Abstract
Chemical warfare nerve agents (CWNAs) are highly toxic compounds that cause a cascade of symptoms and death, if exposed casualties are left untreated. Numerous rodent models have investigated the toxicity and mechanisms of toxicity of CWNAs, but most are limited to male subjects. Given the profound physiological effects of circulating gonadal hormones in female rodents, it is possible that the daily cyclical fluctuations of these hormones affect females’ sensitivity to the lethal effects of CWNAs, and previous reports that included female subjects did not control for the stage of the hormonal cycle. The aim of the current study was to determine the 24-hour median lethal dose (LD50) of the CWNA sarin in male, ovariectomized (OVEX) female, and female rats during different stages of the estrous cycle (diestrus, proestrus, and estrus). Additionally, baseline activity levels of plasma acetylcholinesterase, butyrylcholinesterase, and carboxylesterase were measured to determine differences among the groups. Results indicated that females in proestrus had a significantly higher LD50 of sarin compared to OVEX and estrous females. Although some sex differences were observed in the activity levels of plasma esterases, they were not consistent and likely not large enough to significantly affect the LD50s. These results suggest that hormonal cyclicity can influence the outcome of CWNA-related studies using female rodents, and that this variability can be minimized by controlling for the stage of the cycle. Additional research is necessary to determine the precise mechanism of the observed differences because it is unlikely to be solely explained by plasma esterase activity.
Keywords: sarin, nerve agents, sex differences, estrous cycle, LD50
1. Introduction
Chemical warfare nerve agents (CWNAs), such as sarin, irreversibly bind to acetylcholinesterase and induce a “cholinergic crisis” that causes numerous physiological events including miosis, salivation, respiratory failure, tremors, seizures, and death. Treatment regimens that typically include an anticholinergic (atropine), oxime (2-PAM chloride), and anticonvulsant (diazepam) reduce symptoms and lethality when administered before or quickly after an exposure (Lee, 2003). Limited efficacy and a short therapeutic window of these treatments, however, require the development of new, more effective medical countermeasures. Numerous animal models are currently used to identify novel countermeasures (for review, see (Pereira et al., 2014)). However, these are largely limited to male animals, and relatively little is known regarding the toxic effects of CWNAs and the therapeutic efficacy of countermeasures in females. Furthermore, developing medical countermeasures exclusively in males may result in a product that is not as safe for use in females. There is precedence for this safety concern because past FDA-approved pharmaceuticals have been withdrawn because of unintended adverse effects in women (Heinrich et al., 2001). These facts underscore the need to include female subjects in scientific research, and to incentivize this inclusion, the National Institutes of Health plans to mandate the use of female subjects for preclinical trials (Clayton and Collins, 2014). Thus, understanding how biological differences between males and females can affect outcomes is necessary for developing safe and effective medical countermeasures for CWNA exposure.
The relative sensitivity to the lethal effects of CWNAs between male and female rats is consistent in some reports but not others, and depends on factors including route of exposure, agent, and species. For example, the median lethal concentration (LC50) for whole-body sarin (Mioduszewski et al., 2002) and cyclosarin (Anthony et al., 2004) exposures is lower in female rats compared to males. In at least one report, female rats are also more sensitive than males to subcutaneous injections of soman (Sket, 1993). In contrast, no significant sex differences emerge from whole-body exposures of VX (Benton et al., 2006) or subcutaneous injections of tabun (Lundy et al., 1989). The latter study also reported only a small difference in the subcutaneous median lethal dose (LD50) of soman between the sexes (Lundy et al., 1989), but the significance of this difference is difficult to determine because statistical analyses were not performed. Species is also a factor; in guinea pigs, the LD50 of sarin and VX is lower in males compared to females, whereas no sex difference is observed following exposure to soman (Fawcett et al., 2009).
Why some studies report sex differences after CWNA intoxication and others do not is unknown. One contributing factor may involve differences in sex-specific gonadal hormones. Males secrete androgens at a relatively constant rate that slowly declines with age (Mooradian et al., 1987). Females, in contrast, secrete estradiol and progesterone on a cyclical schedule that can significantly change from day to day. For example, the four-day estrous cycle in female rats is defined by two days of diestrus, one day of proestrus, and one day of estrus (Goldman et al., 2007). Estradiol and progesterone secretions remain relatively quiescent during the first two days of diestrus before peaking in proestrus and then declining by the morning of estrus. Given the widespread distribution of estrogen and progesterone receptors throughout the body, and their effects on physiological functions (Jensen and DeSombre, 1972), endogenous fluctuations of these hormones could possibly affect females’ sensitivity to intoxication with CWNAs from day to day. One commonality among studies using females in CWNA research is that none have controlled for the stage of the estrous cycle (Lundy et al., 1989; Sket, 1993; Mioduszewski et al., 2002; Anthony et al., 2004; Benton et al., 2006; Fawcett et al., 2009).
