Abstract
Background
The diabetic phenotype of wound healing is in part characterized by impaired neovascularization and deficient endothelial progenitor cell (EPC) recruitment. Angiopoietin-1 (Ang-1) is a potent mobilizer of EPCs from the bone marrow (BM). A suggested mechanism for EPC mobilization from the BM is mediated by matrix metalloproteinase 9 (MMP-9) and stem cell factor (SCF). Taken together, we hypothesized that overexpression of Ang-1 in diabetic wounds will recruit EPCs and improve neovascularization and wound healing.
Methods
An endothelial lineage BM-labeled murine model of diabetes was developed to track BM-derived EPCs. FVBN mice were lethally irradiated and then reconstituted with BM from syngeneic Tie2/LacZ donor mice. Diabetes was induced with streptozotocin. Dorsal wounds in BM-transplanted (BMT) mice were treated with Ad-Ang-1, Ad-GFP, or PBS. At day 7 post injury, wounds were harvested and analyzed. A similar experiment was conducted in EPC mobilization deficient MMP-9 −/− mice to determine whether the effects of Ang-1 were EPC-dependent.
Results
Overexpression of Ang-1 resulted in significantly improved re-epithelialization, neovascularization, and EPC recruitment in diabetic BMT wounds at day 7. Ang-1 treatment resulted in increased serum levels of proMMP-9 and SCF, but had no effect on vascular endothelial growth factor (VEGF) levels. According to our FACS results, peripheral blood EPC (CD34+/Cd133+/Flk1+) counts at day 3 post wounding showed impaired EPC mobilization in MMP-9 −/− mice, when compared with those of wild type controls. EPC mobilization was rescued by SCF administration, validating this model for EPC-mobilization deficient mechanistic studies. In MMP-9 −/− mice, Ad-Ang-1 accelerated re-epithelialization in a similar manner, but had no effect on neovascularization.
Conclusion
Our results show that Ang-1 administration results in improved neovascularization which is dependent on EPC recruitment and has direct effects on wound re-epithelialization. These data may represent a novel strategy to correct the phenotype of impaired diabetic neovascularization and may improve diabetic wound healing.
Keywords: diabetic wound healing, angiopoietin-1, MMP-9, neovascularization, endothelial progenitor cells, vasculogenesis
INTRODUCTION
Chronic diabetic ulcers are responsible for more than 42,500, non-traumatic lower-limb amputations and 27% of diabetic health care costs in the United States annually (1, 2). The impairments in the phenotype of cutaneous diabetic wound healing are associated with several intrinsic and extrinsic factors (3). Wounds in diabetic patients, as well as in murine models of Type I and Type II diabetes, show a defect in angiogenesis, re-epithelialization, and wound closure (4). The initial re-epithelialization does not depend on angiogenesis, but the complete healing and maturation are regulated closely by vascular responses of several cells and cell–matrix interactions (5). The deficiencies in angiogenesis have been attributed to poorly managed blood glucose levels and to the related vascular defects in both endothelial cells (EC) (6, 7) and endothelial progenitor cells (EPC) (8–14).
A deficit in neovascularization in diabetes is known to be associated with a compromised response to ischemia in wound healing as a consequence of metabolic derangement (3, 15), but beyond this vague understanding, the compromised ability to revascularize ischemic tissues in diabetes is poorly understood (10). A potential mechanism to explain this impairment is a decrease in the expression of angiogenic growth factors, such as vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), and their receptors (16, 17). Skin samples taken from the peri-wound area from lower extremities (skin within 1 cm from wound margin) of type 2 diabetic subjects has significantly less expression of both VEGF (46%) and Ang-1 (36%) than the skin tissue of nondiabetic subjects (18). Many groups have shown evidence linking impaired neovascularization with delayed closure of diabetic wounds, and further, that supplementation of angiogenic growth factors, such as VEGF, Ang-1, EGF, bFGF, and HIF1-α, or a combination of these factors via recombinant growth factor therapy or gene transfer, has positive effects on improving neovascularization and outcomes of diabetic wound closure (8, 19–25).
The angiopoietin family (Ang-1–4) has been shown to play a critical role in the modulation of physiologic angiogenesis and pathologic neovascularization. VEGF and the angiopoietins function together playing independent roles during vascular development and embryogenesis; VEGF acts early during vessel formation (26) and Ang-1 acts later during vessel remodeling, maturation, and stabilization (27, 28). Angiopoietins 1 and 2 have been studied in in vivo and in vitro models, particularly in relation to diabetic retinopathy (29, 30). Ang-1, a well-established secreted 70KDa ligand that shares many of the pro-angiogenic properties of VEGF, protects blood vessels from increased plasma leakage by counteracting transendothelial permeability stimulated by VEGF (31). Ang-1 signals primarily through the transmembrane receptor tyrosine kinase (Tie2), which is expressed ubiquitously in vascular endothelium and is phosphorylated in quiescent vessels. Ang-1 interacts with several cells, such as neutrophils, endothelial cells, and fibroblasts, through integrins to mediate survival, cell adhesion, and migration (32–34). Ang-1 is an essential and critical regulator of blood vessel development, as evidenced by the Ang-1 null mouse, which is embryonically lethal (35). In contrast, Ang-2, an antagonist of Ang-1 and Tie2 signaling, is generally not expressed in tissues of healthy adults, but is expressed in secretory tissues undergoing inflammation and vascular remodeling, such as healing wounds and tumors (36, 37). The systemic levels of Ang-2 increase early in sepsis (38). Compared with wounds in non-diabetic wildtype control mice (39), wounds in diabetic db/db mice show decreased expression of Tie1 and -2 proteins, which is paralleled by increased expression of the ligand Ang-2. Conversely, Ang-1 treatment was associated with suppressed development of diabetic retinopathy and decreased both vascular endothelial injury and breakdown of the blood-retinal barrier in a rat diabetic model (29). Ang-1 gene transfer also improved the delayed wound repair in diabetes by inducing angiogenesis, albeit in a VEGF-independent manner (25). A recent study suggested that angiopoietins 1 and 2 have a specific regulatory role in endothelial development from circulating CD34+ progenitors; Ang-1 regulates the initial commitment of endothelial progenitor cells, whereas Ang-2 enhances expansion of the endothelial cell progeny (39). The effects of Ang-1 on EPC mobilization and vasculogenesis are not completely defined.
