Abstract
Objective
The prognostic significance of lymphovascular space invasion (LVSI) in patients with early-stage endometrial cancer is not established. We sought to determine if LVSI status in patients with early-stage low-risk endometrial cancer correlates with recurrence and survival.
Methods
The records of all women who underwent hysterectomy for primary treatment of endometrial cancer from January 2006 through January 2011 at one academic institution were reviewed. Patients with grade 1 or 2 endometrioid histology, myometrial invasion less than 50%, and disease confined to the uterus (clinical FIGO stage IA) were analyzed. Fisher’s exact test and the Wilcoxon rank-sum test were applied to compare patients with and without LVSI. Recurrence-free survival (RFS) and overall survival (OS) were calculated using the Kaplan-Meier method.
Results
Two hundred forty patients met the inclusion criteria. Forty (16.7%) had LVSI. Ninety-one patients (37.9%) underwent lymphadenectomy. Median tumor size was 30 mm in patients with and 26 mm in patients without LVSI (p=0.150). Thirty patients (12.5%) received adjuvant therapy. Site of recurrence did not differ between patients with and without LVSI. Patients with LVSI were more likely to have myometrial invasion (p<0.001), postoperative pathologic grade 2 disease (p<0.001), to undergo lymphadenectomy (p=0.049) and receive adjuvant therapy (p<0.001). The 5-year cumulative incidence of recurrence was 3.8% in the no-LVSI group and 14.2% in the LVSI group (p=0.053). The presence of LVSI was significantly associated with worse RFS (p=0.002) and OS (p=0.013).
Conclusion
Patients with low-risk endometrial cancer and LVSI have worse RFS and OS despite being more likely to undergo lymphadenectomy and adjuvant therapy.
INTRODUCTION
Endometrial cancer is the most common gynecologic malignancy in the United States, where 52,630 new cases of endometrial cancer and 8,590 deaths from this disease are expected in 2014 [1]. The majority of women with endometrial cancer are diagnosed with early-stage disease, which carries an excellent prognosis. The 5-year overall survival (OS) rate for women with early-stage endometrial cancer exceeds 80% [2]. There are a number of known prognostic factors for endometrial cancer defined by the International Federation of Obstetrics and Gynecology (FIGO), including tumor stage, grade, histologic type, and depth of myometrial invasion [3].
LVSI is defined as the presence of tumor cells inside endothelium-lined channels of uterine specimens, outside the main tumor, and this pathologic finding is directly correlated with lymphatic tumor metastasis. FIGO does not include lymphovascular space invasion (LVSI) as a prognostic factor for endometrial cancer even though LVSI has been described as a predictor of nodal metastases and disease recurrence in some series [4]. The risk of spread to lymph nodes is known to be higher in patients with LVSI, deep myometrial invasion, cervical involvement, or high-grade tumors [5]. Among women with early-stage endometrial cancer, certain tumor characteristics, including age, depth of invasion, grade, and LVSI, are used to stratify women into risk categories [6–10]. In 2009, O’Brien et al. reported that LVSI in patients with stage IA well-differentiated endometrial cancer correlated with a higher risk of death [11], but the authors did not report results on tumor size, performance of lymphadenectomy, or adjuvant treatment. Few studies have evaluated the impact of LVSI on survival in patients with early-stage endometrial cancer, particularly in patients considered to be at low risk for lymph node metastases and recurrence [10,11].
The aim of this study was to evaluate the association of LVSI with other histologic factors and the impact of LVSI on OS and recurrence-free survival (RFS) in patients with low-risk endometrial cancer. We were particularly interested in the patients with stage IA disease where all other factors were equal and only LVSI was the differentiating factor to determine the outcomes based on this finding alone.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. The medical records of all patients who underwent hysterectomy from January 2006 through January 2011 for primary treatment of endometrial cancer were retrospectively reviewed. Patients with grade 1 or 2 endometrioid histology, myometrial invasion of less than 50%, and disease confined to the uterus (clinical FIGO stage IA) were included in the analysis. Exclusion criteria included surgery performed outside MD Anderson, lack of documentation of treatment or incomplete medical records, nonendometrioid histology or grade 3 disease, and disease stage greater than IA.