The aim of the current study was to determine the toxicity of sarin throughout these hormonal stages in rats by comparing the 24-hour LD50 of sarin in male, ovariectomized (OVEX) female, and cycling female rats at different stages of the estrous cycle (diestrus, proestrus, and estrus). In addition, baseline plasma acetylcholinesterase (AChE), butyrylcholinesterase (BuChE), and carboxylesterase (CE) activity levels were measured. These esterases bind to CWNAs, and numerous studies report sex differences in baseline levels (Leeuwin, 1965; Schmidt and Schmidt, 1978; Illsley and Lamartiniere, 1981; Sterri and Fonnum, 1989; Chanda et al., 1997), which may affect sensitivity to sarin. If lethality significantly fluctuates throughout the estrous cycle in female rats, consideration should be taken in future studies to reduce variability by controlling for hormonal state.
2. Material and methods
2.1 Subjects
Age-matched adult (200–300 g) male (n = 23), cycling female (n = 76), and ovariectomized female (OVEX; n = 19) Sprague Dawley rats were purchased from Charles River Laboratories (Kingston, NY) and housed under normal 12:12 hr light cycle with food and water available ad libitum. OVEX females had their ovaries surgically removed by Charles River Laboratories by an incision through the rear dorsum. To control for the surgical procedures, males and cycling females received a sham procedure, whereby an incision was made on the rear dorsum and immediately stapled. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the USAMRICD, and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89–544), as amended.
2.2 Determination of the stage of estrus
The estrous cycle in females was monitored daily by vaginal smears. To perform the smear, a cotton swab moistened in 0.9% saline was briefly inserted into the vaginal opening of female rats. The cotton swab was removed, and the sample applied to a slide. Slides were dipped in a solution of 0.025% methylene blue and examined under a light microscope at 10X. The ratio of cornified, epithelial, and/or leukocytic cells was used to determine the stage of estrus as described in Goldman et al. (2007). Briefly, diestrus was determined by the presence of leukocytes with or without cornified, nucleated epithelial, or non-nucleated epithelial cells; proestrus by the presence of nucleated epithelial cells without leukocytes; and estrus by the presence of cornified cells without leukocytes.
2.3 Blood draws and agent exposures
Approximately 4 weeks before exposure to sarin, OVEX female and cycling female rats received vaginal smears for 8 days to determine their estrous cycle. OVEX females were smeared to confirm the lack of cyclicity. To control for the perturbation of the smearing procedure, male rats were removed from their cages and immediately replaced. At the end of the monitoring period, all rats were habituated to the blood collection procedure by gentle restraint in a chux. Four to five days later between 0830–0930 hrs, a subset of rats received blood draws (250 μL per day) from a tail incision. To minimize the number of animals required for blood draws, cycling females (n = 14) received repeated daily blood draws throughout their 4-day estrous cycle (diestrus I, diestrus II, proestrus, and estrus), while OVEX (n = 10) and male rats (n =10) received a single blood draw. Only cycling females with regular 4-day cycles were included in blood draws. One cycling female was removed from the entire study because evidence of hormonal cyclicity was not observed. OVEX and male rats received “sham” blood draws for the remaining 3 days, where a tail incision was made but no blood collected. All other rats that did not receive blood draws but were included in the LD50 determination were given tail incisions for 4 days. All animals then recovered for 2 weeks before exposure to sarin.
Five days before exposure to sarin, all females received vaginal smears to confirm hormonal stage. On the day of exposures, cycling females were designated to a group by the stage of their estrous cycle into diestrus, proestrus, or estrus. Given the similar hormonal milieu during the morning on the two days of diestrus (Butcher et al., 1974; Schank and McClintock, 1992), rats in diestrus I and diestrus II were combined to reduce the overall number of required animals. Between 0930–1200 hrs, all rats were subcutaneously injected with sarin (65.8 – 239.1 μg/kg; 0.5 mL/kg). Each rat was then continuously monitored by an observer for 2 hrs and then every 30 min thereafter until 1700 hrs. Rats surviving the 24-hour time period were euthanized by injection of a pentobarbital-based euthanasia solution followed by perforation of the diaphragm.
