Abstract
Inhibitory receptors expressed on T cells control immune responses while limiting autoimmunity. However, tumors can hijack these ‘checkpoints’ for protection from immune attack. Tumor-specific T cells that exhibit an exhausted, unresponsive phenotype express high levels of inhibitory receptors including CTLA4, PD1 and LAG3, among others. Intratumoral regulatory T cells promote immunosuppression and also express multiple inhibitory receptors. Overcoming this inhibitory receptor-mediated immune tolerance has thus been a major focus of recent cancer immunotherapeutic developments. Here, we review how boosting the host’s immune system by blocking inhibitory receptor signaling with antagonistic monoclonal antibodies restores the capacity of T cells to drive durable antitumor immune responses. Clinical trials targeting the CTLA4 and PD1 pathways have shown durable effects in multiple tumor types. Many combinatorial therapies are currently being investigated with encouraging results that highlight enhanced antitumor immunogenicity and improved patient survival. Finally, we will discuss the ongoing identification and dissection of novel T-cell inhibitory receptor pathways, which could lead to the development of new combinatorial therapeutic approaches.
Keywords: Cancer immunotherapy, CTLA4, PD1, LAG3, inhibitory receptors, monoclonal antibodies
Introduction
Two signals are required to initiate an adaptive immune response by T cells: antigen recognition by the T-cell receptor (TCR) and costimulation via an array of receptors interacting with cognate ligands on antigen presenting cells (APCs). Under homeostatic conditions, signaling via inhibitory receptors (IRs) is necessary to balance costimulatory receptor activity to ensure a measured response that, without control, would result in exacerbated activation and autoimmunity. However, during cancer progression, tumor-specific T cells have been shown to display increased, chronic expression of multiple IRs, including, but not exclusive to, PD1, LAG3 and TIM3, which causes their functional exhaustion and unresponsiveness [1, 2]. These exhausted CD8+ tumor-infiltrating lymphocytes (TILs) fail to proliferate in response to antigen and lack critical effector functions such as cytotoxicity and cytokine secretion. The resulting immune tolerance creates multiple barriers to tumor elimination, including regulatory T (Treg) cell infiltration into the tumor, coinhibitory signaling via IRs, and release of suppressive cytokines such as IL-10, TGF-β and IL-35 [3, 4].
Recent immunotherapeutic advances have aimed to target IRs to reverse the exhausted state, re-invigorate T cells and promote antitumor immunity. Substantive, early success has been achieved with monoclonal antibodies (mAbs) blocking signaling through IRs such as CTLA4 and PD1, leading to cancer immunotherapy being highlighted as the “Breakthrough of the Year” in 2013 [5]. Although impressive objective response rates (defined as the percentage of patients whose tumor burden shrinks or disappears following treatment) for both CTLA4- and PD1/PDL1-targeted monotherapies have been observed in multiple tumor types, it was the durable responses seen with PD1 blockade in lung cancer patients that have substantially increased interest in this class of immunotherapeutics [6, 7]. Multiple IRs are expressed on TILs, rather than the tumor cells [8, 9], suggesting that targeted, combinatorial mAb blockade may provide improved clinical benefit compared with that of “conventional” treatments, such as chemotherapy and radiation, with reduced hypersensitivity reactions reported [10].
This review will focus mainly on CTLA4, PD1 and LAG3 (Figure 1); three IRs for which blocking mAbs have been approved or are in clinical trials for the treatment of various cancer types. Importantly, clinical trials are ongoing or in development to determine the optimal combinations of immunotherapeutics with or without the inclusion of chemotherapeutic modalities such as gemcitabine/cisplatin and/or radiotherapy for the treatment of a large number of tumor types. Additional IRs and their cognate ligands that have shown potential in preclinical tumor models will also be discussed as potential therapeutic targets. Other novel immunotherapeutic approaches not covered here include agonist mAbs targeting costimulatory molecules such as 4-1BB, OX40 and CD40 (reviewed in [11]); blocking or depleting mAbs targeting inhibitory populations, such as Treg cells and MDSCs (reviewed in [12]); adoptive T-cell therapies using either patient-derived, tumor antigen-expanded T cells or lentivirus-transduced T cells expressing chimeric antigen receptors (CARs) (reviewed in [13]); and vaccination using genetically-modified dendritic cells (DCs) presenting tumor-restricted epitopes (reviewed in [14]). Lastly, this review will address some of the remaining critical questions and the challenges ahead in deriving the optimal combinatorial therapies for cancer.
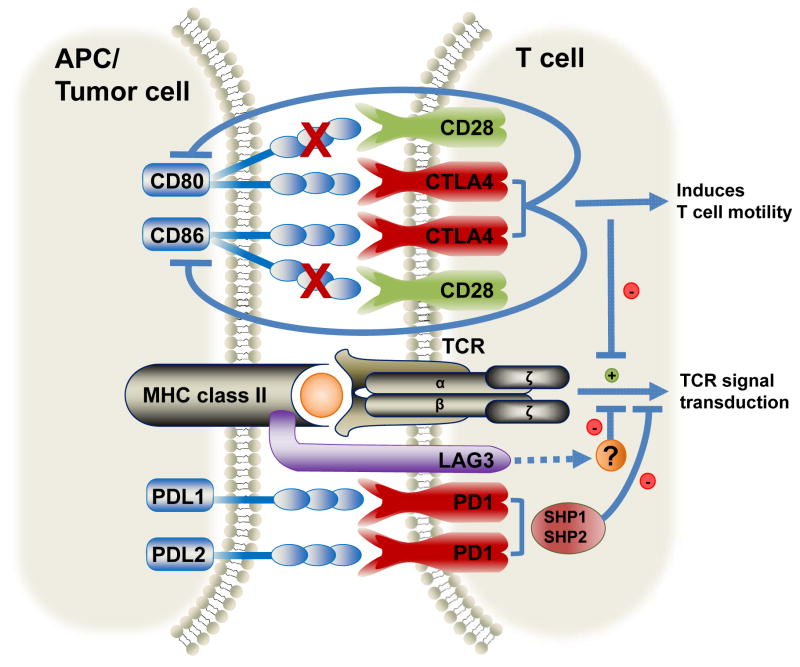
Figure 1.
Recognition of MHC class II-presented antigen by the T-cell receptor on CD8+ T cells initiates a signaling cascade necessary to generate an adaptive immune response. Cytotoxic T-lymphocyte Antigen 4 (CTLA4), Programmed Death-1 (PD1) and Lymphocyte Activation Gene 3 (LAG3) are inhibitory receptors expressed on the surface of T cells, and which interact with their cognate ligands expressed on antigen presenting cells (APCs) or tumor cells to control overt activation. CTLA4 competes to bind to CD80/86, preventing ligation of these ligands with CD28 (depicted by X). This induces T-cell motility attenuating T-cell activation. PD1 binds Programmed Death Ligand-1 (PDL1) and PDL2, recruiting Src homology 2 domain-containing protein tyrosine phosphatase (SHP)-1 and SHP-2 that inhibits downstream signaling and T-cell activation. LAG3 binds to MHC class II molecules and negatively regulates T-cell activation by an unknown mechanism. Together, these inhibitory receptors act as checkpoints to control immune responses and limit autoimmunity.
