Abstract
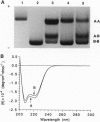
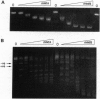
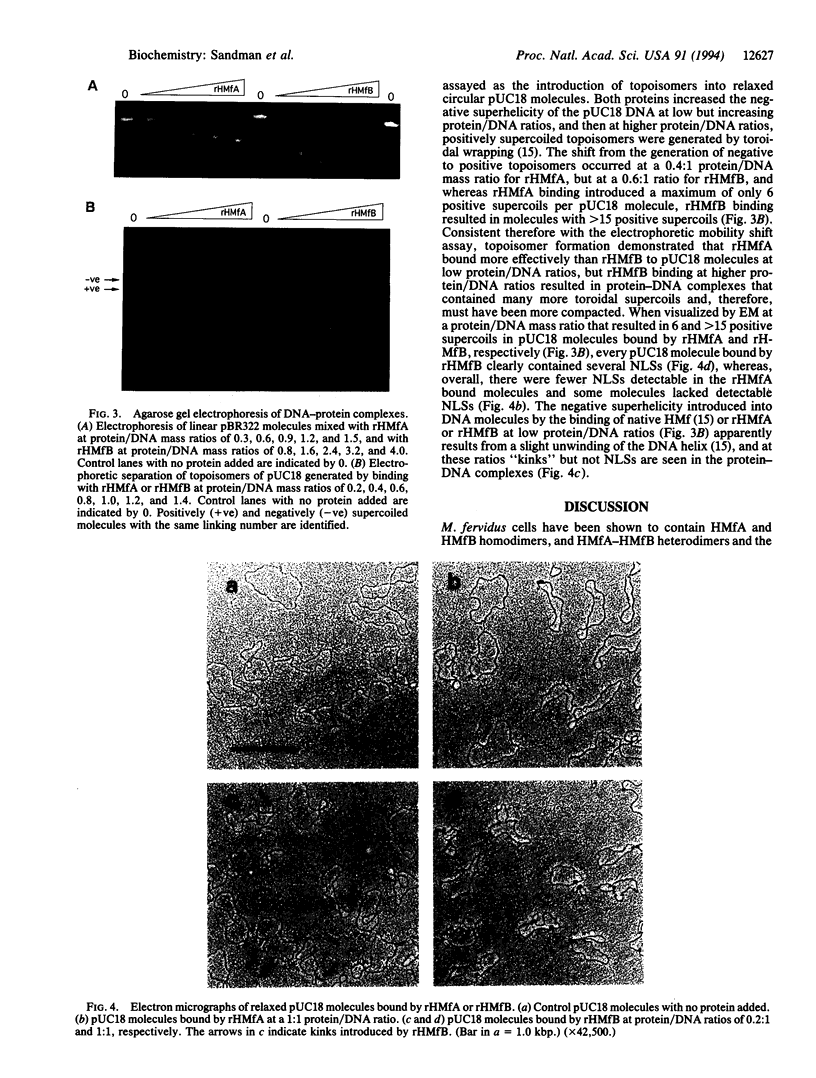
Histone preparations from Methanothermus fervidus (HMf) contain two small polypeptides, HMfA and HMfB, which in solution are dimers and compact DNA to form nucleosome-like structures. These archaeal nucleosome-like structures constrain positive DNA supercoils, in contrast to the negatively supercoiled DNA in eukaryal nucleosomes. HMfA has been found to make up as much as 80% of HMf preparations synthesized by M. fervidus cells during the exponential growth phase of batch cultures but to decrease to approximately 50% as cultures enter the stationary phase. By using a nondenaturing polyacrylamide gel system at pH 6.1, we have demonstrated that HMf preparations contain HMfA homodimers, HMfB homodimers, and HMfA-HMfB heterodimers and that heating a mixture of recombinant HMfA and HMfB homodimers at 95 degrees C for 5 min generates HMfA-HMfB heterodimers. Circular dichroism spectroscopy indicates that HMfA and HMfB have very similar secondary structures, but based on agarose gel electrophoretic mobility shifts, DNA topology assays, and electron microscopy, they have different DNA binding properties. HMfA binding to DNA could be detected at lower protein/DNA ratios than HMfB, but HMfB binding resulted in more extensive DNA compaction. The increased HMfB synthesized in cells approaching the stationary phase and the highly compacted state of HMfB-bound DNA are consistent with preparations for the impending period of limited genome activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersch P., Schmidt K., Bremer E. Synthesis of the Escherichia coli K-12 nucleoid-associated DNA-binding protein H-NS is subjected to growth-phase control and autoregulation. Mol Microbiol. 1993 May;8(5):875–889. doi: 10.1111/j.1365-2958.1993.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Ditto M. D., Roberts D., Weisberg R. A. Growth phase variation of integration host factor level in Escherichia coli. J Bacteriol. 1994 Jun;176(12):3738–3748. doi: 10.1128/jb.176.12.3738-3748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone function in transcription. Annu Rev Cell Biol. 1990;6:643–678. doi: 10.1146/annurev.cb.06.110190.003235. [DOI] [PubMed] [Google Scholar]
- Howard M. T., Sandman K., Reeve J. N., Griffith J. D. HMf, a histone-related protein from the hyperthermophilic archaeon Methanothermus fervidus, binds preferentially to DNA containing phased tracts of adenines. J Bacteriol. 1992 Dec;174(23):7864–7867. doi: 10.1128/jb.174.23.7864-7867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave D. R., Sandman K. M., Reeve J. N. DNA binding by the archaeal histone HMf results in positive supercoiling. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10397–10401. doi: 10.1073/pnas.88.23.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann O., Koch C., Kahmann R. The E.coli fis promoter is subject to stringent control and autoregulation. EMBO J. 1992 Mar;11(3):1075–1083. doi: 10.1002/j.1460-2075.1992.tb05146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Martín J., Rojo F., de Lorenzo V. Promoters responsive to DNA bending: a common theme in prokaryotic gene expression. Microbiol Rev. 1994 Jun;58(2):268–290. doi: 10.1128/mr.58.2.268-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman K., Krzycki J. A., Dobrinski B., Lurz R., Reeve J. N. HMf, a DNA-binding protein isolated from the hyperthermophilic archaeon Methanothermus fervidus, is most closely related to histones. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5788–5791. doi: 10.1073/pnas.87.15.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum R., Sandman K. M., Reeve J. N. HMt, a histone-related protein from Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1992 Dec;174(24):7890–7895. doi: 10.1128/jb.174.24.7890-7895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]