The paradigm to improve therapeutic angiogenesis has focused on enhancing the formation of neovessels from preexisting, terminally differentiated endothelial cells to accelerate neovascularization. Several reports have shown that endothelial progenitor cells (EPCs) incorporate into areas of neovascularization via vasculogenesis. A novel approach to accelerate neovascularization is site-specific recruitment of bone marrow (BM)-derived endothelial progenitor cells to drive both the angiogenic and vasculogenic components of neovascularization. The mechanisms of EPC mobilization have been studied vigorously over the last decade (40). EPCS are primarily BM-derived and are characterized by antigenic markers defining the stemness and hematopoietic lineage (CD34, CD133), in combination with markers showing endothelial commitment (Flk-1) (41, 42). In response to tissue injury, EPCs mobilize from their BM niche into the circulation and home to sites of tissue repair under the guidance of hypoxia, growth factors, and chemokine signaling (40, 43). A suggested mechanism for EPC mobilization from the BM is mediated by matrix metalloproteinase 9 (MMP-9) and stem cell factor (SCF). Under conditions of wound hypoxia, transcription factors like hypoxia inducible factor – 1 (HIF-1) are activated, leading to increased transcription of VEGF (44, 45). VEGF activates MMP-9, which in turn cleaves and activates Kit ligand (KitL, also known as SCF) and induces proliferation and migration of EPCs from their BM niche (46). Treatment with Ang-1 or VEGF results in increased mobilization of EPCs from the BM (47). Ang-1 has been proposed to stimulate angiogenesis via activation of the Akt signaling pathway and the stimulation of eNOS (endothelial NO synthase), which is implicated in HIF-1α pathway and EPC mobilization. All these data notwithstanding, the mechanisms are not completely studied.
After taking all the previous results together, we hypothesized that Ang-1 overexpression in diabetic wounds will result in EPC recruitment and improve neovascularization and wound healing. Here we show that adenoviral-mediated over-expression of Ang-1 to treat diabetic wounds enhances EPC recruitment and corrects the diabetic defect in wound closure in a murine model of STZ-induced diabetes. This process was associated with an increase in MMP-9 and SCF, without an increase in VEGF levels.
METHODS
Mouse wound model
All animal procedures were performed with protocols approved by Cincinnati Children’s Hospital Institutional Animal Care and Use Committee. When the animals were under isoflurane (0.5 ml titrated) inhalational anesthesia, the dorsal skin was shaved, scrubbed with betadine, and then, with an 8 mm dermal biopsy punch, two full-thickness excisional skin wounds were created on the backs of mice, leaving the underlying panniculus carnosus muscle intact. Wounds were covered with sterile, adhesive dressing (Tegaderm™, St Paul, MN). Then, using a Hamilton syringe with a 30 ½ gauge needle, we injected 1×108 PFU Ad-Ang-1 or Ad-GFP (in 50 µl total volume, obtained from Penn Vector Core), or 50 µl PBS into the wound between the panniculus carnosus layer and the dressing. The mice received analgesics and were monitored daily for adverse events and general health.
Animal models
1) Tie2/LacZ BM transfer (BMT) model with STZ-induced diabetes
This murine model was chosen to assess the contribution of BM-derived progenitor cells to the wound healing process (to study the role of vasculogenesis). Transgenic mice carrying a beta-galactosidase reporter gene under the control of the murine Tie2 promoter (Tie2 LacZ; donor mice) were obtained from Jackson Laboratories (BarHarbor, ME). The iliac, tibia, and femur from both legs of male donor mice were harvested, crushed in PBS, and filtered with a 40 µm cell strainer. The red blood cells (RBCs) in the filtered cell solution were lysed with 1× ammonium chloride lysis buffer (BD Pharm Lyse). Cells were centrifuged, resuspended in PBS, and counted. Transplant recipient female FVBN wild type mice (n=12, Jackson Laboratories) were lethally irradiated with 700 cgy and 3 h later with 475 cgy (this is standard procedure performed in the Cincinnati Children’s Hospital Animal facility by trained personnel). Within 2 h after the second irradiation, new BM obtained from a donor was transplanted into these mice via tail vein injection of 1X106 cells in 300 µl PBS. An internal control animal, which did not receive the BM transplant, was included in each experiment of lethal irradiation. The irradiated control animal died within 4 days after lethal irradiation. Mice were placed on either doxycycline food or bactrim food to allow for complete recovery after the irradiation/transplant procedure. Engraftment was assessed with flow cytometric analysis of peripheral blood to check for chimerism (for this, approximately 50–100 µL of blood was collected via retro-orbital bleed). After engraftment (4–6 weeks), an intraperitoneal injection of 160 mg/kg of streptozotocin (STZ, Sigma-Aldrich; approximately 100µL injection volume) was administered to induce diabetes. The experiments of diabetic wound healing were performed 3–10 weeks after injection of STZ (at the time of wounding, mice were approximately 15 weeks old) using animals with serum glucose levels greater than 300 mg/dL. Glucose levels were checked every week with a glucometer using a few drops of animal blood collected via retro-orbital bleed.
2) MMP-9 −/− mice
To determine whether the effects of Ang-1 were EPC-dependent, a similar cutaneous wounding experiment was conducted in female MMP-9 −/− mice (FVB.Cg-Mmp9tm1Tvu/J, from Jackson Laboratories). We used MMP-9 −/− mice because MMP-9 plays a critical role in the mobilization of EPCs from the bone marrow. We and others have shown that mice lacking MMP-9 have impaired EPC mobilization and vasculogenesis (46, 48, 49). To rescue EPC mobilization in MMP-9 −/− mice, we administered IV injections (via tail vein, 0.5 µg/kg, 100 µL, PeproTech, Rocky Hill, NJ) of recombinant murine stem cell factor daily, for seven days, to these mice.
Tissue Processing and Histology
On day 7 post injury, the animals were sacrificed by trained personnel using CO2 inhalation followed by cervical dislocation. Blood was collected, and wounds were harvested and bisected. One half of the wound was fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin-embedded wounds were sliced in 5 µm sections with a RM 2035 microtome (Leica, Heidelberg, Germany), and collected on Superfrost Plus slides (Fisher, Pittsburgh, PA). Epithelial gap closure (defined as the distance between the two advancing epithelial margins) and deposition of granulation tissue were studied with hematoxylin and eosin (H&E) staining and morphometric image analysis using Nikon Elements imaging software (Nikon Instruments, Melville, NY).