Cytotoxic T-Lymphocyte Antigen 4 (CTLA4/CD152)
CTLA4 is an immunoglobulin superfamily member (IgSF) IR that is upregulated on activated T cells, and is constitutively expressed on Treg cells, playing a central role in maintaining cell-intrinsic immune control and peripheral tolerance (reviewed in [15]). Multiple cell-extrinsic mechanisms have been proposed for CTLA4, although controversy remains. In conventional T cells, CTLA4 has been proposed to induce T-cell motility [16, 17]. CTLA4 overrides a stop signal induced by TCR ligation that would otherwise allow an immunological synapse between T cells and DCs to form [16]. Failure of CTLA4+ T cells to perform such prolonged interactions with APCs was shown by in vivo imaging studies, thus modulating T-cell activation [16]. Another mechanism which limits T-cell activation is exhibited by CTLA4-expressing Treg cells, which have been shown to downregulate CD80/86 on APCs, a process which can be reversed by addition of anti-CTLA4 mAb [18, 19]. It has also been suggested that CTLA4 can bind and remove these ligands by a trans-endocytosis mechanism [20].
Early studies showed that CTLA4 blockade in murine melanoma models resulted in tumor regression, demonstrating the potential of IR modulation as an immunotherapeutic approach [21]. CTLA4 was the first IR to be targeted clinically for cancer therapy with Ipilimumab, a fully human IgG1 mAb [22]. Ipilimumab thus releases antitumor effector T cells from CTLA4-mediated inhibition, although the mechanism of action remains unclear. It has been suggested that Ipilimumab mediates FcγR-mediated depletion of Treg cells within the tumor, although this remains controversial [23]. It is also worth noting that Tremelimumab, another CTLA-4 mAb that has a different isotype which does not bind FcγR, has similar therapeutic efficacy [24]. In 2011, Ipilimumab was approved in the USA and Europe for the treatment of patients with advanced (Stage III/IV) melanoma [22]. This followed a randomized, controlled phase III trial in which the mAb was administered as a monotherapy or with a well-utilized glycoprotein 100 (gp100) peptide vaccine, derived from melanoma in order to elicit T-cell-mediated immune responses [25]. Patients receiving both Ipilimumab and gp100 showed a median objective survival (OS) of 10.0 months compared with 6.4 months in patients receiving gp100 alone [25]. In this trial, the median OS of patients receiving Ipilimumab as a monotherapy was 10.1 months, suggesting that Ipilimumab efficacy was not enhanced by the addition of the vaccine. Another large randomized phase III trial showed that Ipilimumab administered with dacarbazine, an anti-neoplastic chemotherapy drug acting as an alkylating agent, enhanced overall survival of previously untreated melanoma patients compared with dacarbazine treatment alone – with median OS rates of 11.2 and 9.1 months, respectively [26].
Ipilimumab was the first blocking mAb found to cause significant regression of metastatic melanoma, with an overall long-term (3 year) survival rate of 21% and impressive results, including complete remission, observed in some patients [27, 28]. However, many immune-related, grade 4 adverse events (defined as life-threatening) were reported to occur following anti-CTLA4 mAb administration, particularly gastrointestinal tract-related complications [29]. This may be due to the high frequency of CTLA4+ Treg cells present at mucosal sites [30]. Adverse events ranged from mild to moderate with the potential to be life-threatening; however, the severity of these side effects was inversely related to patient surveillance and most were treatable and reversible if detected early. Primary side effects included rash (20% of patients), colitis (15%) and thyroiditis (2–5%) [25, 26]. As pre-metastatic solid malignancies are generally spatially restricted, it might be beneficial to target IRs that are preferentially expressed on TILs, such as PD1 or LAG3, rather than peripheral T-cell subsets, in an attempt to limit toxicity.
Clinical evaluation of Ipilimumab is ongoing in prostate, pancreatic, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), gastric and ovarian cancers. There are currently over 200 Ipilimumab-containing clinical trials (Phase I-III) (see clinicaltrials.gov), with many of these combining conventional chemotherapy or radiation with anti-CTLA4 blockade. Other trials combining Ipilimumab with mAbs targeting additional IRs will be discussed in detail below.
Tremelimumab is another fully humanized anti-CTLA4 mAb but has an IgG2 isotype [24]. Phase I/II trials determined that this mAb was safe and that it had antitumor activity against melanoma as a monotherapy or in combination with other agents, with a proportion of patients showing tumor remission. However, in a recent Phase III trial, Tremelimumab failed to statistically improve overall survival of late-stage melanoma patients compared with dacarbazine alone [24]. Nonetheless, 20.7% of patients receiving Tremelimumab alone survived for at least 3 years, which is almost identical to the observations of patients receiving Ipilimumab (20.8%). Administration of Tremelimumab thus shows activity as a monotherapy and trials for treatment of malignant mesothelioma and NSCLC in combination with an anti-EGFR inhibitor (Gefitinib) are being initiated.
In a murine model of melanoma, anti-CTLA4-driven depletion of tumor-infiltrating Treg cells was shown to be FcγRIV-dependent associated with FcγR-expressing macrophages that are highly infiltrated within the tumor microenvironment [23]. However, FcγRIV does not exist in humans, which challenges the translational implications of these findings [31]. Moreover, although only mouse IgG2a and IgG2b anti-CTLA4 mAb isotypes bind to FcγRIV to mediate Treg-cell depletion (and mouse IgG1 does not), human IgG1 mAbs (i.e. Ipilimumab) do bind to FcyRs at a higher affinity than human IgG2 mAbs (i.e. Tremelimumab). This would suggest that Ipilimumab may mediate a more potent FcγR-dependent Treg-cell depletion by antibody-dependent cellular cytotoxicity (ADCC), however this has not been observed in pre-clinical trials [32, 33]. Although further investigation into the mechanism of action of anti-CTLA4 is required, as well as the fact that Ipilimumab and Tremelimumab remain to be formally tested side-by-side, differences in activity may be due to isotype and FcγR-mediated ADCC of Treg cells.
Finally, there are currently no biomarkers to predict whether patients will respond to anti-CTLA4 treatment, although significant increases in Foxp3 and indoleamine 2,3-dioxygenase (IDO) expression in the tumor environment have been shown to correlate with improved clinical outcomes [34]. However, Treg cells utilize IDO as another immunosuppressive mechanism and expression of IDO has been shown to attenuate the efficacy of anti-CTLA4 monotherapy in mice [35].
Programmed Death-1 (PD1/CD279)
PD1 is constitutively expressed on Treg cells and is induced, upon activation, on effector T cells, NK cells, NKT cells and B cells [36]. Enhanced PD1 coexpression with other IRs has been observed on CD8+ and CD4+ TILs, correlating with antigen unresponsiveness and gradual loss of function including decreased cytokine secretion, proliferation and cytolytic capacity [2]. PD1 has previously been shown to be overexpressed on exhausted CD8+ T cells, compared with functional effector and memory T cells, during chronic lymphocytic choriomeningitis virus (LCMV) clone 13 infection, and it was in this model that the functional and phenotypic relevance of PD1-mediated T-cell exhaustion was established [37].
PDL1 (B7-H1/CD274) is the major PD1 ligand, and is constitutively expressed on T cellsT cellsT cells, B-cells, DCs, macrophages and a wide variety of non-hematopoietic cell types [36, 38]. PDL1 can be found on several solid tumor types (gastric, breast, pancreatic, ovarian, lung, prostate, malignant melanoma) and on myeloid cells infiltrating the tumor microenvironment, with expression associated with poor prognosis for melanoma, breast and ovarian cancer [39–42]. The minor ligand, PDL2 (B7-H2/CD273), exhibits restricted expression on DCs and macrophages, as well as in leukemia and B-cell lymphomas [36].
