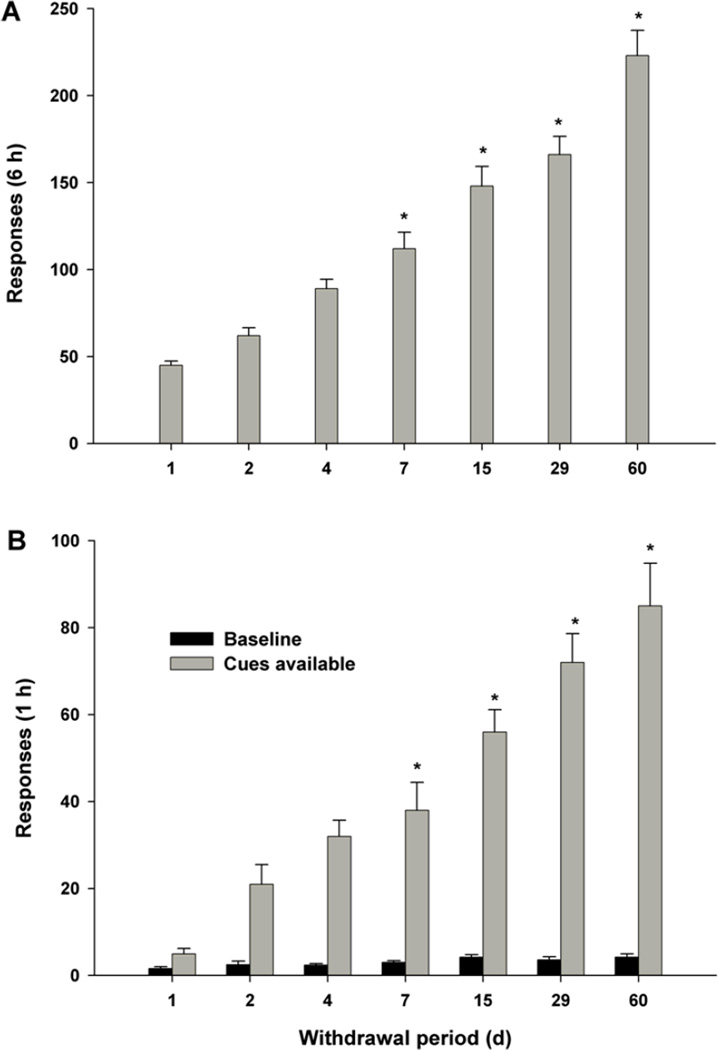
In 2001, Grimm and colleagues described a compelling phenomenon at the laboratory animal level - that relapse vulnerability to drug-seeking behavior incubates (grows more intense) with the mere passage of time [169]. In this “incubation of craving” model, animals are allowed to self-acquire the intravenous drug-taking habit. Once stable drug-taking is established, the animals are returned to their home cages, where they simply wait for varying amounts of time (days, weeks, months). Importantly, nothing is done to the animals during this waiting period. They are not re-exposed to drug nor to stress nor to any drug-associated environmental cues, nor are they exposed to deliberate extinction of the drug-taking habit. Then, on test day, they are returned to the test chamber in which they originally acquired the drug-taking habit and allowed access to the manipulandum (typically, a wall-mounted lever or a nose-poke detector device) that originally activated the infusion pump to deliver the drug. They are then tested for drug-seeking behavior (lever-pressing, nose-poking) in the absence (extinction testing) or presence (cue-triggered testing) of the environmental cues originally associated with the drug-taking behavior. Remarkably, a time-dependent increase (“incubation”) in the intensity of drug-seeking behavior - whether measured as extinction responding or cue-triggered responding - is observed. After 4 days, drug-seeking behavior is approximately 100% greater than after 1 day. After 15 days, drug-seeking behavior is approximately 200% greater than after 1 day. After 60 days, drug-seeking behavior is approximately 400% greater than after 1 day. This remarkable increase in vulnerability to relapse to drug-seeking behavior with the mere passage of time is considered to be an animal model of the extreme fragility of control over relapse vulnerability, and the increase in this vulnerability with the passage of time that is so frequently reported by human addicts.
The same researchers subsequently found that this incubation of craving phenomenon corresponds to a post-cocaine time-dependent increase in brain-derived neurotrophic factor (BDNF) protein levels within the mesolimbic dopaminergic reward and relapse circuits - specifically in the nucleus accumbens and amygdala [170]. This research group also explored the role of the amygdaloid extracellular signal-regulated kinase (ERK) signaling pathway in this incubation [171]. Cocaine seeking induced by exposure to cocaine cues was substantially higher after 30 days of cocaine withdrawal than after 1 day. Exposure to these cues increased ERK phosphorylation in the central, but not the basolateral, amygdala after 30 days of cocaine withdrawal, but not after 1 day. After 30 days of incubation of cocaine craving, inhibition of central (but not basolateral) amygdaloid ERK phosphorylation produced an inhibition of cocaine-seeking behavior. After 1 day of withdrawal, stimulation of central amygdaloid ERK phosphorylation increased cocaine-seeking behavior. All of this suggests that incubation of cocaine craving is mediated by time-dependent increases in the responsiveness of the central amygdaloid ERK pathway to cocaine cues. Central amygdaloid glutamate is also involved [172]. Central amygdala injections of the glutamate mGluR2/3 agonist LY379268 (which decreases glutamate release) attenuated the augmented cue-triggered drug-seeking behavior seen after 21 days of incubation but had no effect after only 3 days of incubation. As the amygdala is critical to cue-triggered relapse [165] and the nucleus accumbens to drug- and stress-triggered relapse [156,157,161,173], these findings are important.
They are especially important in the context of synaptic remodeling. Addiction obviously involves learning (stimulus-reward learning being an important substrate in the acquisition of addiction; stimulus-response learning being an important substrate in relapse responding). Beginning with the pioneering neuropsychological work of Hebb in the 1940s [174], it has been widely appreciated that the neural substrate of learning must involve synaptic remodeling. In recent decades it has come to be appreciated that two important mechanistic substrates of synaptic remodeling are long-term potentiation (LTP) and long-term depression (LTD) [175]. LTP and LTD have been demonstrated to be produced by addictive drugs in precisely those brain loci implicated in reward and relapse - nucleus accumbens [176–179], amygdala [180,181], and hippocampus [182–184]. As a neuronal trophic factor, BDNF may be involved in - indeed, may be an underlying mechanism for - the synaptic remodeling in the nucleus accumbens, amygdala, and hippocampus that may underlie the incubation of craving phenomenon and, by extension, the development of increased vulnerability to relapse among human addicts. Furthermore, such synaptic remodeling may underlie the very transition to the unregulated and compulsive drug-seeking behavior that characterizes addiction, inasmuch as this transition is associated with impaired glutamate receptor-dependent brain LTD [185]. If this be true, an entirely new strategy for anti-addiction, anti-craving, anti-relapse medication development could emerge - medications that target BDNF-mediated synaptic remodeling in the nucleus accumbens, amygdala and hippocampus. Of course, such medication development need not be mutually exclusive to anti-craving and anti-relapse medication development predicated on other neural mechanistic strategies - e.g., glutamate mGluR2/3 agonists [172] or selective dopamine D3 receptor antagonists [83,84,186].
The Disentanglement of Important Addiction-Related Phenomena
From all of the above, it may be appreciated that a number of important phenomena are often confused, even among medical and other professionals who deal routinely with drug addiction and with patients impacted by it. It is important to disentangle these phenomena.
First, abusive drug-taking behavior is initially reward-driven (the drug-induced “high”), and is directly referable to drug-induced activation of the ventral tegmental area-nucleus accumbens reward circuitry.
Second, abusive drug-seeking and drug-taking behavior becomes, with time, habit-driven rather than reward-driven. This transition arguably defines the onset of addictive drug-seeking behavior, and is arguably referable to a transition of the neural locus of control over drug-seeking behavior from the ventral tegmental-ventral neostriatal axis (medial ventral tegmental area and nucleus accumbens) to a more dorsolateral overlying set of neural loop circuits (involving the substantia nigra and dorsolateral neostriatum).
Third, addiction and physical dependence are different phenomena - the first referable to forebrain mesolimbic and mesostriatal reward-related and habit-related circuitry; the second referable to neural loci in the vicinity of the midbrain locus coeruleus and the dorsal mesencephalon. Some drugs are addictive without producing physical dependence; other drugs produce physical dependence without being addictive.
Fourth, opiate- or cannabinoid-induced analgesia are distinct phenomena - having nothing to do with opiate-induced or cannabinoid-induced addictive drug-taking behavior. Opiate-induced analgesia is referable to action on neural mechanisms on peripheral nociceptive neurons, spinal cord dorsal horn pain-gates, dorsal root ganglia, brain-stem tegmental spinothalamic and neospinothalamic pain relay nuclei, and the periaqueductal gray matter of the brain-stem. Cannabinoid-induced analgesia is referable to action on neural mechanisms on peripheral nociceptive neurons, spinal cord dorsal horn pain-gates, dorsal root ganglia, and brain-stem tegmental spinothalamic and neospinothalamic pain relay nuclei. Importantly, appropriate medical use of opiates or cannabinoids for the treatment of pain carries extremely low risk of inducing addiction (see further discussion below).