The other half of the wound was stained to determine β-galactosidase activity. Wounds were rinsed with cold PBS, kept separately, fixed with 0.5% glutaraldehyde for 10 min, and X-gal stained with a solution containing 1 mg/mL of 5-bromo-4-chloro-3-indoyl- -D-galactopyronidase, 5 mmol/L K3Fe(CN)6, 5 mmol/L K4Fe(CN)6, and 1 mmol/L MgCl2 in PBS, pH 7.4, overnight at 37 °C (Sigma-Aldrich, St. Louis, MO). Wounds were then fixed with 10% neutral buffered formalin (Sigma) for 16 h. Post-fixed X-gal stained tissues were processed mechanically and paraffin-embedded. Serial 5 µm sections were cut and counterstained with 0.5% nuclear fast red. The mean number of β-gal positive cells was calculated from 10 high-powered fields distributed evenly throughout the similarly defined wound bed. These cells were denoted as EPCs.
Immunostaining
Serial sections were first immunostained with rat anti-CD31 antibodies (1:20; BD Pharmingen, Franklin Lakes, NJ), and then with biotinylated goat anti-rat antibodies (1:200; Vector laboratories, Burlingame, CA). Capillary lumen density in wounds was measured as the average number of CD31-positive lumens from 6 HPF (high-power fields, 40×) per section, chosen just above the panniculus carnosus and distributed equally between the epithelial margins.
Flow cytometry analysis/FACS
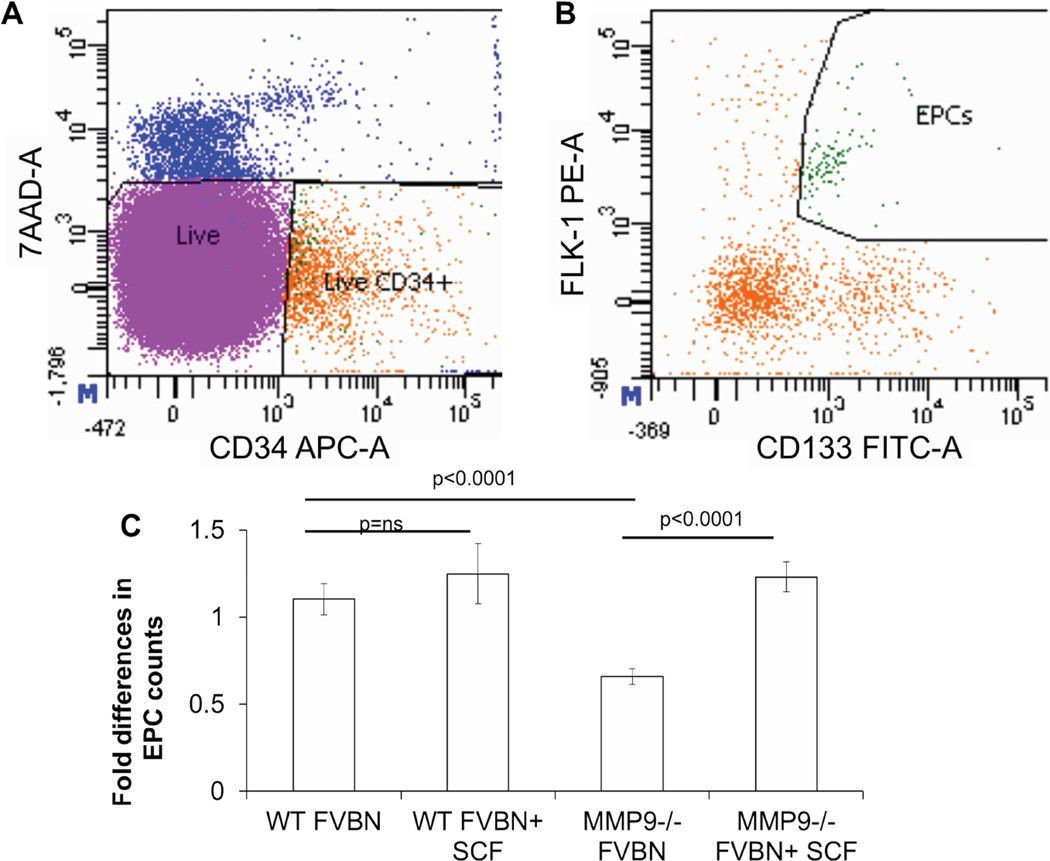
200 µl of whole blood per mouse was collected. Red blood cells were lysed with 1mL lysis solution, as per the manufacturer’s instructions (Qiagen, Alameda, CA). The cells were washed twice with 10mL of 1% BSA-PBS, resuspended in 1mL of 1% BSA-PBS solution, and counted. EPCs were co-labeled using APC-conjugated anti-CD34 (1 µl per 5×106 cells), FITC-conjugated anti-CD133 (1.5 µl per 1×106 cells), and PE-conjugated anti-Flk-1 (2 µl per 1×106 cells) monoclonal antibodies (BD Biosciences, San Jose, CA). Incubations with the antibodies were performed in the dark for 20 min at 4 °C with gentle rocking. Unstained control and individual color controls were also included for gating. Cells were washed and re-suspended in 350 µL of medium containing 7-AAD viability stain for live/dead discrimination (3µL per 1×106 cells, eBioscience, San Diego, CA). With the FACS Canto-II flow cytometer (BD Biosciences, San Jose, CA), single cells were gated to obtain the live CD34 positive population (Figure 5A). Within this population, cells that co-expressed CD133 and Flk-1 were counted as EPCs (Figure 5B–C). Each data point included at least 1,000,000 events. Flow data were then analyzed with FlowJo software (Tree Star Inc., Ashland, OR) by a blinded investigator.
Figure 5.
At 3 days post wounding, peripheral blood was collected from all experimental and controls groups. Circulating levels of EPCs were quantified by counting CD34+CD133+Flk1+ cells using flow cytometry analysis. Representative dot plot distributions are shown where cells are first gated on live CD34+ cells (A). Within this population, EPCs are quantified as cells that express both Flk-1 and CD133 (B). (C) Quantitative analysis demonstrated that after cutaneous wounding MMP-9 −/− mice have a decreased (2-fold) EPC mobilization compared to wildtype FVBN control mice. Stem cell factor (SCF) treatment rescued this phenotype and increased EPC mobilization in MMP-9 −/− mice to levels similar in controls. SCF had no effect on FVBN controls. Bar plots represent mean±SEM, (n=3), p values by ANOVA.