2.4 Enzyme assays
Cholinesterase Assays: Blood AChE and BuChE were measured using a modification of the WRAIR whole blood cholinesterase assay (Gordon et al. 2005). Briefly, fresh rat whole blood was diluted 1:10 in water and 10 μL were added into 96-well plates. The reactions were initiated by the addition of 290 μL of 50 mM sodium phosphate buffer (pH 8.0) containing one of the following substrates at 1 mM: acetylthiocholine, propionylthiocholine, or butyrylthiocholine. The reactions were conducted at 25°C and monitored at 324 nm in a Molecular Dynamics model SpectraMax Plus plate reader (Sunnyvale, CA) under the control of SoftMax Ver. 5.4 software (Molecular Dynamics, Inc.) by tracking hydrolysis of 4,4′-dithiodiopyridine (DTP), added at 0.2 mM. AChE and BChE activities (in μmol/min/mL) were calculated for each sample. The product concentration was determined using the molar extinction coefficient Mε324=12517 cm−1 M−1.
Carboxylesterase (CE) assay: Rat plasma was prepared by centrifugation of whole blood at 3,000 × g for 10 min at 4°C, and then flash frozen on dry ice and stored at −80°C. Plasma CE activities were determined as described by Hashinotsume et al. (1978). Samples (10 μL) were pre-incubated with 10 μM eserine and 10 mM EDTA for 30 min in reaction buffer containing 50 mM HEPES pH 7.4 in a total volume of 280 μL. Treated samples were loaded into a 96-microtiter plate well, and the reaction was initiated by the addition of 20 μL p-nitrophenyl acetate (2.5 mM final concentration). Samples were analyzed at 25°C by monitoring absorbance at 400 nm using a Molecular Dynamics model SpectraMax Plus plate reader with SoftMax Ver. 5.4 software. The product concentration was determined with the molar extinction coefficient at pH 7.4 for p-nitrophenol (Mε400=7860 cm−1 M−1).
2.5 Data analyses
A stagewise, adaptive dose design was used to determine the 24 hr LD50 of sarin (Feder et al., 1991a; Feder et al., 1991b; Feder et al., 1991c). In the first stage, agent doses were selected to span the predicted range of lethality from 0% to 100%. Animals were allocated randomly to agent doses per stage (where the first stage used 2 animals each per 5 agent doses). The lethality results from the first stage were used to select agent doses for the second stage. In the subsequent stages, agent doses were selected to further focus on doses with a 50% lethality response and/or to better estimate the dose response curve. After each stage, probit dose response models using maximum likelihood methods were fitted to the combined data for all stages (Finney, 1971; Feder et al., 1991b). The stage process continued until the half width of the 95% confidence interval (CI) defined as (Upper Bound – Lower Bound)/(2 × LD50) for the 24 hr LD50 was less than 0.4 or a maximum of 32 animals were used. Output from the stagewise adaptive dose program for each group was used to calculate a ratio of the LD50 values for pairs of groups and the respective 95% CI. This ratio and the respective 95% CI was used to compare LD50 values between groups. If the 95% CI did not encompass the value of 1.0, then the LD50 values of the groups were determined to be significantly different.
Statistical comparisons of the activity levels of AChE, BuChE, and CE were tested with one-way ANOVAs. Four separate analyses were conducted with male and OVEX females and one group of cycling females from each stage of the estrous cycle (diestrus I, diestrus II, proestrus, and estrus) because cycling females provided data for each stage of the estrous cycle. Since the male and OVEX female groups were used in all four analyses, statistical significance of the main effect of group was determined using a Bonferroni adjusted p-value (p ≤ 0.0125). Significant main effects of esterase activities were followed by a Tukey’s multiple comparison test to compare all group pairs with a Bonferroni adjusted p-value (p ≤ 0.0167).
3. Results
3.1 24 hr Sarin LD50
Female rats in proestrus had a significantly higher 24 hr sarin LD50 compared to females in estrus or females with their ovaries removed (Table 1; p < 0.05). No differences were observed among any of the female groups compared to the male group.
Table 1.
The 24 hr 1.0 × LD50 of sarin in male, OVEX, diestrous, proestrous, and estrous rats. Protective ratios and confidence bounds as determined by the maximum likelihood model are shown on the right.
| Group | n | LD50 (μg/kg) (95% CI) | Protective Ratio (Confidence Bounds) | |||
|---|---|---|---|---|---|---|
| OVEX | Diestrus | Proestrus | Estrus | |||
|
| ||||||
| Male | 23 | 116 (105–127) | 0.92 (0.82–1.03) | 1.00 (0.85–1.17) | 1.08 (0.98–1.18) | 1.05 (0.91–1.20) |
|
| ||||||
| OVEX | 19 | 106 (99–113) | 0.92 (0.79–1.06) | 1.18* (1.07–1.26) | 0.96 (0.84–1.08) | |
|
| ||||||
| Diestrus | 23 | 116 (102–132) | - | - | 1.08 (0.94–1.23) | 0.95 (0.81–1.13) |
|
| ||||||
| Proestrus | 32 | 125 (122–127) | - | - | - | 1.13* (1.02–1.25) |
|
| ||||||
| Estrus | 21 | 111 (100–122) | - | - | - | - |
indicates a significant difference (p < 0.05).