All patients underwent a pretreatment evaluation, including physical examination and chest radiography. Primary surgery included a total hysterectomy with/without bilateral salpingo-oophorectomy. Pelvic, para-aortic, or both pelvic and para-aortic lymphadenectomy were performed according to the results of the intraoperative frozen section analysis. In our institution, we perform frozen section in the setting of grade I/II endometrioid adenocarcinoma on preoperative evaluation. Most surgeons will consider pelvic and para-aortic lymphadenectomy in all cases unless the frozen section shows the following criteria: grade I/II disease, less than 50% myometrial invasion, and tumor size less than 2 cm. Adjuvant treatment was given at the discretion of a multidisciplinary team. All outside diagnostic pathology slides were reviewed by a pathologist specialized in gynecologic malignancies at MD Anderson prior to surgical treatment.
The demographic, clinical, surgical, and pathologic factors evaluated in this study included age, body mass index, follow-up period, hysterectomy approach (abdominal, laparoscopic, or robotic), lymphadenectomy (yes or no), site of lymphadenectomy (pelvic, para-aortic, or both), lymph node status, histologic type, pre- and postoperative histologic grade, presence of myometrial invasion, tumor size, presence of LVSI, adjuvant treatment (yes or no), and type of adjuvant treatment (brachytherapy or external beam radiation therapy followed by brachytherapy).
The follow-up data were obtained from clinic visits and correspondence with patients and their physicians. Follow-up after treatment included visits every three months for the first year, every four months for the second year, and every six months during years 3 to 5. Pelvic examinations were done at each follow-up visit, and Papanicolaou testing was done once a year (according to the guidelines at the time of patient inclusion in the study). Patients who were no longer being followed clinically by MD Anderson were contacted annually by the institution's Department of Epidemiology to obtain information about cancer status and general medical problems; this information was recorded in each patient's medical record.
We compared patients with and without LVSI with regard to variables of interest by means of Fisher’s exact test for categorical variables. The Wilcoxon rank-sum test was used to compare median age, body mass index, tumor size, and follow-up. OS and RFS were estimated by means of the Kaplan-Meier method [12] stratified by LVSI status. Cox [13] proportional hazards regression was used to model OS and RFS as a function of LVSI and other potential prognostic factors, such as pre-operative grade and tumor size. OS was defined as the time from surgery to the date of death. RFS was defined as the time from surgery to the date of recurrence or death. Patients were censored at the last visit for RFS and at last contact for OS. There were too few events to perform an analysis of disease-specific survival. Multivariate analyses of OS and RFS could not be performed because of the low number of events.
Statistical significance was defined as p<0.05. All statistical analyses were performed using SAS 9.3 for Windows (SAS Institute Inc., Cary, NC), STATA 11.0 for Windows (StataCorp LP, College Station, Texas), and S-PLUS 8.0 for Windows (Insightful Corp., Seattle, Washington).
RESULTS
Two hundred forty patients met the study inclusion criteria. Forty of these patients (16.7%; 95% CI, 12.2%–22.0%) had LVSI. Demographic characteristics are summarized in Table 1. Patients with LVSI were significantly older (p=0.002). Surgical and pathologic characteristics are summarized in Table 2. Patients with LVSI had higher preoperative pathologic grade (p=0.016). There was no difference in surgical approach between patients with and without LVSI. Lymphadenectomy was performed in 91 patients (37.9%) and no patient had positive lymph nodes. Patients with LVSI and patients with pre-operative grade 2 tumors were more likely to have undergone lymphadenectomy at the time of surgery (p=0.049 and p=0.030; respectively). However, on multivariate analysis, neither LVSI (p=0.214), pre-operative grade (p>0.081), nor tumor size (p=0.813) were associated with the decision to perform lymphadenectomy. Only 30 patients (12.5%) received adjuvant therapy. Patients with LVSI were more likely to receive adjuvant therapy (p<0.001). Patients with LVSI were more likely to have myometrial invasion (p<0.001) and a postoperative pathologic grade of 2 (p<0.001).
Table 1.
Demographic characteristics.
| Characteristic | No LVSI (n=200) |
LVSI (n=40) |
Total (N=240) |
p value |
|---|---|---|---|---|
| Age, years | 0.0024 | |||
| Mean (SD) | 55.4 (11.5) | 61.0 (11.6) | 56.4 (11.7) | |
| Median | 56 | 61.5 | 57 | |
| Range | 18–84 | 32–83 | 18–84 | |
| BMI, kg/m2 | 0.4564 | |||
| Mean (SD) | 36.4 (10.2) | 35.3 (10.9) | 36.2 (10.3) | |
| Median | 35.7 | 32.5 | 35.5 | |
| Range | 20.2–64.9 | 18.8–70.8 | 18.8–70.8 | |
| Follow-up, months | 0.5253 | |||
| Mean (SD) | 46.4 (17.6) | 48.1 (18.3) | 46.7 (17.7) | |
| Median | 46.0 | 48.6 | 46.6 | |
| Range | 0.3–83.7 | 0.2–78.2 | 0.2–83.7 | |
| BMI, kg/m2, no. (%) | 0.5749 | |||
| < 25 | 28 (14.0) | 6 (15.0) | 34 (14.2) | |
| 25–30 | 38 (19.0) | 10 (25.0) | 48 (20.0) | |
| > 30 | 134 (67.0) | 24 (60.0) | 158 (65.8) |
SD, standard deviation.