Enzyme Linked Immunosorbent Assays (ELISA)
Serum levels of VEGF, proMMP-9, and SCF were assessed with ELISA. On day 7 post wounding, serum was collected, and protein expression measured with the ELISA kit (R&D Systems, Minneapolis, MN), as per manufacturer’s protocol. Data were normalized to total protein measured with the Bradford method.
Statistical analyses
The results are reported as mean ± standard error of mean (mean±SEM). Statistical comparisons between experimental groups were performed with either two-way ANOVA or Student t-test, as appropriate. Results were considered statistically significant when p<0.05.
RESULTS
Over-expression of Ang-1 corrected diabetic impaired wound healing
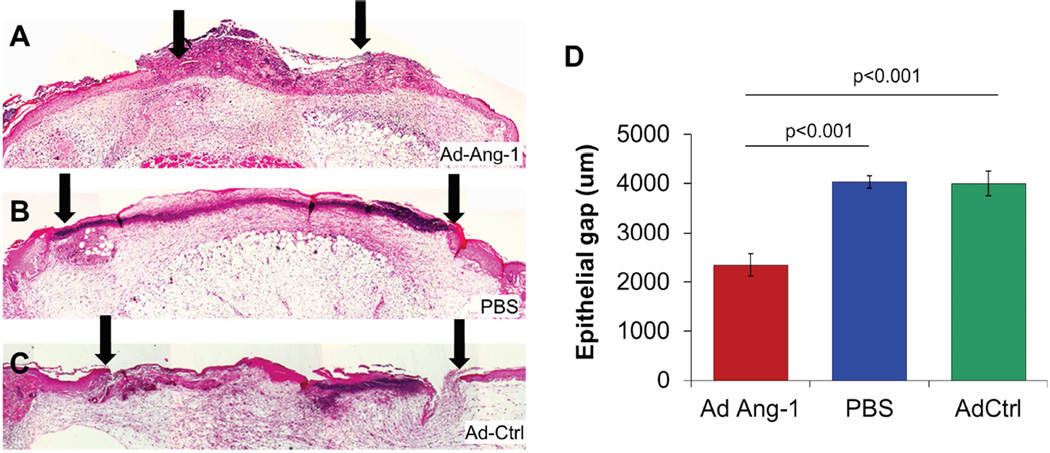
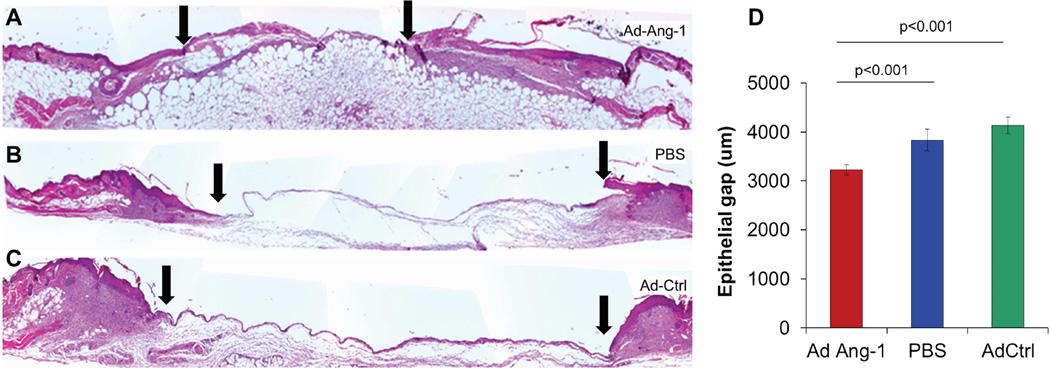
All mice were alive at 4 weeks post BMT. Mice with STZ-induced diabetes had blood glucose levels >350mg/dL. In diabetic BMT mice, excisional flank wounds (8 mm dermal punch biopsies) were created and treated with Ad-Ang-1, or Ad-GFP, or PBS as vehicle control. Most of the Ad-Ang-1-treated wounds were nearly healed by 7 days, in marked contrast to the control Ad-GFP-treated or PBS-treated wounds, which had not healed. Wounds treated with Ad-Ang-1 showed enhanced re-epithelialization at day 7 post wounding and a smaller epithelial gap (Ang-1 2.3±0.2mm; GFP 3.9±0.2; PBS 4.0±0.1, p<0.0001) overlying a bed of robust granulation tissue, as shown in Figure 1.
Figure 1.
(A–C) H&E stained BMT diabetic murine sections of the wound at day 7 post wounding demonstrated increased wound closure (distance between arrowheads represents epithelial gap) in Ad-Ang-1-treated wounds compared to Ad-GFP or PBS controls, with (D) statistical differences in epithelial gap between the three treatments. Bar plots represent mean±SEM, Ad-Ang-1- or PBS-treated wounds (n=4); Ad-GFP-treated wounds (n=3), p values by ANOVA.