Can One Induce Addiction by Long-Term Treatment of Pain with Opiates?
Far too many physicians and other health care professionals have uncritically accepted the false allegation that opiate addiction can be induced by medically appropriate long-term treatment of pain with opiates [186]. This leads to medical malpractice and the ethically unacceptable undertreatment of pain in millions of suffering patients. Equally unacceptably, this misconception permeates the law-enforcement, judicial, and legislative branches of government - with many egregious consequences. It also permeates ordinary society, causing many pain patients to refuse medically appropriate (indeed, often essential) treatment of pain - with often horrible consequences for quality of life.
The truth of the matter is that, although some chronic pain patients are at risk for addiction, they are a very small percentage of the total number of chronic pain patients.
Reliable evidence exists to support the contention that appropriate medical treatment of pain with opiates does not incur a risk of addiction in the vast majority of pain patients. First, chronic pain inhibits opiate-seeking behavior in animal models [186,187]. Second, chronic pain inhibits opiate-enhanced dopamine in the ventral tegmental area-nucleus accumbens reward/relapse neural circuitry [186,188]. Third, chronic pain inhibits reward signaling through the ventral tegmental area-nucleus accumbens reward circuitry, as assessed using the electrical brain-stimulation reward animal model [186,189]. Fourth, chronic pain inhibits the development of opiate-induced physical dependence [186,190]. These preclinical animal model and neurobiological data - together with an impressive corpus of human clinical data - has prompted the World Health Organization to issue the following guideline on the treatment of chronic pain - “When opioids are used - even at heroic doses - in the appropriate medical control of chronic pain, addiction and drug abuse are not a major concern” [191].
Brain Reward/Anti-Reward Pathways and Mechanism-Based Medication Development for Treatment of Addiction
At present, a number of effective pharmacotherapies exist for the treatment of drug addiction. The opiate agonist methadone has been shown to be effective for opiate addiction [192,193], as has the partial opiate agonist buprenorphine [192–194]. Even heroin itself has been used successfully as a maintenance pharmacotherapy for opiate addiction [195]. Although heroin’s short half-life makes it a less than optimal choice for maintenance therapy for many patients, there appears to be a subset of opiate addicts who do better on heroin maintenance than on other opioid agonist therapies [195]. For highly motivated individuals with a strong desire to achieve abstinence from the opiate-taking habit, the long-acting opiate antagonist naltrexone has proven effective [193,196].
Naltrexone has also proven to be effective for some alcoholics wishing to quell alcohol cravings [196–198]. The reason for naltrexone’s effectiveness in alcoholism is not clearly understood, but may relate to the fact that the dopaminergic reward circuitry of the brain is heavily innervated by endogenous opioid peptide (endorphin and enkephalin) circuits, and this opioid peptidergic innervation is known to modulate the reward enhancing properties of alcohol [199–201] and other addictive compounds (e.g., delta-9-tetrahydrocannabinol, the addictive constituent in marijuana and hashsish; see [202–204]).
Acamprosate is also claimed to have effectiveness against alcohol addiction [205], as is the GABA-B receptor agonist baclofen [206–208]. The underlying mechanisms for acamprosate’s putative effectiveness appear to involve re-normalization of alcohol-perturbed glutamatergic neurotransmission [209]. The underlying mechanisms for baclofen’s effectiveness are not fully clear, but baclofen’s presumptive effectiveness at the human level is amply and well supported by many years of work with baclofen in preclinical animal models of addiction by Roberts and colleagues [210–215]. This work with baclofen in preclinical animal models of addiction suggests a broad potential clinical efficacy for baclofen in treating addiction at the human level, but thus far only preliminary studies on human patients have been undertaken with baclofen [208].
The acetylcholine nicotinic α4β2 receptor partial agonist/α7-receptor full agonist varenicline is clearly effective for treating nicotine addiction at the human level [216,217]. Furthermore, varenicline appears superior to other treatments - the one-year continuous abstinence rate from tobacco use is 23% for varenicline, as compared to 15% for bupropion and 10% for placebo [216]. Varenicline’s effectiveness appears to be due to its action at the nicotinic α4β2 receptor subtype; its action at the α7-receptor subtype appears irrelevant to its efficacy [218]. Nicotine replacement therapy (e.g., the nicotine patch) is also effective in some patients for smoking cessation. Bupropion also appears to have efficacy in treating nicotine addiction [219], which may be partly due to bupropion’s action as a dopamine reuptake inhibitor [220] and partly due to its action as a nicotinic receptor antagonist [221].
Yet, none of the above-listed pharmacotherapies is effective for a majority of the patients for whom the therapy should produce clinical benefit, and with the possible exception of baclofen, none of the above therapies is broadly effective against multi-drug addiction.
Thus, there is real need for further medication development in the field of addiction medicine. Fortunately, by reference to current knowledge of the involvement of brain reward/anti-reward pathways and substrates in addiction, mechanism-based medication development for the treatment of addiction is both rational and practical.
The fact that cocaine and other psychostimulants derive their addictive efficacies from inhibiting the synaptic reuptake of dopamine or by causing presynaptic release of dopamine in the reward-related and relapse-related nucleus accumbens makes the development of slow-onset long-acting dopamine transporter inhibitors for treatment of psychostimulant addiction rational [222–226].
The fact that inhibition of medium spiny GABAergic neurons in the nucleus accumbens may constitute a final common pathway for addictive-drug-induced reward [227] makes development of GABAergic agonist therapies rational. As noted above, extraordinary promise has been seen with the GABA-B receptor agonist baclofen in preclinical animal models [210–215], with some supporting evidence from preliminary human use [206–208]. Similarly, considerable promise has been seen with the indirect GABA agonist gamma-vinyl GABA in preclinical animal models [228–237], again with some supporting evidence from preliminary human trials [238].
The fact that cannabinoids activate, and endocannabinoids mediate, brain reward and brain relapse circuits and substrates [202,203,205,239–247] provides a mechanistic rationale for the development of cannabinoid CB1 receptor antagonists as anti-addiction medications [40,248– 252].
The facts that glutamate circuits innervate and modulate brain reward mechanisms [253–260], and that glutamatergic circuits mediate at least some forms of relapse to drug-seeking behavior [164,165], make the development of drugs acting on the glutamate circuitry of the brain attractive as potential anti-addiction medications [41,261–265].
The fact that CRF is the neurotransmitter of one of the stress-triggered relapse circuits in the brain makes the development of anti-CRF medications attractive [167,266–278].
A Final Comment
Addiction medicine has made enormous strides in recent decades. Arguably, addiction medicine has animal models with face-validity, predictive-validity, and construct-validity that are as good or better than animal models in other medical specialties. More has been learned in the last few decades about the neurobiology of addiction, craving, and relapse than would have been thought possible when Olds and Milner first described the reward circuits of the brain in 1954 [6]. These tremendous advances in knowledge bode well for the development of definitive therapies for an illness that is as old as recorded human history.
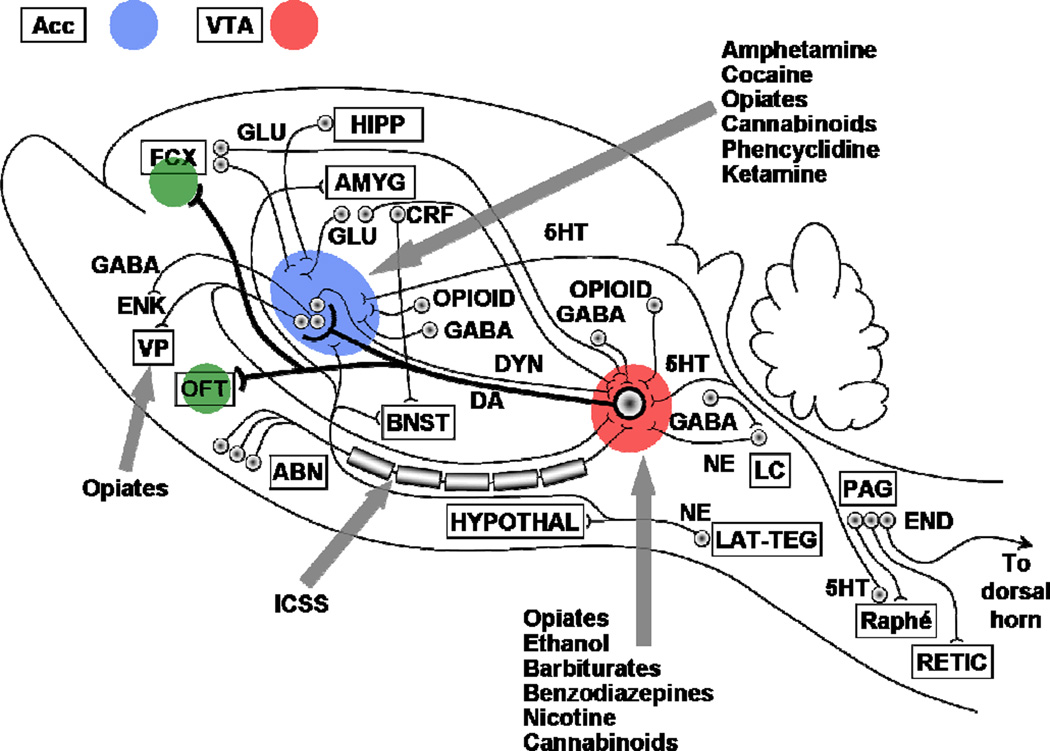
Fig. 1. BRAIN REWARD CIRCUITRY.