Table 2.
Surgical and pathologic characteristics.
| No LVSI (n=200) | LVSI (n=40) | Total (N=240) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p value |
| Mode of surgery | 0.2898 | ||||||
| Abdominal hysterectomy | 65 | 32.5 | 10 | 25.0 | 75 | 31.3 | |
| Laparoscopic hysterectomy | 59 | 29.5 | 17 | 42.5 | 76 | 31.7 | |
| Robotic hysterectomy | 76 | 38.0 | 13 | 32.5 | 89 | 37.1 | |
| Lymphadenectomy | 0.0490 | ||||||
| No | 130 | 65.0 | 19 | 47.5 | 149 | 62.1 | |
| Yes | 70 | 35.0 | 21 | 52.5 | 91 | 37.9 | |
| Site of lymphadenectomy | 0.5730 | ||||||
| Pelvic | 37 | 18.5 | 10 | 25.0 | 47 | 19.6 | |
| Para-aortic | 4 | 2.0 | 0 | 0.0 | 4 | 1.7 | |
| Both | 29 | 14.5 | 11 | 27.5 | 40 | 16.7 | |
| Not performed | 130 | 65.0 | 19 | 47.5 | 149 | 62.1 | |
| Preoperative pathologic grade | 0.0164 | ||||||
| Hyperplasia | 12 | 6.0 | 1 | 2.5 | 13 | 5.4 | |
| Grade 1 | 76 | 38.0 | 7 | 17.5 | 83 | 34.6 | |
| Grade 2 | 112 | 56.0 | 32 | 80.0 | 144 | 60.0 | |
| Tumor size, mm | 0.1502 | ||||||
| N | 141 | 30 | 171 | ||||
| Mean (SD) | 32.7 (22.0) | 36.9 (20.6) | 33.5 (21.7) | ||||
| Median | 26 | 30 | 30 | ||||
| Range | 3–160 | 6–90 | 3–160 | ||||
| Myometrial invasion | <0.001 | ||||||
| No | 90 | 45.0 | 2 | 5.0 | 92 | 38.3 | |
| Yes | 110 | 55.0 | 38 | 95.0 | 148 | 61.7 | |
| Postoperative pathologic grade | <0.001 | ||||||
| Grade 1 | 71 | 35.5 | 3 | 7.5 | 74 | 30.8 | |
| Grade 2 | 129 | 64.5 | 37 | 92.5 | 166 | 69.2 | |
| Adjuvant therapy | <0.001 | ||||||
| No | 191 | 95.5 | 19 | 47.5 | 210 | 87.5 | |
| Yes | 9 | 4.5 | 21 | 52.5 | 30 | 12.5 | |
| Mode of adjuvant therapy | 0.9999 | ||||||
| Brachytherapy | 9 | 100.0 | 19 | 90.5 | 28 | 93.3 | |
| Pelvic + brachytherapy | 0 | 0.0 | 2 | 9.5 | 2 | 6.7 | |
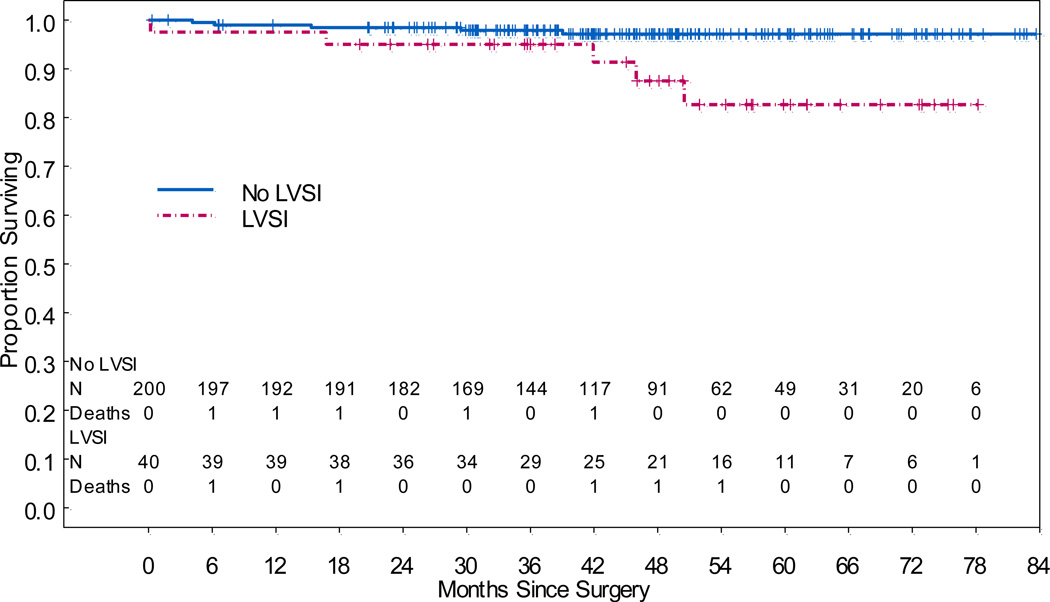