3.2 Plasma AChE, BuChE, and CE
Baseline activity levels of plasma AChE, BuChE, and CE varied throughout the estrous cycle when compared to male and OVEX rats (Figure 1). Significant main effects of esterase activities after Bonferroni adjustment (p ≤ 0.0125; Table 2) were observed for most of the esterases in all four comparisons (male, OVEX, and one stage of the estrous cycle) except for the levels of AChE in proestrous and CE in diestrous II females when compared to males and OVEX females. Tukey’s multiple comparison test revealed that females in diestrus I (p = 0.004), diestrus II (p < 0.001), and estrus (p = 0.001) had significantly lower plasma AChE activity than males. Activities of AChE were not different in OVEX females compared to males or cycling females. Females at each stage of the estrous cycle had significantly more BuChE activity than males (p < 0.001), while levels of BuChE (p = 0.001) were lower in OVEX females compared to females in diestrus II. Activities of CE did not differ in cycling females compared to males, but OVEX females had lower CE (on average, p = 0.012) than did males.
Figure 1.
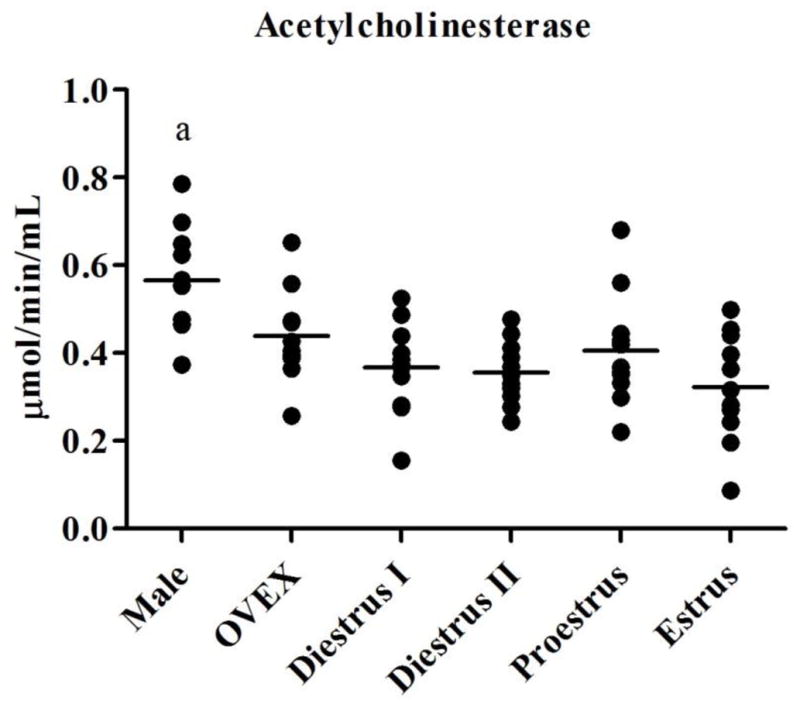
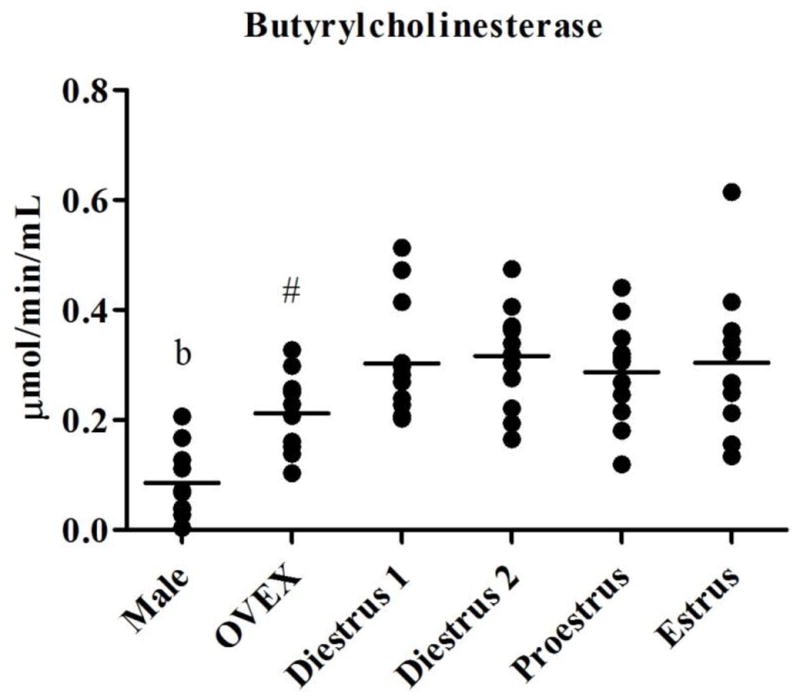
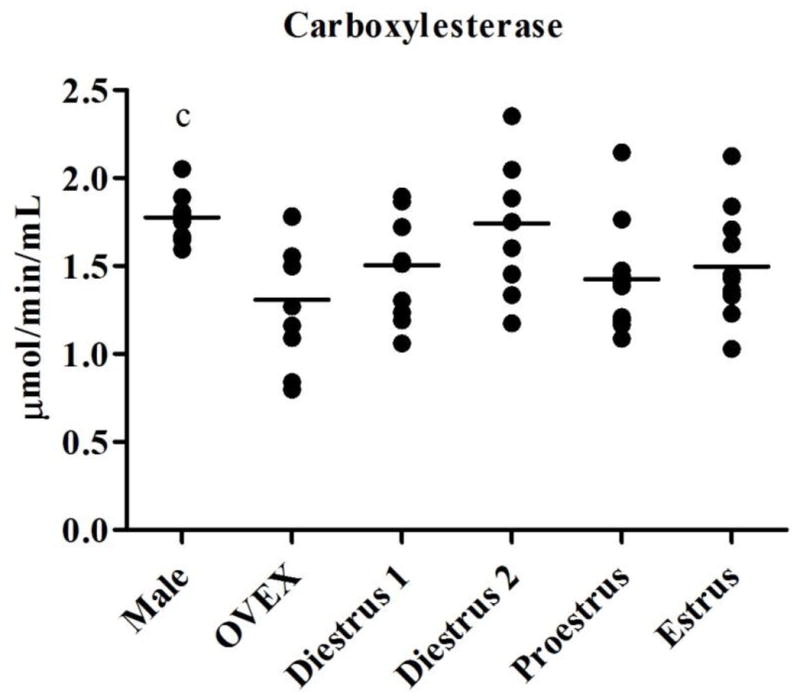
Activity levels of (1.1) AChE, (1.2) BuChE, and (1.3) CE in male, OVEX, diestrous I, diestrous II, proestrous, and estrous rats. Significant post-hoc comparisons are shown in letters and symbols.
a: AChE in males differed from females in diestrus I, diestrus II, and estrus
b: BuChE in males differed from all females
c: CE in males differed from OVEX females
#: BuChE in OVEX females differed from females in diestrus II
Table 2.
Means and standard deviations of AChE, BuChE, and CE plasma activity levels (μmol/min/mL) in male, OVEX, diestrous I, diestrous II, proestrous, and estrous rats. The two right columns show F and p values for esterase comparisons among each cycling female group with the male and OVEX rats.