Over-expression of Ang-1 enhanced neovascularization and recruitment of EPCs from BM, in diabetic wounds
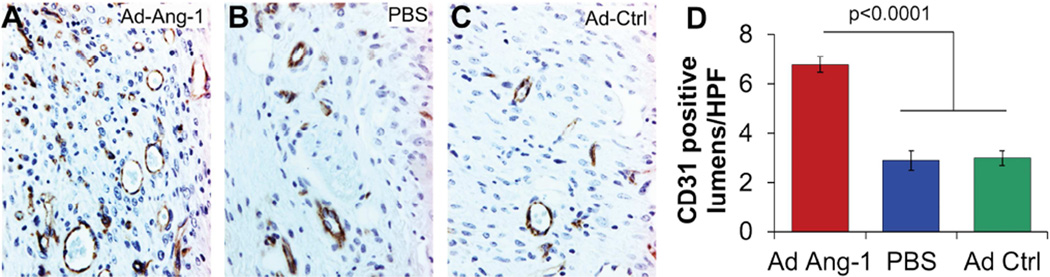
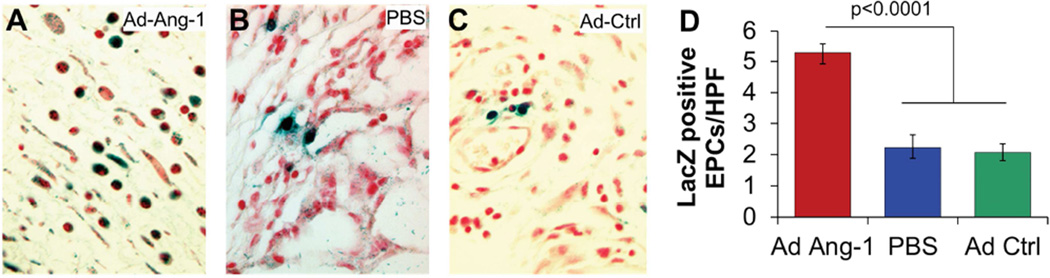
In addition, the Ad-Ang-1-treated wounds in diabetic BMT mice showed enhanced neovascularization, as indicated by an increase in capillary density (Figure 2: Ang-1 6.8±.3 Caps/HPF; GFP 3.0±.4; PBS 2.9±.3, p<0.0001) and an increase in EPC recruitment (Figure 3: Ang-1 5.3±.4 EPCs/HPF; GFP 2.1±.3; PBS 2.2±.3, p<0.0001) at day 7 post wounding, when compared with wounds treated with either Ad-GFP or PBS. Wounds treated with Ad-Ang-1 also had fewer inflammatory cells in the wound. This finding is particularly paradoxical given the ability of Ang-1 to specifically act as a recruitment cytokine for EPCs, and yet its presence resulted in fewer inflammatory cells being recruited to the wound.
Figure 2.
(A–C) CD31-stained sections of the wound at day 7 post wounding demonstrated increased density of capillary lumens in Ad-Ang-1-treated wounds compared to PBS or Ad-GFP controls, with (D) statistical differences in capillaries per high powered field (40×, CAPS/HPF) between the three treatments at day 7 post wounding. Bar plots represent mean±SEM, Ad-Ang-1- or PBS-treated wounds (n=4); Ad-GFP-treated wounds (n=3), p values by ANOVA.
Figure 3.
(A–C) β-gal stained sections of the wound at day 7 post wounding demonstrated increased LacZ-positive cells in Ad-Ang-1-treated wounds, signifying that they are BM derived cells that express the endothelial marker Tie2 which were quantified as EPCs, in comparison to fewer LacZ-positive cells in PBS or Ad-GFP control wounds with statistical differences in LacZ-positive EPCs per high powered field (40×, EPCs/HPF) between the three treatments at day 7 post wounding. Bar plots represent mean±SEM, Ad-Ang-1- or PBS-treated wounds (n=4); Ad-GFP-treated wounds (n=3), p values by ANOVA.
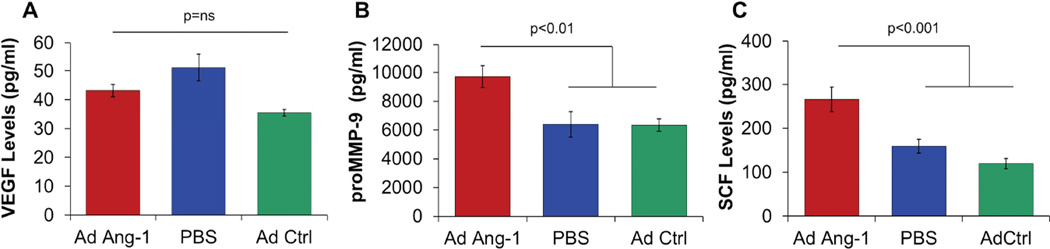
Over-expression of Ang-1 enhanced serum levels of MMP-9 and SCF, but not VEGF, at day 7 after wounding in BMT diabetic mice
Serum levels of VEGF were analyzed to determine whether Ang-1 treatment results in up-regulation of VEGF and whether the observed effects of Ang-1 on wound closure and neovascularization were mediated then via a downstream VEGF-dependent signaling. The serum VEGF levels in diabetic BMT wounded animals treated with Ang-1 gene transfer were not different from serum VEGF levels in control animals (Figure 4A). ProMMP-9 and SCF levels were then determined to study whether Ang-1 effects were mediated through an MMP-9 and SCF-dependent pathway. In contrast, treatment with Ang-1 resulted in an increase in both proMMP-9 (Figure 4B: Ang-1 9.7±.8 ng/mL; GFP 6.3±.9; PBS 6.4±.4, p<.01) and SCF (Figure 4C: Ang-1 265±28 pg/mL; GFP 119±16, PBS 159±12, p<.001) levels in serum at day 7 post-wounding, in BMT diabetic mice.
Figure 4.
There was no difference in serum VEGF levels at day 7 between Ad-Ang-1-treated and control groups (A). Ad-Ang-1 treatment resulted in a significant increase in MMP-9 levels (B) and SCF levels (C) compared to Ad-GFP or PBS controls at day 7. Bar plots represent mean±SEM, Ad-Ang-1- treated wounds (n=4); PBS- or Ad-GFP-treated wounds (n=3), p values by ANOVA.
Cutaneous wounding in MMP-9 −/− murine model showed decreased mobilization of EPCs, which was rescued by SCF treatment
Peripheral blood EPC (CD34+/Cd133+/Flk1+) counts at day 3 post wounding showed impaired EPC mobilization in MMP-9 −/− mice, when compared with the cell counts in wild type controls (Figure 5C: MMP-9 −/− 0.66±0.02; WT 1.1±0.05, p<0.001). SCF administered intravenously for 7 days rescued this phenotype and significantly increased mobilization of EPCs to the levels observed in FVBN wildtype controls (Figure 5C: MMP-9 −/−+SCF 1.23±0.05; WT 1.1±0.05, p=ns), validating this model for the study of mechanisms for MMP-9- and SCF-dependent EPC loss-of-function.
Over-expression of Ang-1 enhanced re-epithelialization of MMP-9 −/− wounds at day 7 post wounding
In MMP-9 −/− mice, treatment with Ad-Ang-1 accelerated re-epithelialization of the wounds at 7 days (Figure 6: Ang-1 3.2±0.1mm; GFP 4.1±0.2; PBS 3.8±0.2; p<.05). The epithelial gap was smaller than that of controls, suggesting that, in part, the effects of Ang-1 on re-epithelialization may be independent of MMP-9. Interestingly, at day 7 post wounding, cutaneous wounds in MMP-9 −/− mice (Figure 6A–C) showed an overall decrease in cellular density and in robustness of granulation tissue, when compared with the wounds of BMT diabetic mice (Figure1A–C) at day 7.
