Abstract
During early patterning of the neural plate, a single region of the embryonic forebrain, the eye field, becomes competent for eye development. The hallmark of eye field specification is the expression of the eye field transcription factors (EFTFs). Experiments in fish, amphibians, birds and mammals have demonstrated largely conserved roles for the EFTFs. Although some of the key signaling events that direct the synchronized expression of these factors to the eye field have been elucidated in fish and frogs, it has been more difficult to study these mechanisms in mammalian embryos. In this study, we have used two different methods for directed differentiation of mouse embryonic stem cells (mESCs) to generate eye field cells and retina in vitro to test for a role of the PDZ domain-containing protein GIPC1 in the specification of the mammalian eye primordia. We find that the overexpression of a dominant-negative form of GIPC1 (dnGIPC1), as well as the downregulation of endogenous GIPC1, is sufficient to inhibit the development of eye field cells from mESCs. GIPC1 interacts directly with IGFR and participates in Akt1 activation, and pharmacological inhibition of Akt1 phosphorylation mimics the dnGIPC1 phenotype. Our data, together with previous studies in Xenopus, support the hypothesis that the GIPC1-PI3K-Akt1 pathway plays a key role in eye field specification in vertebrates.
INTRODUCTION
The eye development begins at gastrula stages during the regionalization of the anterior neural plate (ANP). The current model proposes that master-regulatory genes expressed in these committed progenitors are sufficient to initiate fate-determining developmental programs. At a molecular level, Otx2 is required for the formation of the whole anterior neural region including the eyes [1], and the eye field is subsequently specified by the expression of a network of transcription factors that include Rax (retina and anterior neural fold homeobox), Pax6 (paired box gene 6), Lhx2 (LIM homeobox-2) and Six3 (Sine oculis homeobox 3) [2–4].
The regulatory mechanisms that define the domain of EFTF expression are not well understood. Most of what we know about this has been discovered in organisms with accessible embryos like zebrafish and Xenopus. These studies have revealed a network of signals upstream of the EFTFs that involve inhibition of BMP and Wnt signaling [5–8]. However, it is not known how much of this model is conserved in mammals. Signaling pathways are difficult to manipulate in mammals at early embryonic stages without affecting other functions of the same molecules. Therefore, in vitro model systems that recapitulate key aspects of embryogenesis might provide an approach to understand ANP patterning and retinal specification in mammals.
Recently, embryonic stem cells (ESCs) have emerged as an alternative method to study the earliest steps of mammalian ontogeny. ESCs are pluripotent cells derived from the inner cell mass of pre-implantation blastocysts. These cells behave similarly to those present in the developing embryo and can be differentiated under defined conditions into a broad range of cell types. The differentiation paradigms towards eye field progenitors and mature retinal cells from mouse ESCs (mESCs), human ESCs (hESCs) and induced-pluripotent SCs (iPSCs) are well established [9–16]. Upon differentiation, the cells acquire characteristics of retinal differentiation, progressing through a succession of stages that recapitulates normal in vivo development. Hence, ESCs provide a potential model for testing hypotheses concerning forebrain patterning and eye field specification in vitro.
Here, we directed mESCs towards eye field and retinal fates, to test whether a specific component of the signaling network in frog embryos, Kermit2, is important in mammalian eye development. In a previous report, Kermit2, the Xenopus homolog of GAIP-interacting protein, C terminus (GIPC) was shown to be required for eye formation [17]; morpholino knockdown of this gene led to embryos lacking eyes, but were otherwise apparently normal. GIPC1 is a small adaptor protein that interacts with multiple cytoplasmic proteins and transmembrane receptors and likely plays a role in endosome signaling and membrane recycling [18–21]. In the present study, we use mESC cultures to analyze the function of GIPC in the specification and differentiation of eye field and retinal fates. Our results indicate that GIPC plays a key role in the specification of the eye field, and likely acts through the regulation of PI3K-Akt1 pathway downstream of IGFR.
RESULTS
GIPC1 is expressed in the developing murine retina and upregulated upon retinal differentiation
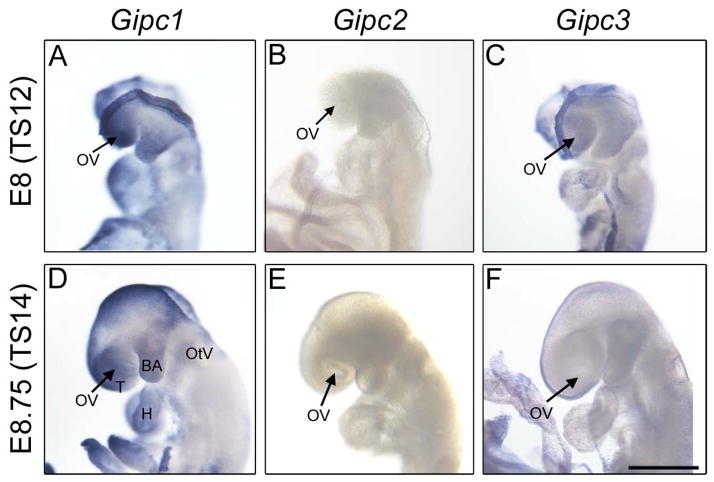
To determine whether GIPC proteins are required for mouse eye development as has been reported for XGIPC/Kermit2 in Xenopus [17], we analyzed the developmental expression of the three members of this family: Gipc1, Gipc2 and Gipc3. The closest mouse homolog to Xenopus Kermit2 is the mouse Gipc1, with 69.9% total amino-acid identity, but Gipc2 and Gipc3 had nearly the same degree of sequence conservation (Gipc2, 69.4% amino-acid identity and Gipc3, 67.8% amino-acid identity) (Supplementary Figure 1). All the Gipc family members, analogous to Kermit2, contain a PDZ domain with a highly conserved carboxylate-binding pocket. We analyzed the expression pattern of Gipc1,2,3 gene family members in mouse embryos, using in situ hybridization (Figure 1). Gipc1 expression was detected at E8 (Theiler stage 12, 2–7 somite pairs), and persisted until later stages. By contrast, Gipc2 was not detected at early embryonic stages and we only detected low levels of Gipc3. At E8.75 (Theiler stage 14, 8–12 somite pairs), Gipc1 was widely expressed in the developing embryo. The highest expression levels were detected in the optic vesicles as well as in the telencephalon, the otic vesicle and the branchial arches. Some other regions like the heart and the midbrain also showed moderate levels of Gipc1 expression (Figure 1).