Although initial studies suggested that PDL1 and PDL2 costimulated T cells [43], further studies found that ligation with PD1 rather coinhibits pre-activated T cells by suppressing TCR signaling through recruitment of Src homology 2 (SH2) domain-containing protein tyrosine phosphatase (SHP)-1 and SHP-2 [44]. SHP-1 and SHP-2 decrease the phosphorylation status of CD3ζ chain immunoreceptor tyrosine-based activation motifs (ITAMs), attenuating ZAP-70 activation and inhibiting downstream signaling and T-cell activation [45].
Pembrolizumab (formerly known as Lambrolizumab/MK-3475) is a fully humanized anti-PD1 IgG4 mAb that obtained FDA approval in September 2014 for the treatment of patients with metastatic melanoma in the USA [46]. Pembrolizumab has also shown promising activity and tolerability in studies of advanced NSCLC, melanoma, and head and neck cancer [47–49]. In a Phase 1b trial, 37% of patients with advanced melanoma had objective response rates to Pembrolizumab treatment [47]. Moreover, 38% of patients who were previously non-responsive to Ipilimumab responded to Pembrolizumab, suggesting that patients who fail one form of IR blockade may be amenable to another IR-blocking mAb therapy [47]. Results from a recent randomized trial reported that treatment with Pembrolizumab gave prolonged overall and progression-free survival in patients with advanced melanoma compared with Ipilimumab, suggesting that anti-PD1 blockade is superior to targeting CTLA4 [50]. Moreover, only low-grade toxicities were reported with Pembrolizumab including fatigue, rash and diarrhea.
Nivolumab was the second fully human IgG4 anti-PD1 mAb to be approved in the USA (in December 2014), which has shown efficacy in targeting multiple cancer types including NSCLC, melanoma and renal cell carcinoma (RCC), with reports of rapid and durable tumor regression in some patients [6]. In a Phase III clinical trial of patients with advanced melanoma receiving Nivolumab, objective responses were seen in 31% of patients with tumor shrinkage of at least 30% [6]. Of those that did respond, 45% demonstrated rapid tumor regression at 8 weeks and 71% maintained anticancer responses of tumor regression for at least 16 weeks after the drug was discontinued [51]. Striking results have also been achieved with Nivolumab in pretreated patients with Hodgkin’s lymphoma [52]. Objective response rates occurred in 20 out of 23 patients (87%), including four (17%) with a complete response and 16 (70%) with a partial response. Like Pembrolizumab, Nivolumab only induced low-grade mild and moderate side effects; however, these were not cumulative as observed in the patients that remained on the drug for longer [6]. In fact, severity of adverse event rates have been shown to decline for unknown reasons; an encouraging scenario for prolonged or combinatorial therapies targeting PD1 [6]. Overall, consistent activity is achieved with anti-PD1 mAbs currently in clinical trials with a broad spectrum of different cancers responsive to PD1 blockade.
Antibodies targeting PD1’s dominant ligand have also been developed with similar clinical observations. For instance, the PDL1 mAb MPDL3280A, a fully human IgG1 mAb that inhibits PDL1 binding to both PD1 and CD80, has shown efficacy in clinical trials. This mAb contains an engineered Fc domain to minimize antibody-dependent cell-mediated cytotoxicity (ADCC) and improve safety. In a Phase I study, patients with previously-treated metastatic urothelial bladder cancer, with tumors identified as PDL1-positive, showed a 52% response rate after 12 weeks [53]. Adverse events were low-grade effects including fatigue and nausea, with no evidence of renal toxicity. Durable response rates were also observed in melanoma patients, so MPDL3280A was granted a Breakthrough Therapy designation by the FDA. Further study of MPDL3280A is continuing in patients with advanced RCC or NSCLC [54, 55]. Trials with Avelumab, another fully humanized IgG1 anti-PD1 mAb, are also being initiated for efficacy and safety in patients with metastatic Merkel cell carcinoma [56].
PD1, like other IRs, functions as a negative regulator of effector T-cell responses to prevent immunopathological damage by overactive effector T cells. If a tumor expresses PDL1, PD1-mediated signaling represses T-cell responses. T-cell effector function can thus be rejuvenated when PD1/PDL1 ligation is blocked. Tumors, such as melanoma, have been shown to constitutively express PDL1 as an innate resistance mechanism, which inversely correlates with disease prognosis [57, 58]. However, PDL1 expression can also be induced by IFN-γ, which is produced as part of the ongoing adaptive immune response against the tumor [59]. The caveat is that PDL1 expression induced in this manner may be indicative of improved survival as PDL1 expression, when associated with TIL infiltration, correlates with prolonged OS in metastatic melanoma [60].
Nevertheless, PDL1 expression on tumors is the strongest predictive biomarker to identify patients that may be most responsive to anti-PD1/PDL1 immunotherapy [61]. However, in other studies, some patients identified as PDL1-negative did respond to anti-PD1/PDL1 mAb therapy, suggesting against the stratification of patients based solely on PDL1 expression [6]. It is not clear whether PDL1-negative patients respond to anti-PD1 due to misdiagnosis of their PDL1 expression (due to assay insensitivity, heterogeneous expression, etc.), or if PD1:ligand interactions with APCs are sufficient to mediate T-cell exhaustion [57]. Sensitivity and specificity of PDL1 detection varies between assay types, assay suppliers, and staining protocols. Although the use of PDL1 as a predictive biomarker is appealing, there are a number of pitfalls and concerns with the assays used to detect PDL1 expression and further studies to evaluate its usefulness are ongoing.
Lymphocyte Activation Gene 3 (LAG3/CD223)
LAG3 is an IR that is structurally similar to CD4; it contains four Ig-like domains and binds to MHC class II molecules, but with greater affinity than CD4. Like PD1, LAG3 has a broad expression profile and is constitutively expressed on Treg cells and induced on activated T cellsT cellsT cells and NK cells [62–64]. Unlike PD1, which is expressed on myeloid DCs, LAG3 is expressed by plasmacytoid DCs [65]. LAG3 has been shown to negatively regulate T-cell activation and proliferation, homeostatic expansion and to control the size of the memory T-cell pool in mice [66, 67]. However, its mechanism of action remains unknown. LAG3 lacks common inhibitory motifs, although a KIEELE motif in the cytoplasmic tail appears to be required for its inhibitory function [68]. LAG3 function also appears to be regulated by cell surface cleavage by ADAM10 and ADAM17 disintegrin/metalloproteases, although soluble LAG3 appears to have no biological function in mice [69, 70]. Lastly, LAG3 is required for maximal suppressive activity of both thymus-derived and peripherally-derived Treg cells [62, 67].
LAG3 has been shown to be coexpressed on TILs with other IRs, including PD1, and is indicative of a highly exhausted T-cell phenotype [71]. Increased expression of LAG3, coexpressed with PD1, was also found to correlate with CD8+ TIL dysfunction in patient tumor samples, and so clinical trials with a LAG3 mAb (BMS-986016) started in 2013 which should provide some initial insight into the potential utility of LAG3 blockade. [72].