Figure 6.
(A–C) H&E stained MMP-9 −/− murine sections of the wound at day 7 post wounding demonstrated increased wound closure (distance between arrowheads represents epithelial gap) in Ad-Ang-1-treated wounds compared to Ad-GFP or PBS controls, with (D) statistical differences in epithelial gap between the three treatments. Bar plots represent mean±SEM, Ad-Ang-1- or PBS-treated wounds (n=4); Ad-GFP-treated wounds (n=3), p values by ANOVA.
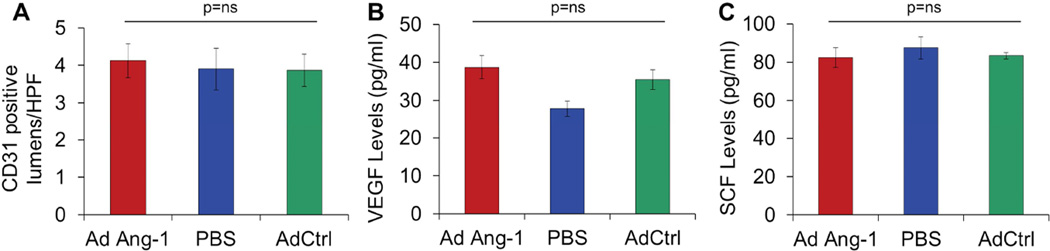
Over-expression of Ang-1 did not have an effect on the neovascularization of wounds in MMP-9 −/− mice, at day 7 post wounding
In MMP-9 −/− mice, administration of Ad-Ang-1 had no effect on wound neovascularization at day 7 post wounding (Figure 7A: Ang-1 4.1±0.5 Caps/HPF; GFP 3.9±0.4; PBS 3.9±0.6). Not surprisingly, neither the VEGF levels (Figure 7B) nor the SCF levels (Figure 7C) in serum at day 7 post wounding were affected by Ang-1 gene transfer in MMP-9 −/− mice.
Figure 7.
In MMP-9 −/− animals, there was no difference in neovascularization (A), serum VEGF levels (B), or serum SCF levels (C) at day 7 between Ad-Ang-1-treated and control groups. Bar plots represent mean±SEM, Ad-Ang-1- or PBS-treated wounds (n=4); Ad-GFP-treated wounds (n=3), p values by ANOVA.
DISCUSSION
Our results show that adenoviral-mediated gene transfer of Ang-1 improved re-epithelialization, enhanced the impaired angiogenesis, and increased BM-derived EPC recruitment to cutaneous wounds in diabetic mice, which was associated with an increase in serum levels of MMP-9 and SCF. In contrast, Ang-1 gene transfer did not modify the disrupted pattern of VEGF expression. We then studied the local effects of Ang-1 on wound healing to determine whether this effect was EPC-dependent. We performed experiments in mice deficient in EPC mobilization (MMP-9 −/− mice) and found that Ang-1 administration resulted in significant acceleration of wound closure in the MMP-9 −/− mice, albeit without an effect on wound neovascularization or on VEGF and SCF serum levels, as expected.
EPCs contribute to vessel growth in embryonic and damaged tissues in adults (50). One of the more potent factors that mobilize EPCs from their BM niche is VEGF (51–53). Tissue hypoxia after trauma or during wound healing has been shown to increase VEGF concentrations (54). Increased VEGF levels further activate MMP-9, which in turn cleaves and activates Kit ligand (SCF) and induces proliferation and migration of EPCs from their BM niche (46). Our previous data showed that Ang-1 is one of the most potent growth factors for the recruitment of EPCs, even more so than VEGF, among a number of tested cytokines. Moreover, our previous study showed that Ang-1 can enhance neovascularization and accelerate re-epithelialization of excisional wounds in genetically diabetic (db/db) mice (unpublished data). Over-expression of Ang-1 can attenuate cardiac apoptosis and promote cardiac repair in db/db mice after myocardial infarction by promoting the recruitment of CD133+/c-kit+ endothelial progenitor cells and restoring their function, as well as by improving angiogenesis (55). We asked the question of whether the underlying mechanism of the observed effects of Ang-1 on increased EPC mobilization in our wound model worked via a similar MMP-9- and SCF-dependent pathway. Our results showed that, indeed, the effects of Ang-1 on EPC mobilization and wound neovascularization were mediated via an MMP-9- and SCF-mediated pathway, because the effects were not observed in MMP-9 −/− animals. There was no increase in neovascularization, or in VEGF, or SCF levels; however, the effect of Ad-Ang-1 on improving wound closure persisted in MMP-9 −/− mice. We hypothesize that Ang-1 has a direct stimulatory effect on keratinocyte migration, which results in increased wound closure. Our preliminary in vitro data show a direct effect of Ang-1 on murine keratinocyte proliferation, measured by an increase in Ki-67 expression in these cells (unpublished data). To the best of our knowledge, there are no other reports on the direct effect of Ang-1 on keratinocyte migration, but there are data showing that angiopoietin like-4 plays a role in keratinocyte migration. Mice deficient in angiopoietin like-4 have impaired keratinocyte migration and delayed wound re-epithelialization (56).
In diabetes, the need for neovascularization arises from inadequate VEGF production and its release into wounds. Therefore, current attempts at targeting growth factors to accelerate wound healing in a diabetic setting have, for the most part, targeted the loss of VEGF protein. Several groups have shown that topical application of VEGF protein or VEGF over-expression in wounds with gene therapy significantly increases angiogenesis and accelerates diabetic wound healing in murine models of diabetes (23). But it has been suggested that increased VEGF also results in increased permeability, which may lead to leaky vessels, and decrease the efficacy of this therapy. The results of the present study, however, suggest that enhanced formation of wound capillaries may also be induced in diabetes with over-expression of Ang-1, which has in fact been shown previously to maintain vascular integrity and vessel maturation. More specifically, our present findings suggest strongly that Ang-1 causes, at least in diabetes, angiogenesis without increasing VEGF levels, possibly through an EPC-dependent increase in neovascularization and/or vascular remodeling. Because diabetic wounds have prolonged and persistent inflammation, granulation tissue formation is impaired, which further results in impaired angiogenesis. Under these specific circumstances, vasculogenesis may represent an alternative pathway to induce the formation of new blood vessels, and Ang-1 may represent a novel target to induce therapeutic vasculogenesis.