Patient outcomes are summarized in Table 3. The median follow-up time for all patients was 46.6 months (range, 0.2–83.7 months). Twelve patients had recurrent disease, and 2 of these patients later died. There was no difference in location of recurrence (vaginal cuff or pelvic) between patients with LVSI and those without. No patient had distant recurrence. Ten patients died, but only 2 died of disease. There were 5 deaths among the 200 patients without LVSI and 5 deaths among the 40 patients with LVSI. There was 1 death from disease in each group. Patients with LVSI had significantly worse OS (hazard ratio [HR], 4.78; 95% CI, 1.38–16.5; p=0.013) (Figure 1). Neither pre-operative grade (p>0.533) nor tumor size (p=0.872) were associated with OS. The 5-year OS rate for patients without LVSI was 97.2% (95% CI, 94.7%–99.7%), while the 5-year OS rate for patients with LVSI was 82.7% (95% CI, 69.4%–98.4%; p=0.05). For patients without LVSI, lymphadenectomy was not significantly associated with OS (p=0.852).
Table 3.
Patient outcomes.
| No LVSI (n=200) | LVSI (n=40) | Total (N=240) | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p value |
| Recurrence | 0.0325 | ||||||
| No | 193 | 96.5 | 35 | 87.5 | 228 | 95.0 | |
| Yes | 7 | 3.5 | 5 | 12.5 | 12 | 5.0 | |
| Site of recurrence | 0.9999 | ||||||
| Vaginal cuff | 4 | 57.1 | 3 | 60.0 | 7 | 58.3 | |
| Pelvis | 3 | 42.9 | 2 | 40.0 | 5 | 41.7 | |
| Treatment at recurrence | 0.5303 | ||||||
| Pelvic RT | 1 | 14.3 | 0 | 0.0 | 1 | 8.3 | |
| Chemotherapy | 1 | 14.3 | 0 | 0.0 | 1 | 8.3 | |
| Chemoradiotherapy | 1 | 14.3 | 2 | 40.0 | 3 | 25.0 | |
| Resection + chemotherapy | 0 | 0.0 | 2 | 40.0 | 2 | 16.7 | |
| Pelvic RT + brachytherapy | 1 | 14.3 | 1 | 20.0 | 2 | 16.7 | |
| Pelvic RT + chemotherapy | 2 | 28.6 | 0 | 0.0 | 2 | 16.7 | |
| Other | 1 | 14.3 | 0 | 0.0 | 1 | 8.3 | |
| Outcome at last follow-up | 0.0341 | ||||||
| No evidence of disease | 192 | 96.0 | 35 | 87.5 | 227 | 94.5 | |
| Alive with disease | 3 | 1.5 | 0 | 0.0 | 3 | 1.3 | |
| Died of disease | 1 | 0.5 | 1 | 2.5 | 2 | 0.8 | |
| Died of other cause | 4 | 2.0 | 4 | 10.0 | 8 | 3.3 | |
RT, external beam radiation therapy.
Figure 1.