| Group | Enzyme Activity (μmol/min/mL) | |||||
|---|---|---|---|---|---|---|
| AChE | BuChE | CE | ||||
|
|
||||||
| Male | 0.57 ± 0.13 | 0.086 ± 0.066 | 1.77 ± 0.15 | |||
|
|
||||||
| OVEX | 0.44 ± 0.11 | 0.21 ± 0.073 | 1.31 ± 0.37 | F | p | |
|
|
||||||
| AChE | 6.57 | 0.005 | ||||
| Diestrus I | 0.37 ± 0.098 | 0.30 ± 0.11 | 1.50 ± 0.28 | BuChE | 12.14 | < 0.001 |
| CE | 5.71 | 0.009 | ||||
|
|
||||||
| AChE | 9.93 | 0.001 | ||||
| Diestrus II | 0.36 ± 0.071 | 0.32 ± 0.090 | 1.74 ± 0.39 | BuChE | 26.84 | < 0.001 |
| CE | 5.09 | 0.014 | ||||
|
|
||||||
| AChE | 4.00 | 0.031 | ||||
| Proestrus | 0.41 ± 0.13 | 0.29 ± 0.094 | 1.42 ± 0.30 | BuChE | 12.61 | < 0.001 |
| CE | 5.75 | 0.009 | ||||
|
|
||||||
| AChE | 9.03 | 0.001 | ||||
| Estrus | 0.32 ± 0.12 | 0.30 ± 0.13 | 1.50 ± 0.31 | BuChE | 9.77 | 0.001 |
| CE | 5.33 | 0.012 | ||||
Significant main effects were Bonferroni corrected at p ≤ 0.0167.
4. Discussion
The purpose of this study was to determine if the 24 hr sarin LD50 changes throughout different hormonal states in female rats and in male rats. In addition, we measured the baseline activity levels of AChE, BuChE, and CE during all hormonal states (male, OVEX, diestrus I, diestrus II, proestrus, and estrus) to determine if activity of these esterases fluctuated among the groups. Our results indicated that the sarin LD50 changed throughout the estrous cycle. Females in proestrus were significantly less sensitive to the lethal effects of sarin compared to OVEX or estrous females, although no overall sex difference was observed. In addition, levels of AChE were generally lower, while BuChE levels were generally higher, in all females compared to males, and OVEX females had lower levels of CE than did males.