Diagram of the brain-reward circuitry of the mammalian (laboratory rat) brain, with sites at which various addictive drugs act to enhance brain reward mechanisms and thus to produce drug-seeking behavior, drug-taking behavior, drug-craving, and relapse to drug-seeking behavior. ABN, anterior bed nuclei of the medial forebrain bundle; Acc, nucleus accumbens; AMYG, amygdala; DA, subcomponent of the ascending mesocorticolimbic dopaminergic system that appears to be preferentially activated by addictive drugs; DYN, dynorphinergic neuronal fiber bundle outflow from the nucleus accumbens; ENK, enkephalinergic neuronal fiber bundle outflow from the nucleus accumbens; FCX, frontal cortex; GABA, GABAergic inhibitory fiber systems synapsing in the ventral tegmental area, the nucleus accumbens, and into the vicinity of the locus coeruleus, as well as the GABAergic neuronal fiber bundle outflow from the nucleus accumbens; GLU, glutamatergic neural systems originating in frontal cortex and synapsing in both the ventral tegmental area and the nucleus accumbens; 5HT, serotonergic (5-hydroxytryptamine) fibers, which originate in the anterior raphé nuclei and project to both the cell body region (ventral tegmental area) and terminal projection field (nucleus accumbens) of the DA reward neurons; ICSS, the descending, myelinated, moderately fast conducting component of the brain-reward circuitry that is preferentially activated by electrical intracranial self-stimulation (electrical brain-stimulation reward); LC, locus coeruleus; NE, noradrenergic fibers, which originate in the locus coeruleus and synapse into the general vicinity of the ventral mesencephalic neuronal cell fields of the ventral tegmental area; Opioid, endogenous opioid peptide neural systems synapsing into both the ventral tegmental DA cell fields and the nucleus accumbens DA terminal projection loci; Raphé, brainstem serotonergic raphé nuclei; VP, ventral pallidum; VTA, ventral tegmental area. After [1].
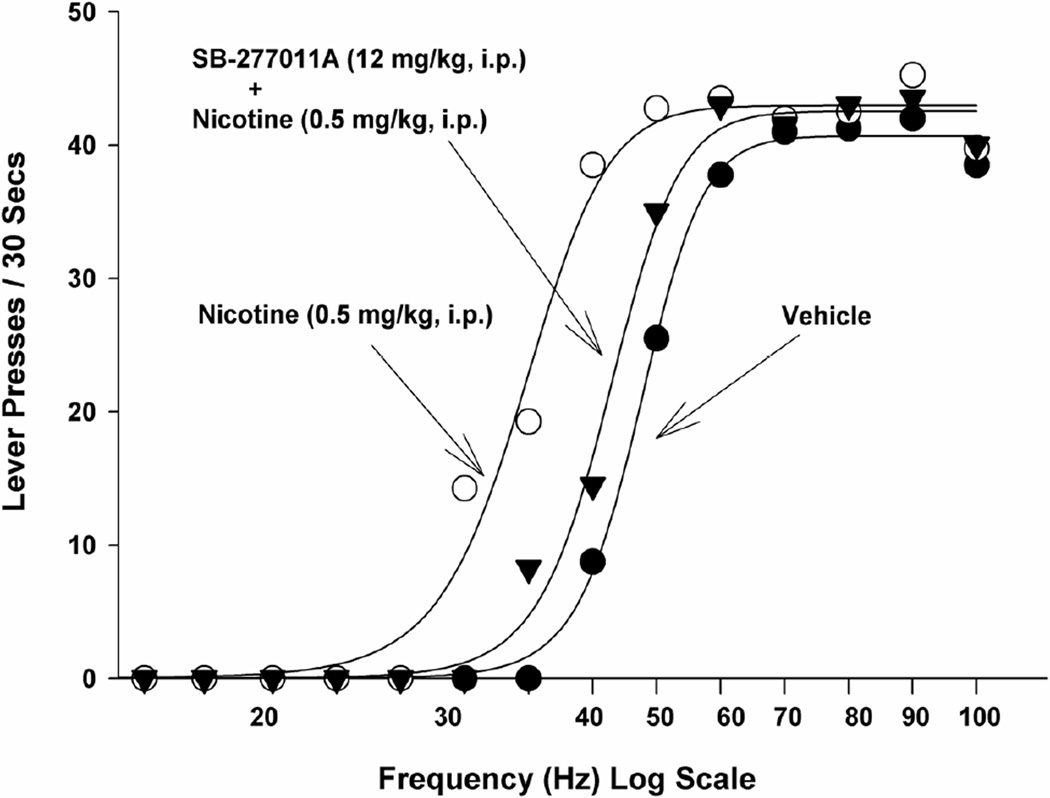
Fig. 2. LEFT SHIFT WITH ADDICTIVE DRUG, COUNTERED BY SELECTIVE D3 RECEPTOR ANTAGONIST.
Enhancement of electrical brain-stimulation reward by an addictive drug (nicotine) and attenuation of that enhancement by a highly selective dopamine D3 receptor antagonist (SB-277011A). Left-shifts in such rate-frequency brain-reward functions denote enhancement of brain reward (the drug-induced “high”); right-shifts denote inhibition of brain reward. After [38].
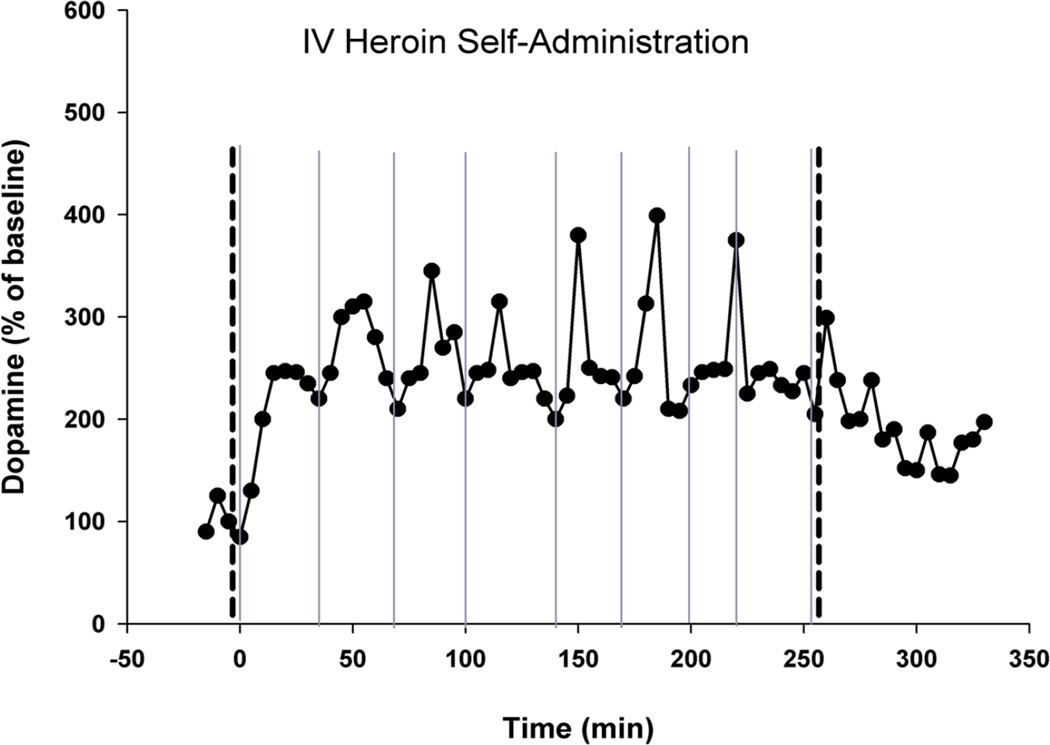
Fig. 3. REAL-TIME DOPAMINE BRAIN MICRODIALYSIS DURING INTRAVENOUS OPIATE SELF-ADMINISTRATION.