Overall Survival
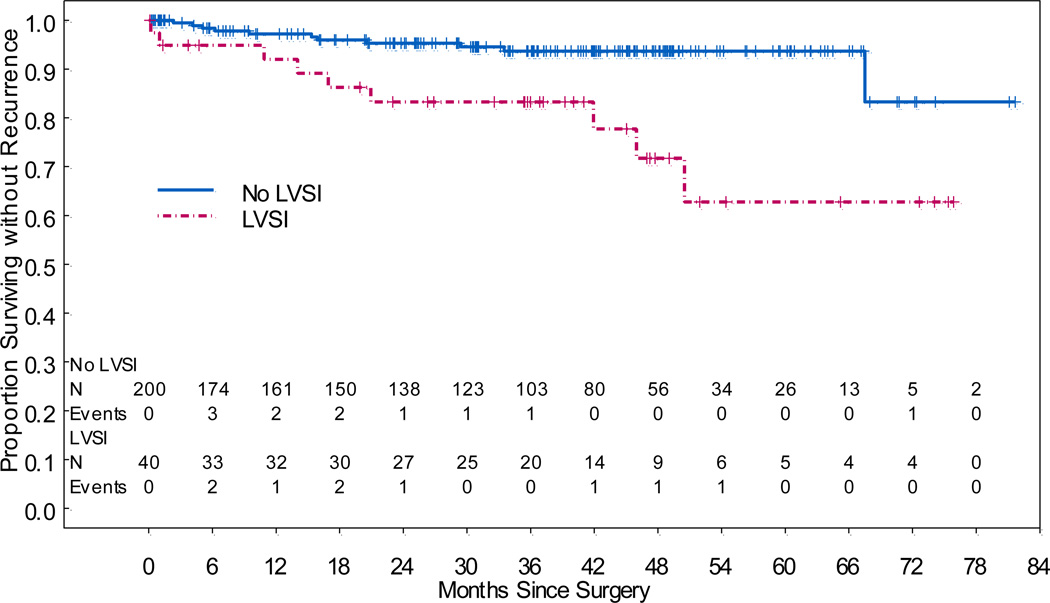
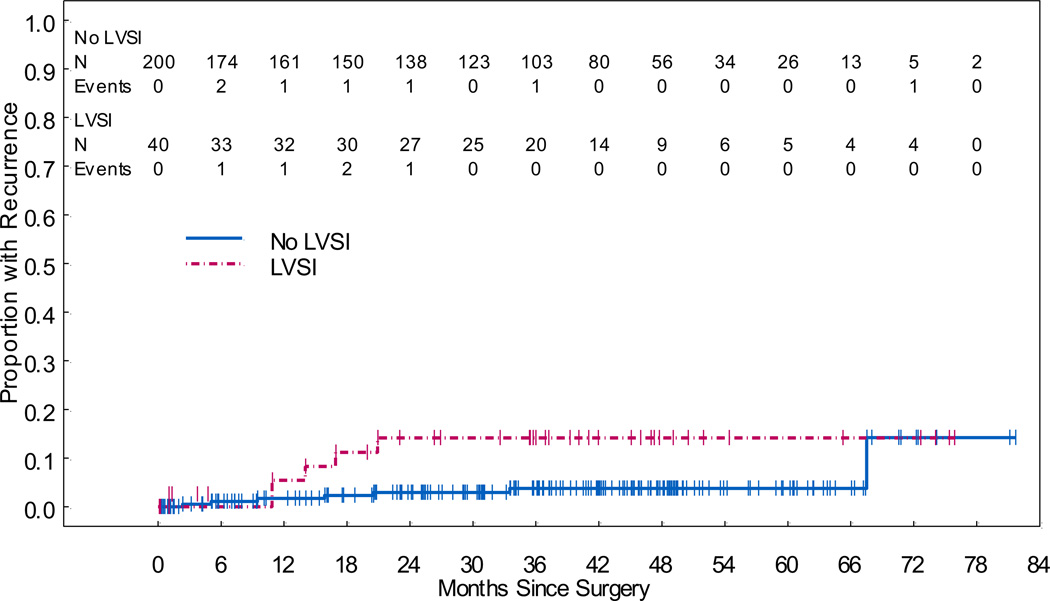
Patients with LVSI had significantly worse RFS (HR, 3.98; 95% CI, 1.64–9.63; p=0.002) (Figure 2). Neither pre-operative grade (p>0.161) nor tumor size (p=0.105) was associated with RFS. The 5-year RFS rate for patients without LVSI was 93.7% (95% CI, 89.9%–97.6%), while the 5-year RFS rate for patients with LVSI was 62.8% (95% CI, 43.6%–90.4%). The difference in 5-year RFS rates was significant (p=0.009). The cumulative incidence of recurrence at 5 years was 3.8% (95% CI, 1.5%–7.7%) in the no-LVSI group and 14.2% (95% CI, 5.2%–27.5%) in the LVSI group (p=0.053) (Figure 3). We aimed to determine the impact of adjuvant treatment on RFS in patients with LVSI. In patients with LVSI and adjuvant therapy, the 5-year RFS was 66.8% (95% CI, 43.9–100) and in those with LVSI and no adjuvant therapy the 5-year RFS was 70.6% (95% CI, 52–96.1) (p=0.192). However, we evaluated the 3-year RFS and found that for patients with LVSI and adjuvant treatment it was 94.4% (95 CI, 84.4–100) and for those with LVSI and no adjuvant therapy it was 70.6% (95% CI, 52–96.1) (p=0.054). For patients without LVSI, lymphadenectomy was not significantly associated with RFS (p=0.785).