Although no sex differences were observed in the LD50 values, the variability in the cycling females could affect outcomes in CWNA-related toxicity or treatment studies. If one conducted a toxicity study in females, and all subjects were in the same day of the estrous cycle, results from that study could skew future experiments. For example, if all of our female subjects were in estrus during the sarin LD50 determination, we may have concluded that the female LD50 of sarin is ~110 μg/kg. If, however, all of the female subjects were in proestrus, we may have concluded that the female LD50 of sarin is ~124 μg/kg. Although a relatively small difference, using an LD50 of sarin of 110 μg/kg in proestrus female rats may be too low to fully or reliably induce toxicity. If one tested potential therapeutics in this scenario, one may incorrectly conclude that the therapeutics possess a higher level of efficacy than actually exists. In light of this possibility, the probability of females cycling together is determined by more than simply chance. Some strains of rats (Schank and McClintock, 1992) but not others (Schank, 2001) exhibit partial estrous cycle synchrony by the third cycle (~12 days), which is likely mediated by pheromones. Environmental stimuli, such as initiating vaginal smears or introducing females to a novel environment, also influence synchrony (Shirley, 1978), suggesting that it is not uncommon for female rats to exhibit similar cycles.
As indicated by LD50 values, protection from sarin in proestrous females compared to OVEX and estrous females may be imparted by the presence of high levels of circulating gonadal hormones, particularly estradiol. Indeed, plasma levels of estradiol peak in the morning of proestrus and decline rapidly by the morning of estrus (Gorski et al., 1975; Schank and McClintock, 1992). It is difficult to determine if progesterone exhibits a protective effect; progesterone levels rapidly peak and decline during the evening of proestrus and early morning of estrus (Gorski et al., 1975), and none of the cycling females received sarin during this time period. Incidentally, the LD50 of sarin in diestrous rats was in between those of proestrus and estrus females, which could have resulted from the gradual increase of estradiol secretion throughout the two days of diestrus. The LD50 of males resembled that of diestrous rats, which suggests that androgens may also impart some protection against sarin.
The exact mechanism underlying the observed differences in LD50 values remains to be determined, but it is unclear if the toxic effects of sarin are affected by variation in levels of plasma AChE, BuChE, and/or CE. These esterases varied little throughout the estrous cycle and, as deduced from Sweeney et al. (2006), endogenous levels of AChE and BuChE in the plasma are likely not high enough to significantly influence the LD50 of sarin. In addition, the levels of esterase activity do not appear to be associated with LD50 values. Males had higher AChE levels, and lower BuChE levels, than females, even though no difference between sexes existed in LD50 values. Furthermore, OVEX females only differed in BuChE activity compared to diestrous II females but the LD50 of these two hormonal states did not differ, and none of the esterase levels differed between OVEX and proestrous females. Nevertheless, these results do not necessarily preclude endogenous esterases from affecting the observed LD50 values. Additional studies examining esterase activity throughout the estrous cycle in tissues could address this issue.
Alternatively, the presumably high levels of estradiol in the proestrous rats may have imparted other protective effects that are independent of esterase activity. It is well established that estradiol has potent protective properties against a variety of physiological insults by reducing inflammation, inhibiting apoptotic messengers, and improving mitochondrial function (for review, see Spence and Voskuhl (2012)). Respiratory failure, a major contributing factor to CWNA-induced lethality (Rickett et al., 1986), may be delayed or reduced in severity by estradiol. Indeed, estradiol increases bronchodilation by relaxing smooth muscle in the airway (Townsend et al., 2010) possibly by potentiating the effects of norepinephrine on acetylcholine-induced contractions (Townsend et al., 2012). Testosterone also relaxes the airway (Montano et al., 2014), which may account for the higher LD50 of sarin in males compared to OVEX females.
Additional research is required to elucidate the precise underlying mechanisms affecting females’ sensitivity to the lethal effects of sarin throughout the estrous cycle. Future studies could examine the potential role of estradiol and progesterone, as well as other hormones that fluctuate with the estrous cycle, such as follicle stimulating hormone and luteinizing hormone. Gonadal hormones are also known to affect seizures (Scharfman and MacLusky, 2006), and the stage of the estrous cycle may affect how the epileptogenic properties of CWNAs manifest in females. Finally, the efficacy of currently fielded (atropine, 2-PAM, and diazepam) and potentially novel therapeutics could be examined to determine if their therapeutic properties fluctuate throughout the cycle. Nevertheless, the current study supports the hypothesis that the lethality of sarin in female rats is affected by the stage of the estrous cycle. If including female subjects in CWNA research, these results suggest that controlling for the stage of the estrous cycle can reduce potential variability most likely due to cycling gonadal hormones.