Minute-by-minute measurements - using in vivo brain microdialysis - of extracellular nucleus accumbens dopamine in a laboratory rat voluntarily self-administering intravenous heroin. The thick dashed vertical lines indicate the start (left thick dashed vertical line) and end (right thick dashed vertical line) of availability of heroin. The thin solid vertical lines indicate a voluntary self-administration of heroin (a heroin “hit”) by the test animal. At the beginning of every drug self-administration session, extracellular nucleus accumbens dopamine is tonically enhanced approximately 200% by the initial “hit(s)” of heroin. Thereafter, extracellular nucleus accumbens dopamine fluctuates phasically - with the animal becoming motivated to give itself another drug “hit” whenever extracellular nucleus accumbens dopamine drops to approximately 100% over its initial pre-heroin level. Thus, the animal appears to self-administer the addictive drug to enhance extracellular nucleus accumbens dopamine and to keep it within a preferred (and elevated) range – thus keeping hedonic tone enhanced over its normal basal state when unaffected by addictive drugs. After [49,50].
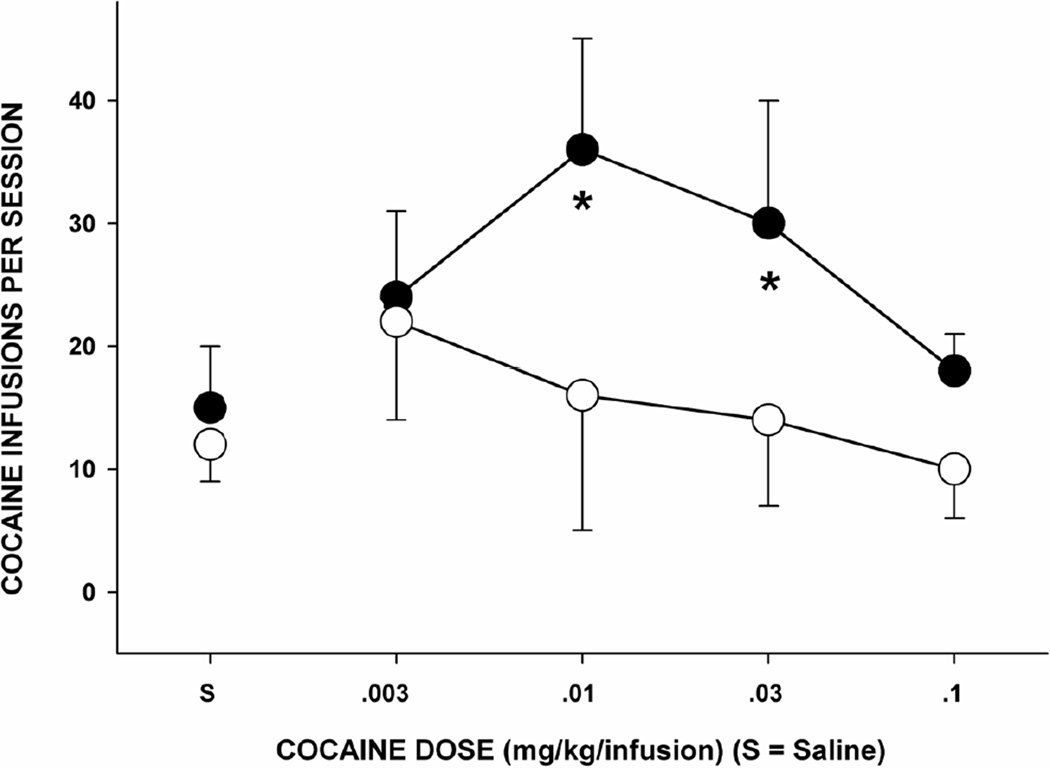
Fig. 5. RANK IN A SOCIAL HIERARCHY CONTRIBUTES TO BRAIN DA DEFICIENCY AND VULNERABILITY TO ADDICTIVE DRUG-TAKING BEHAVIOR.
Intravenous cocaine self-administration in monkeys of differing social hierarchy status within the social group. The solid black circles indicate monkeys with low social status; the open circles indicate moneys with high social status. Low social status produces a dopamine deficit in the mesotelencephalic reward circuitry, and a proclivity to self-administer addictive drugs. After [77].
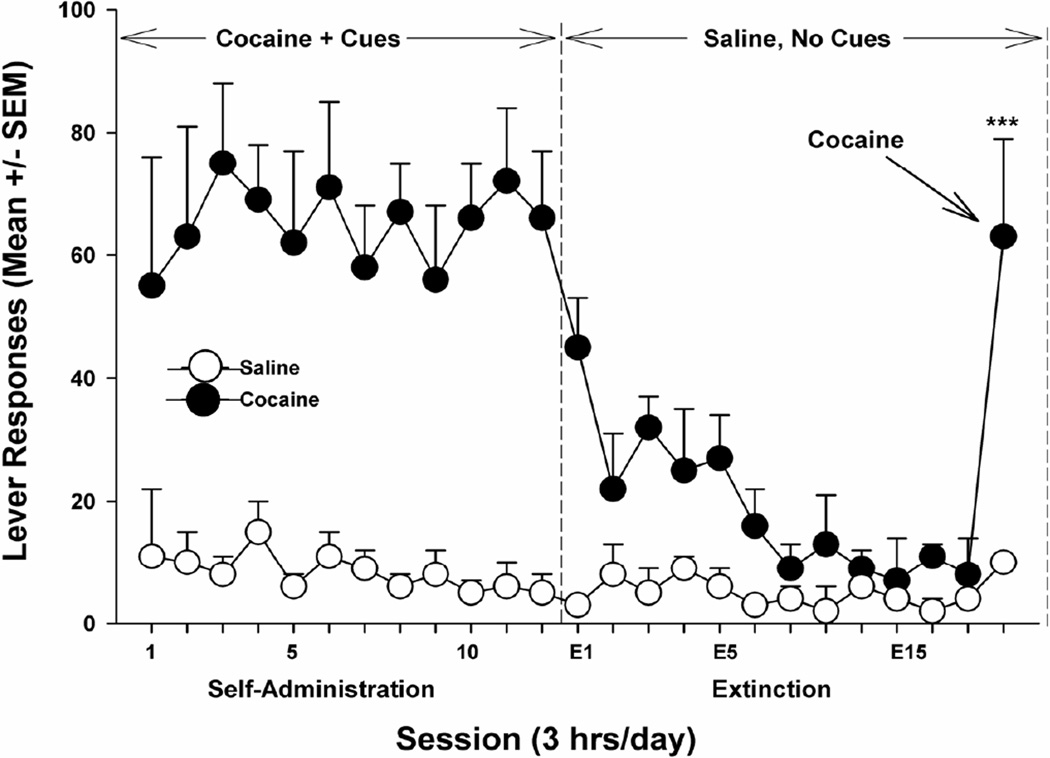
Fig. 6. TIME-LINE GRAPH OF REINSTATEMENT (RELAPSE) TO ADDICTIVE DRUG-SEEKING BEHAVIOR.
Schematic diagram of the “reinstatement” animal model of relapse to drug-seeking behavior. Initially, animals are allowed to freely self-administer intravenous cocaine in the presence of environmental cues that indicate the availability of cocaine. At the point indicated by the first vertical dashed line, the cue lights are turned off and saline is substituted for cocaine in the infusion apparatus. This causes behavioral extinction of the drug-taking habit and, perforce, pharmacological “detoxification” from cocaine. On the last day of the extinction phase of the experiment, the animal is given a single non-contingent intravenous “hit” of cocaine. This causes immediate and robust relapse to intense levels of drug-seeking behavior (although the infusion apparatus is filled with saline, as during the extinction and detoxification stage). After [37].
Fig. 7. INCUBATION OF DRUG CRAVING.
Incubation (enhancement) of drug-craving with the mere passage of time. Panel A: Drug-seeking behavior measured as extinction responding. Panel B: Drug-seeking behavior measured as cue-triggered relapse to drug seeking behavior. After [169].
Acknowledgments
The author is indebted to Ms. Stacey Saunders for editorial assistance, and to Dr. Zheng-Xiong Xi for assistance with the figures.