Figure 2.
Recurrence-Free Survival
Figure 3.
Cumulative Incidence of Recurrence
DISCUSSION
Few studies have focused on the prognostic significance of LVSI in patients with low-risk endometrial cancer. Our study demonstrated that in patients with early-stage, low-risk endometrial cancer, the presence of LVSI was associated with worse RFS and OS even though patients with LVSI were more likely to undergo lymphadenectomy and adjuvant therapy.
The presence of LVSI is a known risk factor for recurrence and death in many other types of cancer, including vulvar cancer [14] and cervical cancer [15,16]. FIGO does not include LVSI as a prognostic factor for endometrial cancer [3]. LVSI has not been established as a predictor of recurrence in patients with endometrial cancer. Important clinical trials, such as PORTEC-1 and GOG-99, have established risk factors associated with increased recurrence rates in patients with EC, such as older age, higher grade, and greater depth of invasion [17,18]. LVSI is very difficult to assess during intraoperative frozen section analysis, and it is often reported only at the final pathology report. Currently, LVSI is considered a prerequisite for tumor dissemination by the lymphatic system.
The rate of LVSI observed in our series, 16.7%, is in line with results of other studies evaluating patients with low-risk endometrial cancer, which have found incidences of LVSI of 12% to 16.9% [11,19]. In studies evaluating all FIGO stages, LVSI has been observed in 8% to 42% of cases [6–10, 20]. Alexander-Sefre et al. found an association between stage and LVSI incidence: LVSI as detected by hematoxylin and eosin staining was not detected in any patients with stage IA disease but was found in 12% and 50% of patients with stage IB and IC disease, respectively [6].
Our results showed no difference in tumor size between patients with and without LVSI among patients with low-risk endometrial cancer. Conversely, a previous multivariate analysis that included patients with FIGO stage IA and IB (2009 staging system) endometrioid type histology and in addition, patients with stage IB and grade 3 EC (high-risk EC) showed that the presence of LVSI was associated with tumor diameter larger than 2 cm [9].
Our finding that patients with LVSI had higher preoperative and postoperative pathologic grade than patients without LVSI is consistent with previous series [4,20,21]. Narayan et al. studied high-intermediate risk and high-risk endometrial cancer and found that in patients with lymph node-negative disease, the presence of LVSI may be a more powerful prognostic marker than grade and histologic type. They proposed that irrespective of histologic type, patients without LVSI or lymph node metastasis should be regarded as having a very low risk of recurrence, and patients with LVSI without lymph node metastasis should be regarded as having an intermediate to high risk of recurrence [22]. We also found that patients with LVSI were more likely to have myometrial invasion.
An interesting finding of our study was that patients with LVSI were more likely to have undergone lymphadenectomy and received adjuvant therapy. This finding is perhaps due to the fact that since patients with LVSI were more likely to have myometrial invasion and higher grade, those patients then underwent a lymphadenectomy much more commonly than when these factors were not present. Another study also showed a significant correlation between LVSI and postoperative adjuvant therapy [23]. The role of adjuvant treatment immediately after surgery in patients with LVSI remains elusive. In our study, we did find that there was a trend towards a benefit of adjuvant therapy in patients with LVSI in the 3-year RFS (94.4% vs. 70.6%). These findings might be a result of the small number of patients with LVSI who underwent adjuvant therapy and a significant difference would have been noted if the number of patients were larger.