Combinational therapies with mAbs targeting CTLA4, PD1 and LAG3
Although CTLA4 or PD1/PDL1 blockade as a monotherapy has shown impressive tumor regression in a proportion of patients, the overall long-term survival rate needs to improve. While it is widely recognized that combinatorial immunotherapy is likely to yield significant improvement, there is substantial debate over the optimal combination of immunotherapeutic modalities. It is generally perceived that greater efficacy may be obtained by combining modalities that target distinct pathways and/or cell types. As anti-CTLA4 has been shown to target the interface between T cells and DCs (by blocking CTLA4:CD80/86 interactions) and as anti-PD1 can also interfere with interactions between the tumor and TILs (by blocking PD1:PDL1 ligation, expressed on tumor cells) it was suggested that combined targeting of these two pathways might be efficacious. Indeed, studies in murine tumor models had previously revealed a synergistic impact on tumor growth with a combination of PD1 and CTLA4 mAbs, resulting in complete B16 tumor regression in a high percentage of mice [73].
A Phase I clinical trial with CTLA4-targeting Ipilimumab plus PD1-targeting Nivolumab was conducted in patients with metastatic melanoma [74]. A rapid and durable regression of target lesions was seen with the combination, with the majority of patients responding to treatment showing tumor regression at the initial tumor assessment. The objective response rate for concurrent Ipilimumab and Nivolumab immunotherapy in this study was 40%, much greater than either monotherapy [74]. Combined Ipilimumab/Nivolumab therapy also affected an increased frequency of Ki67+ CD4+ and CD8+ T cells in the circulation, which is indicative of enhanced cellular proliferation [75]. The majority of patients (62%) experienced some side effects by definition. However, the most common were increases of serum amylase and lipase, although these patients were generally asymptomatic and did not show signs of pancreatitis, suggesting that these are laboratory anomalies [76]. However, ~23% of patients did have to discontinue treatment due to adverse side effects, including one death due to multi-organ failure following an initial event of colitis related to the treatment. Thus, while efficacy can be enhanced by combining immunotherapies, the possibility of increased side effects looms large.
Additional clinical trials combining Ipilimumab and Nivolumab are underway. For instance, combined Ipilimumab and Nivolumab treatment of RCC patients has thus far reported higher objective response rates than either monotherapy [77]. In another example, the initial Phase I safety assessment of the LAG3 mAb, BMS-986016, is patterned on an innovative trial design in which an additional arm combining BMS-986016 with Nivolumab is included; data on the efficacy of this combination will start to accrue once safety and dose tolerability has been determined. This combination is interesting given promising preclinical data in the MC38 murine tumor model, in which dual blockade with anti-PD1 and LAG3 mAbs synergistically limited tumor growth and led to complete tumor clearance in 80% of mice, compared with 40% tumor clearance with anti-PD1 blockade alone [71]. Synergistic cooperation between blockades of PD1 and LAG3 in controlling tumor growth is also supported by observations in PD1/LAG3-deficient mice, which were able to clear high doses of B16 and MC38 transplantable tumors, with only delayed tumor growth displayed in the respective single knockout mice [71].
Clinical trials combining multiple mAbs against IRs as a cancer therapy strategy are summarized in Table 1, but in addition there are a large number of studies ongoing in which checkpoint inhibition is being combined with other immunomodulatory approaches. For instance, several studies combining CTLA4 blockade with therapeutic vaccines are being assessed. Examples include Ipilimumab administered with the gp100 vaccine in advanced melanoma [25], or granulocyte macrophage-colony stimulating factor (GM-CSF) gene-transfected cell vaccine (GVAX) in pancreatic or prostate cancer, which is known to enhance tumor immunogenicity by promoting the differentiation and activation of macrophages and DCs [78, 79]. In a randomized Phase Ib trial, combinational Ipilimumab and GVAX treatment afforded no enhancement in progression-free survival compared with Ipilimumab alone, but there was an improvement in overall survival [80]. Interestingly, the toxic effects of Ipilimumab were reduced in patients receiving the Ipilimumab/GVAX combination compared with Ipilimumab alone [80]. Reduced toxicity was mainly in the pulmonary and gastrointestinal tracts. This may be explained by studies in which GM-CSF-deficient mice develop colitis and pulmonary disorders, which can be prevented by administration of GM-CSF to accelerate mucosal repair [81, 82].
Table 1.
Current cancer clinical trials targeting inhibitory receptors in combination with other monoclonal antibodies.
| Target | Antibody | Type(s) | Phase | Combination Antibody | Target |
|---|---|---|---|---|---|
| CTLA4 | Ipilimumab | Advanced Solid Tumors | I | Lirilumab | KIR |
| Head and Neck Cancer (Stage III/IVb) | I | Cetuximab | EGFR | ||
| Relapsed or Refractory B-cell Lymphoma | I | Rituximab | CD20 | ||
| Recurrent Adult Hodgkin Lymphoma | I | Brentuximab Vedotin | CD30 | ||
| Advanced Melanoma | I/II | Varlilumab | CD27 | ||
| Advanced Melanoma (Stage III/IV) | I/II | Bevacizumab | VEGF-A | ||
| CTLA4 | Tremelimumab | Metastatic Melanoma | I | CP-870,993 | CD40 |
| Advanced Solid Tumors, Head and Neck Cancer or NSCLC | I | MEDI4736 | PDL1 | ||
| Metastatic Melanoma (Stage III/IV) | I | MEDI3617 | Ang2 | ||
| Advanced Solid Tumors | I | Mogamulizumab | CCR4 | ||
| Advanced Solid Tumors or Aggressive B cell Lymphoma | I/II | MEDI6469 | OX40 | ||
| PD1 | Pembrolizumab | Advanced Solid Tumors | I | PF-05082566 | 4-1BB |
| RCC or Advanced Melanoma | I/II | Ipilimumab | CTLA4 | ||
| NSCLC | I/II | Ipilimumab or Bevacizumab | CTLA4/VEGF-A | ||
| Head and Neck Cancer | III | Cetuximab | EGFR | ||
| Advanced Melanoma | III | Ipilimumab | CTLA4 | ||
| PD1 | Nivolumab | Advanced Melanoma (Stage III/IV) | I | Ipilimumab | CTLA4 |
| NSCLC | I | Ipilimumab or Bevacizumab | CTLA4/VEGF-A | ||
| Lymphoma and Multiple Myeloma | I | Ipilimumab or Lirilumab | CTLA4/KIR | ||
| RCC | I | Ipilimumab | CTLA4 | ||
| Advanced or Metastatic Solid Tumors | I/II | Ipilimumab | CTLA4 | ||
| NSCLC, Melanoma, Head and Neck, Colon, Ovarian Carcinoma | I/II | Varlilumab | CD27 | ||
| Metastatic Colon Cancer | I/II | Ipilimumab | CTLA4 | ||
| Advanced Solid Tumors or B-cell Non-Hodgkin Lymphoma | I/II | Urelumab | 4-1BB | ||
| Metastatic RCC | II | Ipilimumab or Bevacizumab | CTLA4/VEGF-A | ||
| Advanced or Metastatic Melanoma | II/III | Ipilimumab | CTLA4 | ||
| Advanced or Metastatic RCC | III | Ipilimumab | CTLA4 | ||
| Squamous Cell Carcinoma of Head and Neck | III | Cetuximab | EGFR | ||
| Recurrent Glioblastoma | III | Ipilimumab | CTLA4 | ||
| PDL1 | MPDL3280A | Advanced or Metastatic Solid Cancers | I | Ipilimumab or Bevacizumab | CTLA4/VEGF-A |
| Follicular Lymphoma and Diffuse Large B-cell Lymphoma | I | Obinutuzumab | CD20 | ||
| RCC | II | Bevacizumab | VEGF-A | ||
| NSCLC | III | Bevacizumab | VEGF-A | ||
| PDL1 | MEDI4736 | Head and Neck, Unresectable Pancreatic Cancer, Solid Tumors, NSCLC | I | Tremelimumab | CTLA4 |