References
- 1.Gardner EL. Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse: A Comprehensive Textbook. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 48–97. [Google Scholar]
- 2.Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Gardner EL, David J. The neurobiology of chemical addiction. In: Elster J, Skog O-J, editors. Getting Hooked: Rationality and the Addictions. Cambridge, England: Cambridge University Press; 1999. pp. 93–136. [Google Scholar]
- 5.Wise RA, Gardner EL. Functional anatomy of substance-related disorders. In: D'haenen H, den Boer JA, Willner P, editors. Biological Psychiatry. New York: Wiley; 2002. pp. 509–522. [Google Scholar]
- 6.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 7.Olds J. Pleasure centers in the brain. Sci Am. 1956;95(4):105–116. [Google Scholar]
- 8.Olds J. Hypothalamic substrates of reward. Physiol Rev. 1962;42:554–604. doi: 10.1152/physrev.1962.42.4.554. [DOI] [PubMed] [Google Scholar]
- 9.Olds ME, Olds J. Approach-avoidance analysis of rat diencephalon. J Comp Neurol. 1963;120:259–295. doi: 10.1002/cne.901200206. [DOI] [PubMed] [Google Scholar]
- 10.Olds ME, Olds J. Drives, rewards and the brain. In: Newcomb TM, editor. New Directions in Psychology. New York: Holt, Rinehart & Winston; 1965. pp. 329–410. [Google Scholar]
- 11.Routtenberg A, Gardner EL, Huang YH. Self-stimulation pathways in the monkey, Macaca mulatta. Exp Neurol. 1971;33:213–224. doi: 10.1016/0014-4886(71)90115-4. [DOI] [PubMed] [Google Scholar]
- 12.Gallistel CR, Shizgal P, Yeomans JS. A portrait of the substrate for self-stimulation. Psychol Rev. 1981;88:228–273. [PubMed] [Google Scholar]
- 13.Wise RA, Bozarth MA. Brain reward circuitry: four circuit elements “wired” in apparent series. Brain Res Bull. 1984;12:203–208. doi: 10.1016/0361-9230(84)90190-4. [DOI] [PubMed] [Google Scholar]
- 14.Stuber GD, van Leeuwen WA, Sparta DR, Zhang F, Deisseroth K, Bonci A. Optogenetic control of brain reward circuitry. Paper presented at meetings of the Society for Neuroscience; October 2009; Chicago. 2009. (Abstract published in 2009 Abstract Viewer/Itinerary Planner CD-ROM, the Thirty-Ninth Annual Meeting of the Society for Neuroscience, Chicago, October 17–21, 2009, Society for Neuroscience, Washington: DC, Abstract Number 686.8) [Google Scholar]
- 15.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia inominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 16.Hubner CB, Koob GF. The ventral pallidum plays a role in mediating cocaine and heroin self-administration in the rat. Brain Res. 1990;508:20–29. doi: 10.1016/0006-8993(90)91112-t. [DOI] [PubMed] [Google Scholar]
- 17.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 18.Kalivas PW, Churchill L, Klitenick MA. The circuitry mediating the translation of motivational stimuli into adaptive motor responses. In: Kalivas PW, Barnes CD, editors. Limbic Motor Circuits and Neuropsychiatry. Boca Raton, Florida: CRC Press; 1993. pp. 237–287. [Google Scholar]
- 19.Napier TC. Transmitter actions and interactions on pallidal neuronal function. In: Kalivas PW, Barnes CD, editors. Limbic Motor Circuits and Neuropsychiatry. Boca Raton, Florida: CRC Press; 1993. pp. 124–153. [Google Scholar]
- 20.Gong WH, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- 21.Hasenrohrl RU, Frisch C, Huston JP. Evidence for anatomical specificity for the reinforcing effects of SP in the nucleus basalis magnocellularis region. Neuroreport. 1998;9:7–10. doi: 10.1097/00001756-199801050-00002. [DOI] [PubMed] [Google Scholar]
- 22.McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 23.White NM, Milner PM. The psychobiology of reinforcers. Annu Rev Psychol. 1992;43:443–471. doi: 10.1146/annurev.ps.43.020192.002303. [DOI] [PubMed] [Google Scholar]
- 24.Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 25.Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JW, Mahl GF, Delgado JMR, Hamlin H. Behavioral changes during intracerebral electrical stimulation. AMA Arch Neurol Psychiatry. 1956;76:399–419. doi: 10.1001/archneurpsyc.1956.02330280057006. [DOI] [PubMed] [Google Scholar]
- 27.Delgado JMR, Hamlin H. Spontaneous and evoked electrical seizures in animals and in humans. In: Ramey ER, O'Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. New York: Harper (Hoeber Medical Division); 1960. pp. 133–158. [Google Scholar]
- 28.Heath RG, Mickle WH. Evaluation of seven years' experience with depth electrode studies in human patients. In: Ramey ER, O'Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. New York: Harper (Hoeber Medical Division); 1960. pp. 214–247. [Google Scholar]
- 29.Sem-Jacobsen W, Torkildsen A. Depth recording and electrical stimulation in the human brain. In: Ramey ER, O'Doherty DS, editors. Electrical Studies on the Unanesthetized Brain. New York: Harper (Hoeber Medical Division); 1960. pp. 280–288. [Google Scholar]
- 30.Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;140:394–396. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- 31.Heath RG. Electrical self-stimulation of the brain in man. Am J Psychiatry. 1963;120:571–577. doi: 10.1176/ajp.120.6.571. [DOI] [PubMed] [Google Scholar]
- 32.Stein L, Ray OS. Brain stimulation reward "thresholds" self-determined in rat. Psychopharmacologia (Berl) 1960;1:251–2566. doi: 10.1007/BF00402746. [DOI] [PubMed] [Google Scholar]
- 33.Gardner EL. An improved technique for determining brain reward thresholds in primates. Behav Res Methods Instrum. 1971;3:273–274. [Google Scholar]
- 34.Miliaressis E, Rompré P-P, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- 35.Coulombe D, Miliaressis E. Fitting intracranial self-stimulation data with growth models. Behav Neurosci. 1987;101:209–214. doi: 10.1037//0735-7044.101.2.209. [DOI] [PubMed] [Google Scholar]
- 36.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychol. 1994;2:244–268. [Google Scholar]
- 37.Vorel SR, Ashby CR, Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pak AC, Ashby CR, Jr, Heidbreder CA, Pilla M, Gilbert J, Xi Z-X, Gardner EL. The selective dopamine D3 receptor antagonist SB-277011A reduces nicotine-enhanced brain reward and nicotine-paired environmental cue functions. Int J Neuropsychopharmacol. 2006;9:585–602. doi: 10.1017/S1461145706006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiller K, Xi Z-X, Peng X-Q, Newman AH, Ashby CR, Jr, Heidbreder CA, Gaál J, Gardner EL. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain-stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi Z-X, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng X-Q, Gardner EL. Cannabinoid CB1 receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2008;33:1735–1745. doi: 10.1038/sj.npp.1301552. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Li J, Peng X-Q, Spiller K, Gardner EL, Xi Z-X. Metabotropic glutamate receptor 7 modulates cocaine’s rewarding effects in rats: involvement of a ventral pallidal GABAergic mechanism. Neuropsychopharmacology. 2009;34:1783–1796. doi: 10.1038/npp.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner EL, Lowinson JH. Drug craving and positive/negative hedonic brain substrates activated by addicting drugs. Semin Neurosci. 1993;5:359–368. [Google Scholar]
- 43.Xi Z-X, Spiller K, Gardner EL. Cannabinoid CB1 and CB2 receptors modulate brain reward function in opposite directions in rats. Paper presented at meetings of the Society for Neuroscience; October 2009; Chicago. (Abstract published in 2009 Abstract Viewer/Itinerary Planner CD-ROM, the Thirty-Ninth Annual Meeting of the Society for Neuroscience, Chicago, Illinois, October 17–21, 2009, Society for Neuroscience, Washington, DC, Abstract Number 449.13) 2009. [Google Scholar]
- 44.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 45.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 46.Gardner EL, Wise RA. Animal models of addiction. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 3rd edn. Oxford, England: Oxford University Press; 2009. pp. 757–774. [Google Scholar]
- 47.Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- 48.Gardner EL, Chen J, Paredes W. Overview of chemical sampling techniques. J Neurosci Methods. 1993;48:173–197. doi: 10.1016/0165-0270(93)90091-5. [DOI] [PubMed] [Google Scholar]
- 49.Wise RA. In vivo estimates of extracellular dopamine and dopamine metabolite levels during intravenous cocaine or heroin self-administration. Semin Neurosci. 1993;5:337–342. [Google Scholar]
- 50.Wise RA, Leone P, Rivest R, Leeb K. Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse. 1995;21:140–148. doi: 10.1002/syn.890210207. [DOI] [PubMed] [Google Scholar]
- 51.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology. 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 52.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 53.Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychologist. 1980;35:691–712. doi: 10.1037//0003-066x.35.8.691. [DOI] [PubMed] [Google Scholar]
- 54.Solomon RL. Recent experiments testing an opponent-process theory of acquired motivation. Acta Neurobiol Exp (Warsaw) 1980;40:271–289. [PubMed] [Google Scholar]
- 55.Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- 56.Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- 57.Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philoso Trans R Soc B. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nazzaro JM, Seeger TF, Gardner EL. Morphine differentially affects ventral tegmental and substantia nigra brain reward thresholds. Pharmacol Biochem Behav. 1981;14:325–331. doi: 10.1016/0091-3057(81)90398-1. [DOI] [PubMed] [Google Scholar]
- 59.Broderick PA, Gardner EL, van Praag HM. In vivo electrochemical evidence for a differential enkephalinergic modulation of dopamine in rat nigrostriatal and mesolimbic systems: correlated behavioral stereotypy results. Paper presented at meetings of the International Narcotic Research Conference; June 1983; Garmisch-Partenkirchen, Germany. [Google Scholar]
- 60.Broderick PA, Gardner EL, van Praag HM. In vivo electrochemical and behavioral evidence for specific neural substrates modulated differentially by enkephalin in rat stimulant stereotypy and locomotion. Biol Psychiatry. 1984;19:45–54. [PubMed] [Google Scholar]