We aimed to evaluate whether in this very low risk population of patients, LVSI impacted recurrence rate and survival. In our study, incidence of disease recurrence was in 14.2% in patients with LVSI and 3.8% of patients without LVSI. Other studies have shown a significant difference in the incidence of disease recurrence in patients with LVSI [6,8]. A previous study showed that among patients with recurrence of endometrial cancer, the rate of LVSI ranged from 28.3% to 54%, while among patients without recurrence, the rate of LVSI ranged from 10% to 12.6% [6]. In addition, patients with LVSI may present with more distant recurrences [8] and shorter median time from diagnosis to recurrence [23]. In our study, patients with LVSI had significantly worse OS and RFS. Similarly, O’Brien et al. found in a series of 41 patients with low-risk endometrial cancer (well-differentiated adenocarcinoma, stage IA and IB) that LVSI was associated with increased risk of disease recurrence and death [11]. That study, however, did not present results on tumor size, lymphadenectomy, or adjuvant treatment.
LVSI has been shown to be a predictor of lymph node metastasis and decreased survival in patients with endometrial cancer. Zhang et al. demonstrated that the presence of LVSI in patients with stage I or II endometrial cancer had sensitivity and specificity of 41.7% and 94.5%, respectively, to predict pelvic lymph node metastasis. Furthermore, a combination of deep myometrial invasion and LVSI proved superior to LVSI alone in the prediction of pelvic lymph node metastasis [7]. Vaizoglu et al. observed that LVSI was the only clinic-pathologic factor associated with isolated para-aortic lymph node metastasis. Sensitivity, specificity, positive predictive value, and negative predictive value of LVSI for retroperitoneal lymph node metastasis in patients with endometrial cancer were 84.6%, 83%, 35.5%, and 98%, respectively [10]. Guntupalli et al. analyzed 757 patients with endometrioid endometrial cancer, stages IA to IVB, and found that LVSI was highly predictive of nodal disease and was an independent predictor of both decreased OS and decreased progression-free survival. The absence of LVSI had a negative predictive value of 95% and could therefore be considered as a marker to stratify patients according to the risk of nodal disease [4]. Hachisuga et al. studied the relationship between degree of LVSI (none, mild, or severe) and other histologic prognostic factors in a series of 303 patients with stages IA to IV EC. The degree of LVSI was found to correlate significantly with survival. The greatest difference in survival was found between patients with mild LVSI and severe LVSI [20]. Alexander-Sefre et al. studied patients with stage I EC and found a significant difference in RFS but not OS based on LVSI status. These authors showed the importance of immunohistochemical detection of LVSI in stage I endometrioid EC. LVSI was detected in a higher proportion of patients by immmunohistochemical analysis than by hematoxylin and eosin staining. Immunohistochemical detection of LVSI led to identification of more patients (73%) at risk for recurrent disease than did conventional hematoxylin and eosin staining (53%) [6]. Weinberg et al. showed that 28.3% of patients with LVSI had recurrence, compared to 12.6% of patients without LVSI (p<0.05). These authors also identified more distant recurrences in patients with LVSI [8].
The strengths of our study include the inclusion of only patients with low-risk endometrial cancer from a single institution operated on by a specialized gynecologic oncology team. Further, the evaluation of all specimens was performed by a designated group of pathologists that specialize in gynecologic malignancies.
The major weaknesses of this study are its retrospective nature and the number of events. Given the low incidence of lymph node metastasis in patients with low-risk endometrial cancer, a much larger sample size would be needed to demonstrate a significant association between LVSI and positive lymph nodes in low-risk patients. Another potential weakness of our study is that there was no re-review of the pathology specimens to evaluate for the presence or absence of LVSI.
In conclusion, our study showed that the presence of LVSI was associated with a worse prognosis in patients with low-risk EC, even those who underwent adjuvant therapy. Unfortunately, LVSI status is most commonly not available until after hysterectomy, at the time of preparation of the final pathology report. Therefore, LVSI status may represent additional information to be considered in the decision-making process regarding whether to perform pelvic and para-aortic lymphadenectomy or deliver adjuvant therapy after hysterectomy. Larger studies are needed to determine whether patients with low-risk EC with LVSI may require restaging, adjuvant therapy, or closer surveillance.
Acknowledgments
This research is supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA016672 and an NIH K12CA088084 K12 Calabresi Scholar Award to SNW.
Footnotes
Conflict of interest statement
None of the authors have conflicts of interests to report concerning the manuscript.