| Advanced Solid Tumors | I | Mogamulizumab | CCR4 | ||
| Advanced Solid Tumors or Aggressive B cell Lymphoma | I/II | MEDI6469 | OX40 | ||
| Gastric or Gastroesophageal Junction Adenocarcinoma | I/II | Tremelimumab | CTLA4 | ||
| Glioblastoma | II | Bevacizumab | VEGF-A | ||
| Recurrent/Metastatic Squamous Cell Carcinoma of Head and Neck | II | Tremelimumab | CTLA4 | ||
| Head and Neck Cancer, NSCLC | III | Tremelimumab | CTLA4 | ||
| LAG3 | BMS-986016 | Advanced Solid Tumors | I | Nivolumab | PD1 |
Neoantigens are formed by peptides that are completely absent from the normal human genome, arising from tumor-specific mutations. Recent evidence suggests that IR checkpoint blockade may be most effective in patients with tumors that harbor a high mutational rate, which provides newly generated targets for enhanced immune recognition and clearance. Indeed, this was shown in a recent genome exome analysis study that selected 25 patients who had been previously treated with Ipilimumab [83, 84]. Patients that showed a long-term benefit from Ipilimumab therapy had a greater mutational burden compared with non-/minimal-responders. Although a strong correlation exists between high mutational burdens and response to Ipilimumab therapy, there were also patients with a high mutational load that did not respond to Ipilimumab treatment [83, 84]. Nevertheless, this allows candidate antigens to be discovered to understand the molecular determinants for responsiveness in the neoantigen landscape. Similarly, whole-genome sequencing of NSCLC patients treated with Pembrolizumab was performed, which also showed that a higher nonsynomynous mutational burden in tumors associated with improved objective responses [85]. For patients harboring few or no immunogenic mutations, oncolytic viruses such as Newcastle Disease Virus (NDV) could be utilized to induce mutagenesis. NDV does not integrate into the human genome and humans do not have pre-existing immunity to the virus [86]. NDV readily infects the majority of cancer cells due to the ubiquitous expression of its receptor (sialic acid) and thus infectivity is highly restricted to cancer cells. In mice, combining NDV administration with anti-CTLA4 was shown to enhance rejection of bilateral tumors and improved long-term survival, suggesting that NDV combined with Ipilimumab treatment could enhance efficacy in melanoma patients [87].
Other Inhibitory Receptor Targets
While current clinical trials are focusing on CTLA4, PD1 and LAG3, there is a substantial pipeline of additional inhibitory molecules that are being assessed for their potential utility as immunotherapeutic targets. A few are discussed briefly here.
T-cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM3/CD366)
TIM3, an IR identified in 2002, is found on CD4+ Th1, CD8+ CTLs, and upregulated on T cellsT cells expressing IFN-γ [88]. TIM3 is constitutively expressed on murine Treg cells but is only induced upon activation on human Treg cells. TIM3+ Treg cells express higher levels of Foxp3 and CTLA4, marking a highly suppressive population compared with TIM3− Treg cells [89]. Furthermore TIM3 has been found on functionally exhausted cells in both cancer patients (melanoma, NSCLC, follicular B-cell non-Hodgkin’s lymphoma) and in preclinical murine tumor models [90–93]. TIM3 is coexpressed with other IRs, most notably PD1, identifying the “most exhausted” population of CD8+ T cells within the tumor, which fail to proliferate and produce IL-2, TNF and IFN-γ [94]. Blockade of these IRs can restore ex vivo T-cell function while enhancing antitumor responses [91, 94]. Combined blockade of the TIM3 and PD1 pathways in mice with acute myelogenous leukemia (AML) enhances survival and is more effective than PD1 blockade alone [95]. The inhibitory function of TIM3 has been recently shown to be controlled by interaction with carcinoembryonic antigen adhesion molecule 1 (CEACAM1) [96]. Targeting both TIM3 and CEACAM1 was more effective in delaying tumor growth in a mouse model of colorectal cancer compared with targeting TIM3 alone. TIM3 is also highly expressed on NK cells isolated from melanoma patients, in which overexpression correlated with late stage of disease and poor prognosis [97]. TIM3+ NK cells were shown to be functionally impaired, with reduced proliferative capacity and cytotoxicity, as well as defects in cytokine production, compared with NK cells isolated from healthy patients [97]. Like with CD8+ T cells, in vitro antibody blockade of TIM3 led to the reversal of this exhausted phenotype, enhancing proliferation and NK-cell cytotoxicity [97]. Finally, TIM3 was recently shown to be upregulated on tumor-infiltrating DCs in murine and human tumors [98]. It is speculated that anti-TIM3 blockade could promote pro-inflammatory cytokines from tumor-associated DCs in order to prime antitumor immunity.
There are currently no ongoing clinical trials involving blockade of TIM3; however, given its expression pattern and the exciting preclinical data, TIM3 is an attractive target for future cancer immunotherapy approaches.
CD200
CD200 is a broadly-expressed membrane glycoprotein found on thymocytes, activated T cells, B cells and DCs as well as on vascular endothelial cells, CNS neurons and in the eye [99, 100]. It serves as a stem cell marker for the hair follicle and various pluripotent stem cell types. CD200 also marks a population of squamous cell carcinoma with stem cell-like properties and increased tumorigenic potential [101]. It is overexpressed on melanoma, B-cell chronic lymphocytic leukemia (CLL) and hairy cell leukemia as well as colon, breast, brain and prostate cancers [102]. Its expression acts as a poor prognostic indicator in acute myeloid leukemia and multiple myeloma [100, 103, 104].
CD200 binds specifically to its receptor, CD200R, an IgSF IR expressed on myeloid cells such as granulocytes, macrophages, mast cells and DCs, as well as T cells, B cells and NK cells. CD200R ligation regulates Treg-cell induction and macrophage activation as well as mast cell degranulation and cytokine production in both humans and mice [105]. While inhibition of T-cell effector activity by CD200:CD200R signaling has been largely studied in vitro, expression of CD200 on tumor cells has been shown to directly inhibit NK-cell cytotoxic and cytokine-producing activity [103].
Multiple studies have explored the manipulation of the CD200/CD200R pathway in mice using blocking antibodies or genetic modification. While transgenic tumor cells overexpressing CD200 grew more aggressively, blockade of CD200:CD200R signaling led to decreased tumor metastases and increased cytotoxic CD8+ T-cell responses in the transplantable EMT6 breast cancer model [106], suggesting that the CD200:CD200R axis may be a viable therapeutic target in cancer.
T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory domain (TIGIT)
TIGIT, like many previously-discussed IRs, is expressed on regulatory, memory and activated T cells as well as NK cells. In particular, TIGIT is expressed on both human and mouse TILs, and co-blockade of TIGIT with PDL1 enhances T-cell effector function, alleviating T-cell exhaustion in both murine tumor and viral models. Co-blockade was shown to synergistically mediate a CD8+ T-cell-dependent rejection of the CT26 tumor, which was more efficacious than targeting PDL1 alone [107].