5. Conclusion
The 24 hr LD50 of sarin changes throughout the estrous cycle in female rats. Females in proestrus exhibit a significantly higher LD50 compared to females in estrus or those females with their ovaries removed. Furthermore, this finding does not appear to be dependent on differences in plasma activity levels of acetylcholinesterase, butyrylcholinesterase, or carboxylesterase. The mechanism underlying the higher LD50 in proestrus females is unclear but may be related to the presence of estradiol during that stage of the cycle. Although future research is required to elucidate these mechanisms, results from this study underscore the importance of monitoring the estrous cycle when using female rats in CWNA-related research.
Highlights.
The LD50 of sarin was determined in female rats throughout the stages of the estrous cycle.
Females in proestrus had a significantly higher LD50 compared to estrous or ovariectomized females.
No sex differences were observed between male and female rats.
It is unlikely that plasma esterase activity underlies the observed differences in LD50s
Acknowledgments
This research was supported by the NIH CounterACT Administrative Supplement Program #U01NS058183-07S1. The authors would like to thank Curtis Bradley, Conor Jennings, Caroline Schultz, and Amanda Furman for their assistance with animal handling, and Dr. Heidi Hoard-Fruchey, Dr. Douglas Cerasoli, and Dr. Erik Johnson for their review of this paper.
Footnotes
Disclaimers
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government. The experimental protocol was approved by the Animal Care and Use Committee at the United States Army Medical Research Institute of Chemical Defense and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony JS, Haley M, Manthei J, Way R, Burnett D, Gaviola B, Sommerville D, Crosier R, Mioduszewski R, Thomson S, Crouse C, Matson K. Inhalation toxicity of Cyclosarin (GF) vapor in rats as a function of exposure concentration and duration: potency comparison to sarin (GB) Inhalation toxicology. 2004;16:103–111. doi: 10.1080/08958370490265031. [DOI] [PubMed] [Google Scholar]
- Benton BJ, McGuire JM, Sommerville DR, Dabisch PA, Jakubowski EM, Matson KL, Mioduszewski RJ, Thomson SA, Crouse CL. Effects of whole-body VX vapor exposure on lethality in rats. Inhalation toxicology. 2006;18:1091–1099. doi: 10.1080/08958370600945598. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Chanda SM, Mortensen SR, Moser VC, Padilla S. Tissue-specific effects of chlorpyrifos on carboxylesterase and cholinesterase activity in adult rats: an in vitro and in vivo comparison. Fundamental and applied toxicology: official journal of the Society of Toxicology. 1997;38:148–157. [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett WP, Aracava Y, Adler M, Pereira EF, Albuquerque EX. Acute toxicity of organophosphorus compounds in guinea pigs is sex- and age-dependent and cannot be solely accounted for by acetylcholinesterase inhibition. The Journal of pharmacology and experimental therapeutics. 2009;328:516–524. doi: 10.1124/jpet.108.146639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder PI, Hobson DW, Olson CT, Joiner RL, Matthews MC. Stagewise, adaptive dose allocation for quantal response dose-response studies. Neuroscience and biobehavioral reviews. 1991a;15:109–114. doi: 10.1016/s0149-7634(05)80101-0. [DOI] [PubMed] [Google Scholar]
- Feder PI, Olson CT, Hobson DW, Matthews MC. Statistical analysis of dose-response experiments by maximum likelihood analysis and iteratively reweighted nonlinear least squares techniques. Drug Information Journal. 1991b;25:323–334. [Google Scholar]
- Feder PI, Olson CT, Hobson DW, Matthews MC, Joiner RL. Stagewise, group sequential experimental designs for quantal responses. one-sample and two-sample comparisons. Neuroscience and biobehavioral reviews. 1991c;15:129–133. doi: 10.1016/s0149-7634(05)80104-6. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Statistical aspects of monitoring for dangers in drug therapy. Methods of information in medicine. 1971;10:1–8. [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth defects research. Part B, Developmental and reproductive toxicology. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Gordon RK, Haigh JR, Garcia GE, Feaster SR, Riel MA, Lenz DE, Aisen PS, Doctor BP. Oral administration of pyridostigmine bromide and huperzine A protects human whole blood cholinesterases from ex vivo exposure to soman. Chemico-biological interactions. 2005;157–158:239–246. doi: 10.1016/j.cbi.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Mennin SP, Kubo K. The neural and hormonal basis of the reproductive cycle. In: Hedlund LW, Franz JM, Kenny AD, editors. Biological rhythms and endocrine function. Plenum Press; New York, NY: 1975. pp. 115–153. [Google Scholar]