- 61.Kokkinidis L, McCarter BD. Postcocaine depression and sensitization of brain-stimulation reward: analysis of reinforcement and performance effects. Pharmacol Biochem Behav. 1990;36:463–471. doi: 10.1016/0091-3057(90)90242-a. [DOI] [PubMed] [Google Scholar]
- 62.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 63.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardner EL, Lepore M. Withdrawal from a SINGLE small dose of marijuana elevates baseline brain-stimulation reward thresholds in rats. Paper presented at Winter Conference on Brain Research; January 1996; Aspen, Colorado. [Google Scholar]
- 65.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 66.Blum K, Cull JG, Braverman ER, Comings DE. Reward deficiency syndrome. Am Scientist. 1996;84:132–145. [Google Scholar]
- 67.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJH, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gardner EL, Blum K. Neurobiology and genetics of addiction: implications of “reward deficiency syndrome” for therapeutic strategies in chemical dependency. Paper presented at Russell Sage Foundation Conference on Addiction; June 1997; New York. [Google Scholar]
- 69.Gardner EL. Neurobiology and genetics of addiction: implications of “reward deficiency syndrome” for therapeutic strategies in chemical dependency. In: Elster J, editor. Addiction: Entries and Exits. New York: Russell Sage Foundation; 1999. pp. 57–119. [Google Scholar]
- 70.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 71.Gardner EL. Reward behaviors as a function of hypo-dopaminergic activity: animal models of reward deficiency syndrome. Mol Psychiatry. 2001;6(suppl. 1):S4. [Google Scholar]
- 72.Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jadadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of the human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–2059. [PubMed] [Google Scholar]
- 73.Volkow ND, Wang G-J, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding Y-S, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- 74.Volkow ND, Fowler JS, Wang G-J, Ding Y-S, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 75.Volkow ND, Wang G-J, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding Y-S, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 76.Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 78.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Lääne K, Peña Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron J-C, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mash DC. Are neuroadaptations in D3 dopamine receptors linked to the development of cocaine dependence? Mol Psychiatry. 1997;2:7–8. [PubMed] [Google Scholar]
- 81.Gardner EL. Use of animal models to develop antiaddiction medications. Curr Psychiat Rep. 2008;10:377–384. doi: 10.1007/s11920-008-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xi Z-X, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xi Z-X, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin. 2009;30:723–739. doi: 10.1038/aps.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heidbreder CA, Gardner EL, Xi Z-X, Thanos PK, Mugnaini M, Hagan JJ, Ashby Cr., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heidbreder CA, Andreoli M, Marcon C, Hutcheson DM, Gardner EL, Ashby CR., Jr Evidence for the role of dopamine D3 receptors in oral operant alcohol self-administration and reinstatement of alcohol-seeking behavior in mice. Addict Biol. 2007;12:35–50. doi: 10.1111/j.1369-1600.2007.00051.x. [DOI] [PubMed] [Google Scholar]
- 86.Beitner-Johnson D, Guitart X, Nestler EJ. Dopaminergic brain reward regions of Lewis and Fischer rats display different levels of tyrosine hydroxylase and other morphine- and cocaine-regulated phosphoproteins. Brain Res. 1991;561:147–150. doi: 10.1016/0006-8993(91)90759-o. [DOI] [PubMed] [Google Scholar]
- 87.Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12:242–253. doi: 10.1002/syn.890120310. [DOI] [PubMed] [Google Scholar]
- 88.Berrettini WH, Ferraro TN, Alexander RC, Buchberg AM, Vogel WH. Quantitative trait loci mapping of three loci controlling morphine preference using inbred mouse strains. Nat Genet. 1994;7:54–58. doi: 10.1038/ng0594-54. [DOI] [PubMed] [Google Scholar]
- 89.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 90.Brodkin ES, Carlezon WA, Jr, Haile CN, Kosten TA, Heninger GR, Nestler EJ. Genetic analysis of behavioral, neuroendocrine, and biochemical parameters in inbred rodents: initial studies in Lewis and Fischer 344 rats and in A/J and C57BL/6J mice. Brain Res. 1998;805:55–68. doi: 10.1016/s0006-8993(98)00663-5. [DOI] [PubMed] [Google Scholar]
- 91.McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- 92.Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. [DOI] [PubMed] [Google Scholar]
- 93.Nestler EJ. Genes and addiction. Nat Genet. 2000;26:277–281. doi: 10.1038/81570. [DOI] [PubMed] [Google Scholar]
- 94.Dole VP, Nyswander M. A medical treatment for diacetylmorphine (heroin) addiction. A clinical trial with methadone hydrochloride. JAMA. 1965;193:646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 95.Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade - A medical technique for stopping heroin use by addicts. Trans Assoc Am Physicians. 1966;79:122–136. [PubMed] [Google Scholar]
- 96.Wise RA. The dopamine synapse and the notion of “pleasure centers” in the brain. Trends Neurosci. 1980;3:91–95. [Google Scholar]
- 97.Lee R-S, Criado JE, Koob GF, Henriksen SJ. Cellular responses of nucleus accumbens neurons to opiate-seeking behavior. I. Sustained responding during heroin self-administration. Synapse. 1999;33:49–58. doi: 10.1002/(SICI)1098-2396(199907)33:1<49::AID-SYN5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 98.Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang J-Y, Sawyer SF, Lee R-S, Woodward DJ. Electrophysiological and pharmacological evidence for the role of the nucleus accumbens in cocaine self-administration in freely moving rats. J Neurosci. 1994;14:1224–1244. doi: 10.1523/JNEUROSCI.14-03-01224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci. 2008;28:7837–7846. doi: 10.1523/JNEUROSCI.1600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gregorios-Pippas L, Tobler PN, Schultz W. Short-term temporal discounting of reward value in human ventral striatum. J Neurophysiol. 2009;101:1507–1523. doi: 10.1152/jn.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitalfrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peoples LL, Uzwiak AJ, Gee F, Fabbricatore AT, Muccino KJ, Mohta BD, West MO. Phasic accumbal firing may contribute to the regulation of drug taking during intravenous cocaine self-administration sessions. Ann NY Acad Sci. 1999;877:781–787. doi: 10.1111/j.1749-6632.1999.tb09322.x. [DOI] [PubMed] [Google Scholar]
- 104.Peoples LL, Cavanaugh D. Differential changes in signal and background firing of accumbal neurons during cocaine self-administration. J Neurophysiol. 2003;90:993–10101. doi: 10.1152/jn.00849.2002. [DOI] [PubMed] [Google Scholar]
- 105.Uhl GR. Molecular genetics of substance abuse vulnerability: remarkable recent convergence of genome scan results. Ann NY Acad Sci. 2004;1025:1–13. doi: 10.1196/annals.1316.001. [DOI] [PubMed] [Google Scholar]
- 106.Uhl GR, Drgan T, Johnson C, Fatusin OO, Liu Q-R, Contoreggi C, Li C-Y, Buck K, Crabbe J. “Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochem Pharmacol. 2008;75:98–111. doi: 10.1016/j.bcp.2007.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khokhar JY, Ferguson CS, Zhu AZX, Tyndale RF. Pharmacogenetics of drug dependence: role of gene variations in susceptibility and treatment. Annu Rev Pharmacol Toxicol. 2010;50:39–61. doi: 10.1146/annurev.pharmtox.010909.105826. [DOI] [PubMed] [Google Scholar]
- 108.Piazza PV, Maccari S, Deminière JM, Le Moal M, Mormède P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA. 1991;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deminière JM, Piazza PV, Guegan G, Abrous N, Maccari S, Le Moal M, Simon H. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 1992;586:135–139. doi: 10.1016/0006-8993(92)91383-p. [DOI] [PubMed] [Google Scholar]
- 110.Redolat R, Pérez-Martinez A, Carrasco MC, Mesa P. Individual differences in novelty-seeking and behavioral responses to nicotine: a review of animal studies. Curr Drug Abuse Rev. 2009;2:230–242. doi: 10.2174/1874473710902030230. [DOI] [PubMed] [Google Scholar]
- 111.Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68:818–829. [PubMed] [Google Scholar]
- 112.Zuckerman M, Kuhlman DM. Personality and risk-taking: common biosocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev. 2004;3:261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- 114.Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, Wilson AS. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. 2008;200:529–544. doi: 10.1007/s00213-008-1231-7. [DOI] [PubMed] [Google Scholar]
- 115.Crowley TJ, Mikulich SK, Macdonald M, Young SE, Zerbe GO. Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome. Drug Alcohol Depend. 1998;49:225–237. doi: 10.1016/s0376-8716(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 116.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith D, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 117.Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;53:232–240. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 118.Wilson JJ, Levin FR. Attention deficit hyperactivity disorder (ADHD) and substance use disorders. Curr Psychiat Rep. 2001;3:497–506. doi: 10.1007/s11920-001-0044-8. [DOI] [PubMed] [Google Scholar]
- 119.Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiat Clin North Am. 2004;27:283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 120.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 121.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 122.Koob GF. Hedonic homeostatic dysregulation as a driver of drug-seeking behavior. Drug Discov Today Dis Models. 2008;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lepore M, Vorel R, Gardner EL. Studies on the neurobiological interrelationship between vulnerability to depression and vulnerability to drug abuse in animal models. Behav Pharmacol. 1995;6([suppl. 1]):82–84. [Google Scholar]