REFERENCES
- 1.Cancer facts & figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 2.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120(2 Pt 1):383–397. doi: 10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
- 3.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Guntupalli SR, Zighelboim I, Kizer NT, Zhang Q, Powell MA, Thaker PH, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol. 2012;124:31–35. doi: 10.1016/j.ygyno.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 6.Alexander-Sefre F, Nibbs R, Rafferty T, Ayhan A, Singh N, Jacobs I. Clinical value of immunohistochemically detected lymphatic and vascular invasions in clinically staged endometrioid endometrial cancer. Int J Gynecol Cancer. 2009;19:1074–1079. doi: 10.1111/IGC.0b013e3181abb0c0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Wang C, Feng W. Clinicopathological risk factors for pelvic lymph node metastasis in clinical early-stage endometrioid endometrial adenocarcinoma. Int J Gynecol Cancer. 2012;22:1373–1377. doi: 10.1097/IGC.0b013e318269f68e. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg LE, Kunos CA, Zanotti KM. Lymphovascular space invasion (LVSI) is an isolated poor prognostic factor for recurrence and survival among women with intermediate- to high-risk early-stage endometrioid endometrial cancer. Int J Gynecol Cancer. 2013;23:1438–1445. doi: 10.1097/IGC.0b013e3182a16c93. [DOI] [PubMed] [Google Scholar]
- 9.Laufer J, Scasso S, Papadia A, Sosa C, Cirillo F, Raspagliesi F. Association between tumor diameter and lymphovascular space invasion among women with early-stage endometrial cancer. Int J Gynaecol Obstet. 2013;123:142–145. doi: 10.1016/j.ijgo.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Vaizoglu F, Yuce K, Salman MC, Basaran D, Calis P, Ozgul N, et al. Lymphovascular space involvement is the sole independent predictor of lymph node metastasis in clinical early stage endometrial cancer. Arch Gynecol Obstet. 2013;288:1391–1397. doi: 10.1007/s00404-013-2913-x. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien DJ, Flannelly G, Mooney EE, Foley M. Lymphovascular space involvement in early stage well-differentiated endometrial cancer is associated with increased mortality. BJOG. 2009;116:991–994. 12. doi: 10.1111/j.1471-0528.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Cox DR. Regression models and life tables (with discussion) Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 14.Paladini D, Cross P, Lopes A, Monaghan JM. Prognostic significance of lymph node variables in squamous cell carcinoma of the vulva. Cancer. 1994;74:2491–2496. doi: 10.1002/1097-0142(19941101)74:9<2491::aid-cncr2820740916>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 16.Milam MR, Frumovitz M, dos Reis R, Broaddus RR, Bassett RL, Jr., Ramirez PT. Preoperative lymph-vascular space invasion is associated with nodal metastases in women with early-stage cervical cancer. Gynecol Oncol. 2007;106:12–15. doi: 10.1016/j.ygyno.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355(9213):1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 18.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 19.Momeni M, Kolev V, Cardenas-Goicoechea J, Getrajdman J, Fishman D, Chuang L, et al. Does the type of surgery for early-stage endometrial cancer affect the rate of reported lymphovascular space invasion in final pathology specimens? Am J Obstet Gynecol. 2013;208 doi: 10.1016/j.ajog.2012.10.009. 71.e1-6. [DOI] [PubMed] [Google Scholar]
- 20.Hachisuga T, Kaku T, Fukuda K, Eguchi F, Emoto M, Kamura T, et al. The grading of lymphovascular space invasion in endometrial carcinoma. Cancer. 1999;86:2090–2097. [PubMed] [Google Scholar]
- 21.Koskas M, Bassot K, Graesslin O, Aristizabal P, Barranger E, Clavel-Chapelon F, et al. Impact of lymphovascular space invasion on a nomogram for predicting lymph node metastasis in endometrial cancer. Gynecol Oncol. 2013;129:292–297. doi: 10.1016/j.ygyno.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Narayan K, Khaw P, Bernshaw D, Mileshkin L, Kondalsamy-Chennakesavan S. Prognostic significance of lymphovascular space invasion and nodal involvement in intermediate- and high-risk endometrial cancer patients treated with curative intent using surgery and adjuvant radiotherapy. Int J Gynecol Cancer. 2012;22:260–266. doi: 10.1097/IGC.0b013e318230c264. [DOI] [PubMed] [Google Scholar]
- 23.Puljiz M, Puljiz Z, Danolic D, Alvir I, Tomica D, Mamic I, et al. Prognostic value of lymphovascular space invasion in endometrial cancer. Med Glas (Zenica) 2013;10:288–292. [PubMed] [Google Scholar]