TIGIT binds the poliovirus receptor (PVR/CD155) on DCs and CD112 which is expressed on tumor cells [108, 109]. Ligation of TIGIT with CD155 leads to increased IL-10 and decreased IL-12 production by DCs, which in turn inhibits T-cell activation and proliferation [108]. CD226 also binds to CD155 in humans but delivers a costimulatory signal, inducing T-bet and IFN-γ production. However, TIGIT competes with CD226 to bind to CD155 and therefore exerts a coinhibitory effect [110]. In melanoma patients, TIGIT coexpresses with PD1 on CD8+ TILs, although there was a reduction of CD226 expression, suggesting this TIGIT/CD226 imbalance also occurs in humans [111].
In both mice and humans, TIGIT has recently been shown to mark a highly active subset of Treg cells producing fibrinogen-like protein 2 (Fgl2), which specifically suppress Th1-cell and Th17-cell, but not Th2-cell differentiation and responses [112]. Signaling through TIGIT on T cells and NK cells results in a strong inhibition of proliferation and effector functions [109, 113]. TIGIT was discovered in 2008 and is not yet in clinical trials [108]. However, due to its highly specific expression and activity, and because it inhibits some immune responses while leaving others intact, TIGIT represents another attractive target for immunotherapeutic blockade.
B and T lymphocyte attenuator (BTLA/CD272)
BTLA exhibits structural and functional similarity to CTLA4 and PD1, although unlike other IRs, its expression is induced on CD4+ T cells during activation and reduced with total CD8+ T-cell differentiation [114]. BTLA is also expressed on naïve T cells, Th1 cells, T follicular cells, DCs, macrophages, NK cells, NKT cells and B cells [114–116]. In addition, BTLA is strongly expressed on B-cell CLL and other hematologic malignancies [114, 116]. BTLA binds herpes virus entry mediator (HVEM/CD270), which unlike other IRs of the IgSF, is a TNF-receptor superfamily member which is constitutively expressed on naïve CD4+ and CD8+ T cells, but is downregulated upon T-cell activation [117]. HVEM is also expressed on immature DCs, monocytes, NK cells, as well as on several types of malignancies [115]. BTLA:HVEM ligation decreases T-cell proliferation and cytokine production, although BTLA-deficient mice display increased generation of memory T cells and memory responses [118]. BTLA and PD1 are highly coexpressed on dysfunctional CD8+ T cells in the tumor microenvironment, suggesting that signaling through BTLA may inhibit the function of tumor antigen-specific CD8+ T cells [119, 120]. This inhibition can at least be partially relieved ex vivo by anti-BTLA mAb blockade [121]. There are currently no clinical trials involving BTLA modulation, but it may be a suitable candidate because of its coexpression with other IRs and the large fraction of cells (i.e., tumor-specific, fully-differentiated TILs) that remain BTLA-positive following Melan-A/MART-1 peptide vaccination [114].
BTLA also binds CD160, a glycophosphatidylinositol (GPI)-anchored protein found on cytolytic cells such as NK cells, NKT cells and CD8+ T cells [122]. Its expression is also induced upon activation of CD4+ T cells, in which engagement of CD160 strongly inhibits T-cell responses by dampening proliferation and cytokine secretion. While CD160 is not found on myeloid cells or B cells, it is highly expressed on multiple types of leukemia, lymphoma and B-cell malignancies [123]. This highly restricted expression pattern suggests it may be a good target for cancer immunotherapy. Preclinical studies showed that active immunization combined with blockade of the BTLA:HVEM/CD160 pathway induces regression of large, established tumor masses in a genetically-engineered murine thyroid adenocarcinoma model [124].
B7 family members: B7-H3 (CD276), B7-H4 (B7S1/B7x) and V-domain Ig suppressor of T-cell activation (B7-H5/VISTA)
B7-H3 is a B7 family member whose transcripts are ubiquitously expressed in many healthy tissues; however, expression of the protein is induced on T cells, NK cells, activated DCs and monocytes [125]. The receptor for murine B7-H3 is Triggering receptor expressed on myeloid cells (TREM)-Like Transcript-2 (TLT-2), which is constitutively expressed on CD8+ T cells, NK cells, B cells, DCs, macrophages, neutrophils and is induced upon CD4+ T-cell activation [126]. While B7-H3:TLT-2 serves a costimulatory role in mice, the role of B7-H3 in humans is controversial as there is no evidence for an interaction with TLT-2 [126]. Expression of B7-H3 is associated with enhanced survival and frequency of CD8+ TILs for patients with gastric cancer, yet expression is correlated with poor prognosis in NSCLC, prostate, breast, colorectal, ovarian and pancreatic cancers [126, 127]. B7-H3 expression on circulating endothelial cells has been correlated with tumor progression, metastases, and decreased TIL numbers in many of these malignancies [128]. In NSCLC patients, it has been suggested that B7-H3 suppresses cancer immune surveillance by differentiating monocytes into suppressive tumor-associated macrophages (TAMs) contributing to an anti-inflammatory phenotype by promoting IL-10 secretion [129].
Despite contrasting effects in different tumor types, blocking mAbs targeting B7-H3 are in clinical development. Radioimmunotherapy using a 131Iodine-labelled anti-B7-H3 mAb (clone 8H9), which specifically targets the 4Ig isoform, showed efficacy in neuroblastoma patients with CNS metastases, as B7-H3 was found to be expressed on brain cancer but not healthy CNS tissues [130]. A second anti-B7-H3 mAb, MGA271, is being investigated in a Phase I safety study in refractory prostate carcinoma, melanoma and glioblastoma to evaluate safety, efficacy and how long the mAb remains in the patient’s blood [131]. Given the expression of B7-H3 on primary tumor samples and tumor cell lines, it is hoped that such targeted immunotherapy will be effective in malignancies outside the CNS [130].
B7-H4 is another B7 superfamily member exhibiting a broad mRNA distribution on mouse and human peripheral tissues but with limited protein expression induced on T cells, B cells, TAMs and APCs [132, 133]. B7-H4 is also found on tumor cells of NSCLC, melanoma, gastric, pancreatic and gynecologic cancers (cervical, uterine, ovarian and breast) [134–137]. Engagement with an as-yet-unknown ligand inhibits T-cell effector activity including proliferation, cytokine secretion and cytolytic activity by cell cycle arrest [132]. B7-H4 expression on tumor cells and TAMs correlates with increased disease progression, poor prognosis and decreased patient survival, as well as decreased infiltration of T cells into tumors [136, 138, 139]. While there are currently no clinical trials targeting B7-H4 in cancer, an anti-B7-H4 single-chain variable fragment was recently shown to inhibit antigen-specific T cells in vitro and exhibited in vivo efficacy in a humanized ovarian cancer model [140].
B7-H5/VISTA is the newest member of the B7 family and was discovered in 2011, expressed on both CD4+ and CD8+ T cells in mice and humans, but not on tumor cells [141, 142]. Its extracellular Ig domain shares significant sequence homology with PDL1 and PDL2, however it has a different structure than other B7 family members. The receptor for VISTA in both mice and humans has not yet been identified. VISTA mRNA in both systems is expressed mostly in hematopoietic tissues such as the thymus, spleen and bone marrow whereas VISTA protein is highly enriched on CD11b+ macrophages and DCs [142]. VISTA signaling was shown to inhibit T-cell, but not B-cell proliferation and cytokine production in vitro and accordingly, anti-VISTA blockade enhanced in vitro T-cell proliferation [141]. Anti-VISTA monotherapy was shown to suppress tumor growth in melanoma models by increasing effector T-cell infiltration and activity, and was also shown to synergize and enhance efficacy of a peptide-based tumor vaccine, consisting of anti-CD40 mAb and TLR agonists [143, 144]. While approaches targeting VISTA are not yet in clinical trials, it has been proposed as a target for cancer immunotherapy due to its structural similarity to PDL1/L2 and promising pre-clinical data.