- 124.Phillips GD, Howes SR, Whitelaw RB, Robbins TW, Everitt BJ. Isolation rearing impairs the reinforcing efficacy of intravenous cocaine or intra-accumbens d-amphetamine: impaired response to intra-accumbens D1 and D2/D3 dopamine receptor antagonists. Psychopharmacology. 1994;115:419–429. doi: 10.1007/BF02245085. [DOI] [PubMed] [Google Scholar]
- 125.Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 126.Ginovart N, Farde L, Halldin C, Swahn CG. Changes in striatal D2-receptor density following chronic treatment with amphetamine as assessed with PET in nonhuman primates. Synapse. 1999;31:154–162. doi: 10.1002/(SICI)1098-2396(199902)31:2<154::AID-SYN9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 127.Ginovart N, Wilson AA, Houle S, Kapur S. Amphetamine pretreatment induces a change in both D2 -receptor density and apparent affinity: a [11C]raclopride positron emission tomography study in cats. Biol Psychiatry. 2004;55:1188–1194. doi: 10.1016/j.biopsych.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 128.Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(suppl.1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 131.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 132.Ikemoto S, Qin M, Liu Z-H. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the nucleus accumbens core, shell, and olfactory tubercle valid? J Neurosci. 2005;25:5061–5065. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and non-automatic processes. Psychol Rev. 1990;97:146–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 134.O’Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- 135.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 136.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 137.Verdejo-García A, Pérez-García M. Substance abusers= self-awareness of the neurobehavioral consequences of addiction. Psychiat Res. 2008;158:172–180. doi: 10.1016/j.psychres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 138.Goldstein R, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 140.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 141.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 142.Robbins TW, Everitt BJ. Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem. 2002;78:625–636. doi: 10.1006/nlme.2002.4103. [DOI] [PubMed] [Google Scholar]
- 143.White NM. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav Brain Res. 2009;199:3–23. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 144.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc B. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Porrino LJ, Daunais JB, Smith HR, Nader MA. The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci Biobehav Rev. 2004;27:813–820. doi: 10.1016/j.neubiorev.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 148.Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma YM, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Takahashi Y, Roesch MR, Stalnaker TA, Schoenbaum G. Cocaine shifts the balance of associative encoding from ventral to dorsolateral striatum. Front Integr Neurosci. 2007;1:11. doi: 10.3389/neuro.07/011.2007. [Published online 30 Dec 2007; see http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2526005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- 151.Pert A, Yaksh T. Sites of morphine induced analgesia in the primate brain: relation to pain pathways. Brain Res. 1974;80:135–140. doi: 10.1016/0006-8993(74)90731-8. [DOI] [PubMed] [Google Scholar]
- 152.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 153.Schaible HG. Peripheral and central mechanisms of pain generation. Handb Exp Pharmacol. 2007;177:3–28. doi: 10.1007/978-3-540-33823-9_1. [DOI] [PubMed] [Google Scholar]
- 154.D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 155.Alcoholics Anonymous. Alcoholics Anonymous Big Book. 1st edn. New York: Alcoholics Anonymous World Services, Inc.; 1939. [Google Scholar]
- 156.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 157.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 158.Aguilar MA, Rodríguez-Arias M, Miñarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 159.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 162.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 163.Itoi K. Ablation of the central noradrenergic neurons for unraveling their roles in stress and anxiety. Ann NY Acad Sci. 2008;1129:47–54. doi: 10.1196/annals.1417.012. [DOI] [PubMed] [Google Scholar]
- 164.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 165.Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL. Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology. 2003;168:75–83. doi: 10.1007/s00213-002-1328-3. [DOI] [PubMed] [Google Scholar]
- 166.Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann NY Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- 167.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- 169.Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grimm JW, Lu L, Hayashi T, Hope BT, Su T-P, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 172.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 173.Xi Z-X, Gilbert J, Campos AC, Kline N, Ashby CR, Jr, Hagan JJ, Heidbreder CA, Gardner EL. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 175.Chen BT, Hopf W, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann NY Acad Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 177.Hoffman AF, Oz M, Caulder T, Lupica CR. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid Exposure. J Neurosci. 2003;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Q-S, Pu L, Poo M-M. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453:1253–1258. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE. Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci. 2010;30:4676–4686. doi: 10.1523/JNEUROSCI.6165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pu L, Bao G-B, Xu N-J, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127:177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 184.Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 186.Gardner EL. Pain management and the so-called “risk” of addiction. In: Smith H, Passik SD, editors. Pain and Chemical Dependency. London: Oxford University Press; 2008. pp. 427–435. [Google Scholar]
- 187.Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T. Direct evidence for the involvement of the mesolimbic κ-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology. 2005;30:11–118. doi: 10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- 188.Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. J Neurochem. 2004;88:1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- 189.Kinshore KR, Desiraju T. Inhibition of positively rewarding behavior by the heightened aggressive state evoked either by pain-inducing stimulus or septal lesion. Indian J Physiol Pharmacol. 1990;34:125–129. [PubMed] [Google Scholar]
- 190.Vaccarino AL, Marek P, Kest B, Ben-Eliyahu S, Couret LC, Jr, Kao B, Liebeskind JC. Morphine fails to produce tolerance when administered in the presence of formalin pain in rats. Brain Res. 1993;627:287–290. doi: 10.1016/0006-8993(93)90332-h. [DOI] [PubMed] [Google Scholar]
- 191.UNAIDS (Joint United Nations Programme on HIV/AIDS) Cancer Pain Relief, with a Guide to Opioid Availability. 2nd edn. Geneva, Switzerland: World Health Organization; 1996. pp. 24–37. [Google Scholar]
- 192.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lobmaier P, Gossop M, Waal H, Bramness J. The pharmacological treatment of opioid addiction - a clinical perspective. Eur J Clin Pharmacol. 2010;66:537–545. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- 194.Wakhlu S. Buprenorphine: a review. J Opioid Manag. 2009;5:59–64. doi: 10.5055/jom.2009.0007. [DOI] [PubMed] [Google Scholar]
- 195.Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, Williams H, Zador D, Evers R, Groshkova T, Charles V, Martin A, Forzisi L. Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet. 2010;375:1885–1895. doi: 10.1016/S0140-6736(10)60349-2. [DOI] [PubMed] [Google Scholar]
- 196.Ross S, Peselow E. Pharmacotherapy of addictive disorders. Clin Neuropharmacol. 2009;32:277–289. doi: 10.1097/wnf.0b013e3181a91655. [DOI] [PubMed] [Google Scholar]
- 197.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 198.Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9:13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- 199.Altshuler HL, Phillips PA, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- 200.Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HWD, Koob GF, Gold LH. μ-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- 202.Gardner EL, Paredes W, Smith D, Zukin RS. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol is mediated by an endogenous opioid mechanism. Adv Biosci. 1989;75:671–674. [Google Scholar]
- 203.Chen J, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Δ9-Tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 204.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 205.Mason BJ, Heyser CJ. Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets. 2010;9:23–32. doi: 10.2174/187152710790966641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ameisen O. Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol. 2005;40:147–150. doi: 10.1093/alcalc/agh130. [DOI] [PubMed] [Google Scholar]
- 207.Ameisen O. The End of My Addiction. New York: Farrar, Straus and Giroux; 2008. [Google Scholar]
- 208.Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16:2113–2117. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- 209.Mason BJ, Heyser CJ. The neurobiology, clinical efficacy and safety of acamprosate in the treatment of alcohol dependence. Expert Opin Drug Saf. 2010;9:177–188. doi: 10.1517/14740330903512943. [DOI] [PubMed] [Google Scholar]
- 210.Roberts DCS, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–423. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 211.Roberts DCS, Andrews MM. Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology. 1997;131:271–277. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- 212.Brebner K, Phelan R, Roberts DCS. Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2000;66:857–862. doi: 10.1016/s0091-3057(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 213.Brebner K, Phelan R, Roberts DCS. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology. 2000;148:314–321. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- 214.Roberts DCS, Brebner K. GABA modulation of cocaine self-administration. Ann NY Acad Sci. 2000;909:145–158. doi: 10.1111/j.1749-6632.2000.tb06680.x. [DOI] [PubMed] [Google Scholar]