Up to now, Ang-1 was not thought to be a dominant factor in neovascularization. Ang-1 signals primarily through Tie1/TEK. Tie2 activity is tightly modulated by its angiopoietin ligands, and this highly coordinated signaling is required for maturation and maintenance of local angiogenesis, and for the integrity of the endothelium (57). Although Ang-1 expression is not affected by skin injury, Ang-2 expression is upregulated transiently during the period of formation of granulation tissue in normal mice, as shown by Kampfer and colleagues. Their study further showed that, under diabetic wound conditions, Ang-2 levels kept increasing even after day 7, a time point at which Ang-2 expression in normal wounds started to decline, and were paralleled by an increase in Tie1 levels, but a complete absence of Tie2 (17). The authors concluded that the overexpression of Ang-2 in the presence of markedly decreased VEGF expression (16) in diabetic wounds was associated with vessel regression and impaired neovascularization. The importance of Tie2 expression has also been shown in a skin flap model in diabetic mice, in which, tissue re-integration was disturbed severely and was associated with markedly decreased expression of endothelium-specific receptors Tie2 and FLT-1 (58). In another diabetes-related pathology, decreased Ang-1/Tie2 and increased Ang-2 expression have been suggested to contribute to diabetes-induced vascular damage after stroke (59). Similar attenuation of Tie2 expression and a significant increase in Ang-2 levels were observed in db/db mice subjected to myocardial ischemia (60).
In contrast, strategies that focused on activation or over-expression of Ang-1 proved to be efficacious to improve diabetic wound healing. In a recent study, Ang-1 activation with hydrogen sulfide restored EPC function and improved wound healing in a murine model of type-II diabetes (18). Similarly, a new Angiopoietin-based peptidomimetic compound, Vasculotide, improved activation of Ang receptor Tie2 and its associated signaling pathways in EC, which resulted in improved survival, migration, and MMP-2 production in vitro, and resulted in improved diabetic wound healing in db/db mice (61). Cartilage oligomeric matrix protein (COMP)-Ang-1, a soluble, stable, and potent form of Ang-1, significantly improved wound closure and epidermal and dermal regeneration in murine diabetic wounds associated with enhanced angiogenesis, lymphangiogenesis, and blood flow (62). These studies suggest that the impaired angiogenic responses in diabetic animals could result from an imbalance in the expression of VEGF and angiopoietins and that over-expression of Ang-1 or a combination therapy with VEGF and Ang-1 may result in mature, non-leaky, functional vasculature in diabetic wounds, which may be beneficial. As with any therapy, while attempting to harness the useful effects of Ang-1, it is also important to fully understand the spectrum of wound healing responses with varying doses of Ang-1. Particularly, the effects on spatial and temporal regulation of other angiopoietin family members and the angiogenic receptors Tie1 and Tie2 should be understood in the context of normal and chronic diabetic wound healing.
In conclusion, our results indicate that Ang-1 has direct effects on re-epithelialization and EPC recruitment and plays a biologically important role in both physiologic neovascularization and in the normal response to tissue injury in normal wound healing which is impaired in diabetic mice. These data suggest a new paradigm for stimulating neovascularization and correcting the impairment in diabetic wound healing by recruiting EPCs via targeted gene therapy.
ACKNOWLEDGEMENTS
The authors sincerely acknowledge the technical support received from the laboratory staff members and the support received from Cincinnati Children’s Hospital Medical Center Flow Cytometry Core. We also thank Dr. Ana Maria Rodriguez for editing the manuscript. This study is supported by NIH-NIDDK awards R01-DK072446 and R01-DK074055 (T.M.C.), and NIH-NIGMS awards K08 GM098831 and 1R01GM111808 (S.G.K).
ABBREVIATIONS
- Ad vectors
Adenoviral vectors
- Ang-1
Angiopoietin-1
- Ang-2
Angiopoietin-2
- bFGF
Basic fibroblast growth factor
- FVB/N
Inbred for the Fv1b allele which confers sensitivity to Friend leukemia virus B strain
- EC
Endothelial cells
- EGF
Epidermal growth factor
- ELISA
Enzyme linked immunosorbent assay
- EPCs
Endothelial progenitor cells
- eNOS
Endothelial NO synthase
- PFU
Particle forming units
- HIF-1α
Hypoxia inducible factor-1 alpha
- MMP-9
Matrix metalloproteinase-9
- NO
Nitric oxide
- PBS
Phosphate buffered saline
- PE
Phycoerythrin
- SCF
Stem cell factor
- Tie2
Receptor tyrosine kinase of the Tie family
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work described here is conducted in Cincinnati, Ohio, USA.
Author contributions: All authors approved the final version of the manuscript. SB-conception and design, analysis and interpretation, data collection, writing the article, critical revision of the article; NH and CM-analysis and interpretation, data collection, writing the article, critical revision of the article; AS- analysis and interpretation, critical revision of the article; PB- analysis and interpretation, critical revision of the article; TMC and SGK-conception and design, analysis and interpretation, data collection, critical revision of the article, obtaining funding.