Future Directions and Perspectives
There is now considerable interest in integrating immunotherapy into the standard of care for a wide range of tumor types, in large part due to the considerable progress made in targeting IRs in the clinic [5]. Durable responses have been observed with multiple therapies targeting CTLA4 and the PD1/PDL1 axis in a growing list of tumor types. The most striking observations have been in NSCLC, as this is a tumor type that was previously thought to be intractable. However, treatment with immune modulators is not without consequence. While most adverse events experienced with immunotherapy, such as dermatologic effects and mild gastrointestinal complications, are manageable in advanced clinics, it remains to be seen if these can be effectively managed in more regional hospitals where expertise might be more limited.
While remarkable progress has been made, many questions and challenges remain. First, although combinatorial immunotherapy is likely to yield significant therapeutic improvement, there is substantial debate over the optimal combination of immunotherapeutic modalities. This will be particularly relevant as we try to combine IR blockade with therapeutic vaccines or agonists for costimulatory receptors (e.g. 4-1BB, OX40, CD40). Alternatively, chemokine receptor-specific antibodies such as Mogamulizumab, targeting CCR4, could be used in combination with IR blockade. Mogamulizumab has been approved in Japan to treat refractory adult T-cell leukemia and lymphoma, targeting CCR4+ Treg cells from migrating into tumors [145]. It is also unclear how we should optimally integrate these new therapies with conventional and targeted chemotherapy and radiotherapy. While there may be some basic paradigms that apply to all malignancies, it is possible that this will need to be determined empirically for each tumor type.
Second, IR blockade can result in spectacular tumor shrinkage and remission in only a proportion of patients. It is critically important to understand why. While a number of factors, such as IR ligand expression and the brevity and immunogenicity of neoantigen expression, could contribute, there may be many other factors that remain unknown. Determining these factors and identifying biomarkers that can predict responsiveness to each immunological modality will be critical. Indeed, it seems likely that cancer care in the future will incorporate immunological as well as genetic analysis of an individual patient’s tumor leading to a personalized treatment plan to maximize efficacy. In this regard, developments towards incorporating Immunoscore, a project designed to include the analysis of immunological biomarkers when evaluating patient tissue samples, into current tumor boards will likely be required to predict optimal treatment combinations, prognosis and response to immunotherapy [146, 147].
Third, while the current immunotherapies in clinical trials represent a significant step forward, it remains important to continue the rapid development of new targets in the pipeline that may be critical components of future combinatorial approaches. This is especially valid if new modalities are similarly efficacious but have reduced adverse events. It is also important to continue to identify new potential immunotherapeutic targets and mechanism, and novel delivery platforms.
In summary, while many unknowns, questions and challenges remain, most agree that we are at the dawn of a new age in cancer immunotherapy.
Acknowledgments
This work was supported by the National Institutes of Health (R01 AI091977 and AI039480 to D.A.A.V.; and F32 CA168294 to M.E.T.).
Footnotes
Conflict of interest
D.A.A.V. has submitted patents on LAG3 that are granted or pending and is entitled to a share in net income generated from licensing of these patent rights for commercial development. M.E.T. and L.P.A. declare no financial or commercial conflict of interest.
References
- 1.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: Focus on hypersensitivity responses. Oncoimmunology. 2013;2:e26333. doi: 10.4161/onci.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol. 2011;77:12–19. doi: 10.1016/j.critrevonc.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39:49–60. doi: 10.1016/j.immuni.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami N, Riella LV. Co-inhibitory pathways and their importance in immune regulation. Transplantation. 2014;98:3–14. doi: 10.1097/TP.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 16.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Schneider H, Rudd CE. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood. 2012;120:4560–4570. doi: 10.1182/blood-2012-04-421420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 22.Ledford H. Melanoma drug wins US approval. Nature. 2011;471:561. doi: 10.1038/471561a. [DOI] [PubMed] [Google Scholar]
- 23.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C, Thomas L, Bondarenko I, O’Day S, MDJ, Garbe C, Lebbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 27.Addeo A, Rinaldi CR. Treatment with ipilimumab: a case report of complete response in a metastatic malignant melanoma patient. Case Rep Oncol. 2013;6:285–288. doi: 10.1159/000351834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della Vittoria Scarpati G, Fusciello C, Perri F, Sabbatino F, Ferrone S, Carlomagno C, Pepe S. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. Onco Targets Ther. 2014;7:203–209. doi: 10.2147/OTT.S57335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324–334. doi: 10.1038/mi.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancardi DA, Iannascoli B, Hoos S, England P, Daeron M, Bruhns P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J Clin Invest. 2008;118:3738–3750. doi: 10.1172/JCI36452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furness AJ, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol. 2014;35:290–298. doi: 10.1016/j.it.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Keler T, Halk E, Vitale L, O’Neill T, Blanset D, Lee S, Srinivasan M, et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J Immunol. 2003;171:6251–6259. doi: 10.4049/jimmunol.171.11.6251. [DOI] [PubMed] [Google Scholar]
- 34.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 40.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, George AJ, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 44.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 46.Poole RM. Pembrolizumab: First Global Approval. Drugs. 2014 doi: 10.1007/s40265-014-0314-5. [DOI] [PubMed] [Google Scholar]
- 47.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garon EB, Leighl NB, Rizvi NA, Blumenschein GR, Balmanoukian AS, Eder JP, Goldman JW, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2014;32:8020. [Google Scholar]
- 49.Seiwert TY, Burtness B, Weiss J, Gluck I, Eder JP, Pai SI, Dolled-Filhart M, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. ASCO Meeting Abstracts. 2014;32:6011. [Google Scholar]
- 50.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 51.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. Journal of Clinical Oncology. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 54.Johnson DB, Rioth MJ, Horn L. Immune Checkpoint Inhibitors in NSCLC. Curr Treat Options Oncol. 2014 doi: 10.1007/s11864-014-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho DC, Sosman JA, Sznol M, Gordon MS, Hollebecque A, Hamid O, McDermott DF, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstracts. 2013;31:4505. [Google Scholar]
- 56.Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, Schlom J. Antibody dependent cellular cytotoxicity (ADCC) activity of a novel anti-PD-L1 antibody, avelumab (MSB0010718C), on human tumor cells. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daud AI. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma(MEL): correlation of tumor PD-L1 expression with outcome [abstract] Proc Ann Meeting AACR. 2014:CT104. [Google Scholar]
- 58.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, et al. IFN-[gamma] from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015 doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saloura V, Zuo Z, Koeppen H, Keck MK, Khattri A, Boe M, Hegde PS, et al. Correlation of T-cell inflamed phenotype with mesenchymal subtype, expression of PD-L1, and other immune checkpoints in head and neck cancer. ASCO Meeting Abstracts. 2014;32:6009. [Google Scholar]
- 61.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015 doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 62.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Huard B, Gaulard P, Faure F, Hercend T, Triebel F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics. 1994;39:213–217. doi: 10.1007/BF00241263. [DOI] [PubMed] [Google Scholar]