- 215.Cousins MS, Roberts DCS, de Wit H. GABAB receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 216.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 217.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin Ther. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 218.Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of α4β2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–66. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Glynn DA, Cryan JF, Kent P, Flynn RA, Kennedy MP. Update on smoking cessation therapies. Adv Ther. 2009;26:369–382. doi: 10.1007/s12325-009-0022-9. [DOI] [PubMed] [Google Scholar]
- 220.Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology. 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- 221.Semmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 222.Froimowitz M, Wu KM, Moussa A, Haidar RM, Jurayj J, George C, Gardner EL. Slow-onset, long-duration 3-(3',4'-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse. J Med Chem. 2000;43:4981–4992. doi: 10.1021/jm000201d. [DOI] [PubMed] [Google Scholar]
- 223.Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25:1889–1893. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gardner EL, Liu X, Paredes W, Giordano A, Spector J, Lepore M, Wu KM, Froimowitz M. A slow-onset, long-duration indanamine monoamine reuptake inhibitor as a potential maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction. Neuropharmacology. 2006;51:993–1003. doi: 10.1016/j.neuropharm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 225.Tanda G, Newman AH, Katz JL. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol. 2009;57:253–289. doi: 10.1016/S1054-3589(08)57007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Peng X-Q, Xi Z-X, Li X, Spiller K, Li J, Chun L, Wu K-M, Froimowitz M, Gardner EL. Is slow-onset long-acting monoamine transport blockade to cocaine as methadone is to heroin? Implication for anti-addiction medications. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.133. in press [Epub ahead of print 08 Sept 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kushner SA, Dewey SL, Kornetsky C. Gamma-vinyl GABA attenuates cocaine-induced lowering of brain stimulation reward thresholds. Psychopharmacology. 1997;133:383–388. doi: 10.1007/s002130050418. [DOI] [PubMed] [Google Scholar]
- 229.Dewey SL, Morgan AE, Ashby CR, Jr, Horan B, Kushner SA, Logan J, Volkow ND, Fowler JS, Gardner EL, Brodie JD. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–129. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 230.Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR., Jr A pharmacologic strategy for the treatment of nicotine addiction. Synapse. 1999;31:76–86. doi: 10.1002/(SICI)1098-2396(199901)31:1<76::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 231.Kushner SA, Dewey SL, Kornetsky C. The irreversible γ-aminobutyric acid (GABA) transaminase inhibitor γ-vinyl-GABA blocks cocaine self-administration in rats. J Pharmacol Exp Ther. 1999;290:797–802. [PubMed] [Google Scholar]
- 232.Gerasimov MR, Ashby CR, Jr, Gardner EL, Mills MJ, Brodie JD, Dewey SL. Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse. 1999;34:11–19. doi: 10.1002/(SICI)1098-2396(199910)34:1<11::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 233.Paul M, Dewey SL, Gardner EL, Brodie JD, Ashby CR., Jr Gamma-vinyl GABA (GVG) blocks expression of the conditioned place preference response to heroin in rats. Synapse. 2001;41:219–220. doi: 10.1002/syn.1078. [DOI] [PubMed] [Google Scholar]
- 234.Gardner EL, Schiffer WK, Horan BA, Highfield D, Dewey SL, Brodie JD, Ashby CR., Jr Gamma-vinyl GABA, an irreversible inhibitor of GABA transaminase, alters the acquisition and expression of cocaine-induced sensitization in male rats. Synapse. 2002;46:240–250. doi: 10.1002/syn.10138. [DOI] [PubMed] [Google Scholar]
- 235.Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse. 2003;50:261–265. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]
- 236.Peng X-Q, Li X, Gilbert JG, Pak AC, Ashby CR, Jr, Brodie JD, Dewey SL, Gardner EL, Xi Z-X. Gamma-vinyl GABA inhibits cocaine-triggered reinstatement of drug-seeking behavior in rats by a non-dopaminergic mechanism. Drug Alcohol Depend. 2008;97:216–225. doi: 10.1016/j.drugalcdep.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, Hammel C, Kothari SK, Kahanda M, Liebling CN, Patel V, Schiffer WK, Brodie JD, Dewey SL. Racemic gamma vinyl-GABA (R,S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse. 2009;63:87–94. doi: 10.1002/syn.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Brodie JD, Case BG, Figueroa E, Dewey SL, Robinson JA, Wanderling JA, Laska EM. Randomized, double-blind, placebo-controlled trial of vigabatrin for the treatment of cocaine dependence in Mexican parolees. Am J Psychiatry. 2009;166:1269–1277. doi: 10.1176/appi.ajp.2009.08121811. [DOI] [PubMed] [Google Scholar]
- 239.Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology. 1988;96:142–144. doi: 10.1007/BF02431546. [DOI] [PubMed] [Google Scholar]
- 240.Gardner EL, Lowinson JH. Marijuana's interaction with brain reward systems: update 1991. Pharmacol Biochem Behav. 1991;40:571–580. doi: 10.1016/0091-3057(91)90365-9. [DOI] [PubMed] [Google Scholar]
- 241.Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by Δ9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 242.De Vries TJ, Homberg JR, Binnekade R, Raasø H, Schoffelmeer ANM. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology. 2003;168:164–169. doi: 10.1007/s00213-003-1422-1. [DOI] [PubMed] [Google Scholar]
- 243.Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci. 2003;17:1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- 244.Anggadiredja K, Nakamichi M, Hiranita T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T. Endocannabinoid system modulates relapse to methamphetamine seeking: possible mediation by the arachidonic acid cascade. Neuropsychopharmacology. 2004;29:1470–1478. doi: 10.1038/sj.npp.1300454. [DOI] [PubMed] [Google Scholar]
- 245.Spano MS, Fattore L, Cossu G, Deiana S, Fadda P, Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br J Pharmacol. 2004;143:343–350. doi: 10.1038/sj.bjp.0705932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yamamoto T, Anggadiredja K, Hiranita T. New perspectives in the studies on endocannabinoid and cannabis: a role for the endocannabinoid-arachidonic acid pathway in drug reward and long-lasting relapse to drug taking. J Pharmacol Sci. 2004;96:382–388. doi: 10.1254/jphs.fmj04003x5. [DOI] [PubMed] [Google Scholar]
- 247.Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005b;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 248.Fattore L, Spano S, Cossu G, Deiana S, Fadda P, Fratta W. Cannabinoid CB1 antagonist SR 141716A attenuates reinstatement of heroin self-administration in heroin-abstinent rats. Neuropharmacology. 2005;48:1097–1104. doi: 10.1016/j.neuropharm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 249.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 250.Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 251.Fagerström K, Balfour DJ. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Exp Opin Investig Drugs. 2006;15:107–116. doi: 10.1517/13543784.15.2.107. [DOI] [PubMed] [Google Scholar]
- 252.Li X, Hoffman AF, Peng X-Q, Lupica CR, Gardner EL, Xi Z-X. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology. 2009;204:1–11. doi: 10.1007/s00213-008-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS Neurol Disord Drug Targets. 2008;7:482–491. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- 254.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 255.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(suppl.1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann NY Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li X, Gardner EL, Xi Z-X. The metabotropic glutamate receptor 7 (mGluR7) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats. Neuropharmacology. 2008;54:542–551. doi: 10.1016/j.neuropharm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Li X, Li J, Gardner EL, Xi Z-X. Activation of mGluR7s inhibits cocaine-induced reinstatement of drug-seeking behavior by a nucleus accumbens glutamate-mGluR2/3 mechanism in rats. J Neurochem. 2010;114:1368–1380. doi: 10.1111/j.1471-4159.2010.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Peng X-Q, Li J, Gardner EL, Ashby CR, Jr, Thomas A, Wozniak K, Slusher BS, Xi Z-X. Oral administration of the NAALADase inhibitor GPI-5693 attenuates cocaine-induced reinstatement of drug-seeking behavior in rats. Eur J Pharmacol. 2010;627:156–161. doi: 10.1016/j.ejphar.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Xi Z-X, Kiyatkin M, Li X, Peng X-Q, Wiggins A, Spiller K, Li J, Gardner EL. N-acetyl-aspartatylglutamate (NAAG) attenuates cocaine-enhanced brain-stimulation reward and cocaine self-administration in rats. Neuropharmacology. 2010;58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Xi Z-X, Li X, Peng X-Q, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 267.Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chu K, Koob GF, Cole M, Zorrilla EP, Roberts AJ. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007;86:813–821. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–171203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gilpin NW, Richardson HN, Koob GF. Effects of CRF1 -receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:1535–1542. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Papaleo F, Ghozland S, Ingallinesi M, Roberts AJ, Koob GF, Contarino A. Disruption of the CRF2 receptor pathway decreases the somatic expression of opiate withdrawal. Neuropsychopharmacology. 2008;33:2878–2887. doi: 10.1038/npp.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;96:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;5:371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]