Presented at the 10th Annual Academic Surgical Congress in Las Vegas, NV, February 3–5, 2015
REFERENCES
- 1.Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26:1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- 2.CDC. Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 3.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, et al. Wound healing: the role of growth factors. Drugs Today (Barc) 2003;39:787–800. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 5.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirola L, Balcerczyk A, Okabe J, El-Osta A. Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol. 2010;6:665–675. doi: 10.1038/nrendo.2010.188. [DOI] [PubMed] [Google Scholar]
- 7.Berlanga-Acosta J, Schultz GS, Lopez-Mola E, Guillen-Nieto G, Garcia-Siverio M, Herrera-Martinez L. Glucose toxic effects on granulation tissue productive cells: the diabetics' impaired healing. Biomed Res Int. 2013;2013:256043. doi: 10.1155/2013/256043. (15 pages). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, et al. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- 9.Capla JM, Grogan RH, Callaghan MJ, Galiano RD, Tepper OM, Ceradini DJ, et al. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2007;119:59–70. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 10.Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 12.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Sartore S, Schiavon M, Albiero M, Baesso I, Cabrelle A, et al. Diabetes impairs progenitor cell mobilisation after hindlimb ischaemia-reperfusion injury in rats. Diabetologia. 2006;49:3075–3084. doi: 10.1007/s00125-006-0401-6. [DOI] [PubMed] [Google Scholar]
- 15.Stehouwer CD, Lambert J, Donker AJ, van Hinsbergh VW. Endothelial dysfunction and pathogenesis of diabetic angiopathy. Cardiovasc Res. 1997;34:55–68. doi: 10.1016/s0008-6363(96)00272-6. [DOI] [PubMed] [Google Scholar]
- 16.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 17.Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and-2 and the tie-1 and-2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361–373. doi: 10.1038/labinvest.3780244. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, et al. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014;63:1763–1778. doi: 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singla S, Singla S, Kumar A, Singla M. Role of Epidermal Growth Factor in Healing of Diabetic Foot Ulcers. Indian J Surg. 2012;74:451–455. doi: 10.1007/s12262-012-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou Z, Nie C, Si Z, Ma Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1alpha. Diabetes Res Clin Pract. 2013;10:62–71. doi: 10.1016/j.diabres.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Losi P, Briganti E, Errico C, Lisella A, Sanguinetti E, Chiellini F, et al. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomater. 2013;9:7814–7821. doi: 10.1016/j.actbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Kwon MJ, An S, Choi S, Nam K, Jung HS, Yoon CS, et al. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J Gene Med. 2012;14:272–278. doi: 10.1002/jgm.2618. [DOI] [PubMed] [Google Scholar]
- 23.Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, et al. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol. 2009;129:2275–2287. doi: 10.1038/jid.2009.26. [DOI] [PubMed] [Google Scholar]
- 24.Allen RJ, Jr, Soares MA, Haberman ID, Szpalski C, Schachar J, Lin CD, et al. Combination therapy accelerates diabetic wound closure. PloS One. 2014;9:e92667. doi: 10.1371/journal.pone.0092667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitto A, Minutoli L, Galeano MR, Altavilla D, Polito F, Fiumara T, et al. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin Sci (Lond) 2008;114:707–718. doi: 10.1042/CS20070250. [DOI] [PubMed] [Google Scholar]
- 26.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 27.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 28.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 29.Joussen AM, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, et al. Suppression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree IA. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89:480–483. doi: 10.1136/bjo.2004.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oubaha M, Gratton JP. Phosphorylation of endothelial nitric oxide synthase by atypical PKC zeta contributes to angiopoietin-1-dependent inhibition of VEGF-induced endothelial permeability in vitro. Blood. 2009;114:3343–3351. doi: 10.1182/blood-2008-12-196584. [DOI] [PubMed] [Google Scholar]
- 32.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 33.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–e24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 34.Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 35.Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M, et al. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- 37.Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, et al. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–124. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Moss A. The angiopoietin:Tie 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24:579–592. doi: 10.1016/j.cytogfr.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Schurmann C, Schmidt N, Seitz O, Pfeilschifter J, Frank S. Angiogenic response pattern during normal and impaired skin flap re-integration in mice: a comparative study. J Craniomaxillofac Surg. 2014;42:1710–1716. doi: 10.1016/j.jcms.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Hildbrand P, Cirulli V, Prinsen RC, Smith KA, Torbett BE, Salomon DR, et al. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104:2010–2019. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 43.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 44.Rafii S, Avecilla S, Shmelkov S, Shido K, Tejada R, Moore MA, et al. Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment. Ann N Y Acad Sci. 2003;996:49–60. doi: 10.1111/j.1749-6632.2003.tb03232.x. [DOI] [PubMed] [Google Scholar]
- 45.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 46.Semenza GL. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. J Clin Invest. 2001;108:39–40. doi: 10.1172/JCI13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang PH, Chen YH, Wang CH, Chen JS, Tsai HY, Lin FY, et al. Matrix metalloproteinase-9 is essential for ischemia-induced neovascularization by modulating bone marrow-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1179–1184. doi: 10.1161/ATVBAHA.109.189175. [DOI] [PubMed] [Google Scholar]
- 50.Hurley JR, Cho H, Sheikh AQ, Balaji S, Keswani SG, Crombleholme TM, et al. Nanofiber Microenvironment Effectively Restores Angiogenic Potential of Diabetic Endothelial Cells. Adv Wound Care (New Rochelle) 2014;3:717–728. doi: 10.1089/wound.2013.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 52.Li B, Sharpe EE, Maupin AB, Teleron AA, Pyle AL, Carmeliet P, et al. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006;20:1495–1497. doi: 10.1096/fj.05-5137fje. [DOI] [PubMed] [Google Scholar]
- 53.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosti V, Massa M, Campanelli R, De Amici M, Piccolo G, Perfetti V. Vascular endothelial growth factor promoted endothelial progenitor cell mobilization into the peripheral blood of a patient with POEMS syndrome. Haematologica. 2007;92:1291–1292. doi: 10.3324/haematol.11455. [DOI] [PubMed] [Google Scholar]
- 55.Heeschen C, Dimmeler S, Hamm CW, Boersma E, Zeiher AM, Simoons ML. Prognostic significance of angiogenic growth factor serum levels in patients with acute coronary syndromes. Circulation. 2003;107:524–530. doi: 10.1161/01.cir.0000048183.37648.1a. [DOI] [PubMed] [Google Scholar]
- 56.Zeng H, Li L, Chen JX. Overexpression of angiopoietin-1 increases CD133+/c-kit+ cells and reduces myocardial apoptosis in db/db mouse infarcted hearts. PloS One. 2012;7:e35905. doi: 10.1371/journal.pone.0035905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–33009. doi: 10.1074/jbc.M110.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milner CS, Hansen TM, Singh H, Brindle NP. Roles of the receptor tyrosine kinases Tie1 and Tie2 in mediating the effects of angiopoietin-1 on endothelial permeability and apoptosis. Microvasc Res. 2009;77:187–191. doi: 10.1016/j.mvr.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–1613. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Slyke P, Alami J, Martin D, Kuliszewski M, Leong-Poi H, Sefton MV, et al. Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A. 2009;15:1269–1280. doi: 10.1089/ten.tea.2007.0400. [DOI] [PubMed] [Google Scholar]
- 62.Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, et al. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A. 2006;103:4946–4951. doi: 10.1073/pnas.0506352103. [DOI] [PMC free article] [PubMed] [Google Scholar]