- Hashinotsume M, Higashino K, Hada T, Yamamura Y. Purification and enzymatic properties of rat serum carboxylesterase. Journal of biochemistry. 1978;84:1325–1333. doi: 10.1093/oxfordjournals.jbchem.a132254. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Gahart MT, Rowe EJ, Bradley L. In: Drug safety: most drugs withdrawn in recent years had greater health risks for women. Office GA, editor. Washington, D.C: 2001. [Google Scholar]
- Illsley NP, Lamartiniere CA. Endocrine regulation of rat serum cholinesterase activity. Endocrinology. 1981;108:1737–1743. doi: 10.1210/endo-108-5-1737. [DOI] [PubMed] [Google Scholar]
- Jensen EV, DeSombre ER. Estrogens and progestins. In: Litwack G, editor. Biochemical actions of hormones. Academic Press, Inc; New York, NY: 1972. [Google Scholar]
- Lee EC. Clinical manifestations of sarin nerve gas exposure. Jama. 2003;290:659–662. doi: 10.1001/jama.290.5.659. [DOI] [PubMed] [Google Scholar]
- Leeuwin RS. Effect of androgenic and anabolic compounds on pseudocholinesterase activity in the liver and serum of the rat. Acta endocrinologica. 1965;50:391–402. doi: 10.1530/acta.0.0500391. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Goulet JC, Hand BT. Hormone- and dose schedule-dependent protection by HI-6 against soman and tabun poisoning. Fundamental and applied toxicology: official journal of the Society of Toxicology. 1989;12:595–603. doi: 10.1016/0272-0590(89)90032-8. [DOI] [PubMed] [Google Scholar]
- Mioduszewski R, Manthei J, Way R, Burnett D, Gaviola B, Muse W, Thomson S, Sommerville D, Crosier R. Interaction of exposure concentration and duration in determining acute toxic effects of sarin vapor in rats. Toxicological sciences: an official journal of the Society of Toxicology. 2002;66:176–184. doi: 10.1093/toxsci/66.2.176. [DOI] [PubMed] [Google Scholar]
- Montano LM, Espinoza J, Flores-Soto E, Chavez J, Perusquia M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. The Journal of endocrinology. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocrine reviews. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Aracava Y, DeTolla LJ, Jr, Beecham EJ, Basinger GW, Jr, Wakayama EJ, Albuquerque EX. Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. The Journal of pharmacology and experimental therapeutics. 2014;350:313–321. doi: 10.1124/jpet.114.214932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickett DL, Glenn JF, Beers ET. Central respiratory effects versus neuromuscular actions of nerve agents. Neurotoxicology. 1986;7:225–236. [PubMed] [Google Scholar]
- Schank JC. Do Norway rats (Rattus norvegicus) synchronize their estrous cycles? Physiology & behavior. 2001;72:129–139. doi: 10.1016/s0031-9384(00)00395-4. [DOI] [PubMed] [Google Scholar]
- Schank JC, McClintock MK. A coupled-oscillator model of ovarian-cycle synchrony among female rats. Journal of theoretical biology. 1992;157:317–362. doi: 10.1016/s0022-5193(05)80614-9. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E, Schmidt FW. Sex differences of plasma cholinesterase in the rat. Enzyme. 1978;23:52–55. doi: 10.1159/000458549. [DOI] [PubMed] [Google Scholar]
- Shirley B. Partial synchrony of the oestrous cycles of rats introduced to a new environment. The Journal of endocrinology. 1978;77:195–202. doi: 10.1677/joe.0.0770195. [DOI] [PubMed] [Google Scholar]
- Sket D. Efficacy of antidotes against soman poisoning in female physostigmine-protected rats. Pharmacology & toxicology. 1993;72:25–30. doi: 10.1111/j.1600-0773.1993.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Frontiers in neuroendocrinology. 2012;33:105–115. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterri SH, Fonnum F. Carboxylesterase - the soman scavenger in rodents: heterogeneity and hormonal influence. In: Reiner E, Aldrige WN, Hoskin FCG, editors. Enzymes hydrolyzing organophosphorus compounds. Ellis Horwood; Chichester, UK: 1989. pp. 155–164. [Google Scholar]
- Sweeney RE, Langenberg JP, Maxwell DM. A physiologically based pharmacokinetic (PB/PK) model for multiple exposure routes of soman in multiple species. Archives of toxicology. 2006;80:719–731. doi: 10.1007/s00204-006-0114-0. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. American journal of physiology Lung cellular and molecular physiology. 2012;303:L923–928. doi: 10.1152/ajplung.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. American journal of physiology Lung cellular and molecular physiology. 2010;298:L521–530. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