- 64.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32:2255–2263. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 65.Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 67.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223) J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 68.Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. 2002;169:5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- 69.Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, Hartmann D, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26:494–504. doi: 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li N, Workman CJ, Martin SM, Vignali DA. Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223) J Immunol. 2004;173:6806–6812. doi: 10.4049/jimmunol.173.11.6806. [DOI] [PubMed] [Google Scholar]
- 71.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Callahan M, Horak C, Curran M, Hollman T, Schaer D, Yuan J, Lesokhin A, et al. Peripheral blood and tumor biomarkers in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab. Journal for ImmunoTherapy of Cancer. 2013;1:O6. [Google Scholar]
- 76.Sznol M, Kluger HM, Callahan MK, Postow MA, Gordon RA, Segal NH, Rizvi NA, et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL) ASCO Meeting Abstracts. 2014;32:LBA9003. [Google Scholar]
- 77.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel T, Harrison MR, Vaishampayan UN, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): Results of a randomized, dose-ranging phase II trial. ASCO Meeting Abstracts. 2014;32:5009. [Google Scholar]
- 78.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13:509–517. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 80.Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312:1744–1753. doi: 10.1001/jama.2014.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Y, Hunt NH, Bao S. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008;18:1220–1229. doi: 10.1038/cr.2008.310. [DOI] [PubMed] [Google Scholar]
- 82.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 83.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snyder Charen A, Makarov V, Merghoub T, Walsh L, Yuan J, Miller M, Kannan K, et al. The neoantigen landscape underlying clinical response to ipilimumab. ASCO Meeting Abstracts. 2014;32:3003. [Google Scholar]
- 85.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schirrmacher V, Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra232. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 89.Gautron AS, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. Eur J Immunol. 2014;44:2703–2711. doi: 10.1002/eji.201344392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res. 2014;2:393–398. doi: 10.1158/2326-6066.CIR-14-0039. [DOI] [PubMed] [Google Scholar]
- 91.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2014 doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 100.Rygiel TP, Meyaard L. CD200R signaling in tumor tolerance and inflammation: A tricky balance. Curr Opin Immunol. 2012;24:233–238. doi: 10.1016/j.coi.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 101.Adhikary G, Grun D, Kerr C, Balasubramanian S, Rorke EA, Vemuri M, Boucher S, et al. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One. 2013;8:e84324. doi: 10.1371/journal.pone.0084324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawasaki BT, Mistree T, Hurt EM, Kalathur M, Farrar WL. Co-expression of the toleragenic glycoprotein, CD200, with markers for cancer stem cells. Biochem Biophys Res Commun. 2007;364:778–782. doi: 10.1016/j.bbrc.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coles SJ, Wang EC, Man S, Hills RK, Burnett AK, Tonks A, Darley RL. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia. 2011;25:792–799. doi: 10.1038/leu.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pillai V, Pozdnyakova O, Charest K, Li B, Shahsafaei A, Dorfman DM. CD200 flow cytometric assessment and semiquantitative immunohistochemical staining distinguishes hairy cell leukemia from hairy cell leukemia-variant and other B-cell lymphoproliferative disorders. Am J Clin Pathol. 2013;140:536–543. doi: 10.1309/AJCPEBK31VQQNDDR. [DOI] [PubMed] [Google Scholar]
- 105.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 106.Gorczynski RM, Chen Z, Khatri I, Podnos A, Yu K. Cure of metastatic growth of EMT6 tumor cells in mice following manipulation of CD200:CD200R signaling. Breast Cancer Res Treat. 2013;142:271–282. doi: 10.1007/s10549-013-2735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 108.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 109.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol. 2013;43:2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188:3869–3875. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015 doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pasero C, Speiser DE, Derre L, Olive D. The HVEM network: new directions in targeting novel costimulatory/co-inhibitory molecules for cancer therapy. Curr Opin Pharmacol. 2012;12:478–485. doi: 10.1016/j.coph.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 116.M’Hidi H, Thibult ML, Chetaille B, Rey F, Bouadallah R, Nicollas R, Olive D, et al. High expression of the inhibitory receptor BTLA in T-follicular helper cells and in B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Am J Clin Pathol. 2009;132:589–596. doi: 10.1309/AJCPPHKGYYGGL39C. [DOI] [PubMed] [Google Scholar]
- 117.Morel Y, Schiano de Colella JM, Harrop J, Deen KC, Holmes SD, Wattam TA, Khandekar SS, et al. Reciprocal expression of the TNF family receptor herpes virus entry mediator and its ligand LIGHT on activated T cells: LIGHT down-regulates its own receptor. J Immunol. 2000;165:4397–4404. doi: 10.4049/jimmunol.165.8.4397. [DOI] [PubMed] [Google Scholar]
- 118.Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- 119.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hobo W, Norde WJ, Schaap N, Fredrix H, Maas F, Schellens K, Falkenburg JH, et al. B and T lymphocyte attenuator mediates inhibition of tumor-reactive CD8+ T cells in patients after allogeneic stem cell transplantation. J Immunol. 2012;189:39–49. doi: 10.4049/jimmunol.1102807. [DOI] [PubMed] [Google Scholar]
- 122.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 123.Farren TW, Giustiniani J, Liu FT, Tsitsikas DA, Macey MG, Cavenagh JD, Oakervee HE, et al. Differential and tumor-specific expression of CD160 in B-cell malignancies. Blood. 2011;118:2174–2183. doi: 10.1182/blood-2011-02-334326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lasaro MO, Sazanovich M, Giles-Davis W, Mrass P, Bunte RM, Sewell DA, Hussain SF, et al. Active immunotherapy combined with blockade of a coinhibitory pathway achieves regression of large tumor masses in cancer-prone mice. Mol Ther. 2011;19:1727–1736. doi: 10.1038/mt.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105:10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer. 2014;134:2764–2771. doi: 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 128.Kraan J, van den Broek P, Verhoef C, Grunhagen DJ, Taal W, Gratama JW, Sleijfer S. Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br J Cancer. 2014;111:149–156. doi: 10.1038/bjc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun J, Mao Y, Zhang YQ, Guo YD, Mu CY, Fu FQ, Zhang XG. Clinical significance of the induction of macrophage differentiation by the costimulatory molecule B7-H3 in human non-small cell lung cancer. Oncol Lett. 2013;6:1253–1260. doi: 10.3892/ol.2013.1586. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, Humm JL, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 132.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 133.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He C, Qiao H, Jiang H, Sun X. The inhibitory role of b7-h4 in antitumor immunity: association with cancer progression and survival. Clin Dev Immunol. 2011;2011:695834. doi: 10.1155/2011/695834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, et al. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu W, Shibata K, Koya Y, Kajiyama H, Senga T, Yamashita M, Kikkawa F. B7-H4 overexpression correlates with a poor prognosis for cervical cancer patients. Mol Clin Oncol. 2014;2:219–225. doi: 10.3892/mco.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B. B7-h4 expression in human melanoma: its association with patients’ survival and antitumor immune response. Clin Cancer Res. 2011;17:3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 138.Chen C, Zhu YB, Shen Y, Zhu YH, Zhang XG, Huang JA. Increase of circulating B7-H4-expressing CD68+ macrophage correlated with clinical stage of lung carcinomas. J Immunother. 2012;35:354–358. doi: 10.1097/CJI.0b013e31824212c4. [DOI] [PubMed] [Google Scholar]
- 139.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, et al. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, Ra HJ, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, et al. B7-H5 costimulates human T cells via CD28H. Nat Commun. 2013;4:2043. doi: 10.1038/ncomms3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S, Ceeraz S, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P, Noelle RJ, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014;74:1933–1944. doi: 10.1158/0008-5472.CAN-13-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L. Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol. 2015;6:12. doi: 10.3389/fimmu.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]