Figure 1. GIPC1, GIPC2, and GIPC3 expression in the developing mouse.
Whole mount RNA in situ hybridization of GIPC1, GIPC2 and GIPC3 in embryonic day 8 and 8.75 embryos. The arrows indicate eye field/optic vesicle (OV) region. T: telencephalon, BA: branchial arches, OtV: Otic vesicle, H: heart. Scale bar: 300 microns.
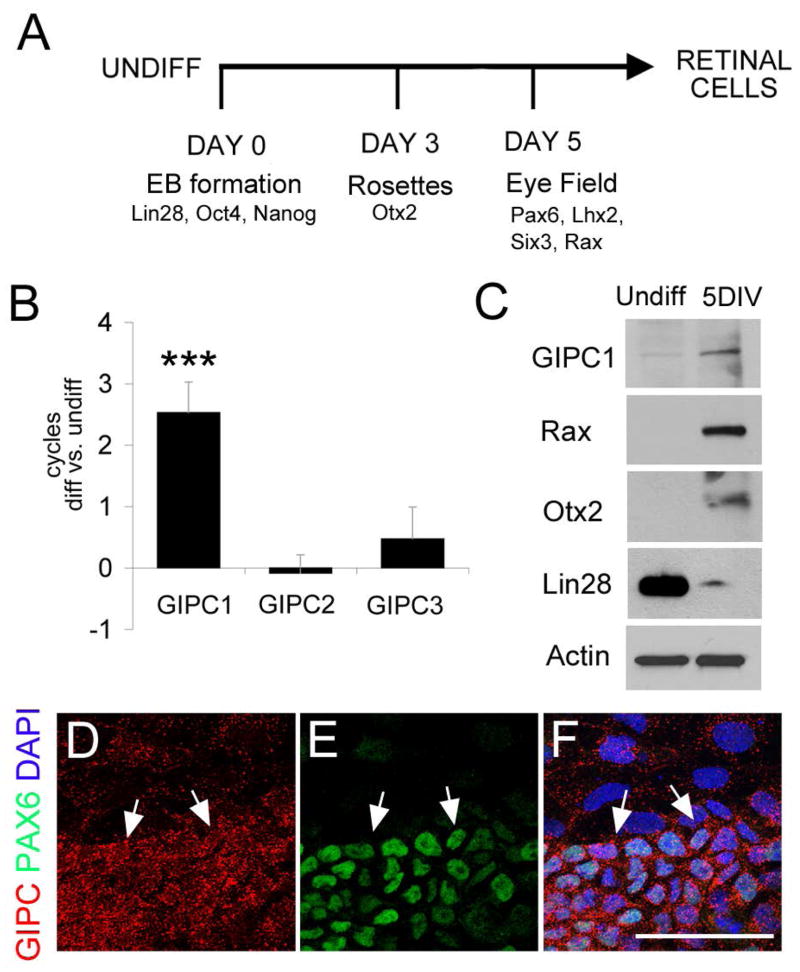
To further analyze the expression of Gipc genes during eye field specification in mouse, we used a previously described protocol for inducing ocular tissues from ESCs [11]. In this protocol, a combination of soluble factors, IGF1, Dkk1 and Noggin1, induces synchronized expression of EFTFs and the undifferentiated mESC colonies undergo a stepwise differentiation process that reproduces the normal retinal developmental timeline (Figure 2A). The mESC initially develop neuroepithelial characteristics (day 3) and, soon after, they yield a highly enriched population of eye field cells (day 5). Subsequently, cells acquire characteristics of retinal differentiation [11], and after 3–7 days of treatment, these cultures contain neuronal rosettes and express multiple eye field markers. To investigate the expression pattern of the Gipc genes in these eye field cells, Gipc1, Gipc2 and Gipc3 were monitored by reverse transcription and quantitative PCR (RT-qPCR). Consistent with the in situ hybridization results, Gipc1 was expressed in mESC-derived eye field cells, but Gipc2 and Gipc3 were not increased after retinal induction (Figure 2B). Western blot analyses detected GIPC1 protein expression as early as day 5 of differentiation in the mESC-derived eye field cells (Figure 2C) and immunofluorescence showed cells co-expressing GIPC1 and Pax6, one of the EFTFs (Figure 2D–F). Together these observations provide evidence that GIPC1 is present in the early stages of eye development in mice.
Figure 2. GIPC1 is upregulated upon mESC retinal differentiation.
(A) Diagram of the mESC 2D differentiation protocol (B) RT-qPCR analyses of the expression profiles of GIPC1, GIPC2 and GIPC3 in undifferentiated mESCs and cells differentiated for 5 days. Data is represented as the relative change (ddCT) between undifferentiated samples and differentiated cultures and indicates mean ± SEM. *** indicates a p-value < 0.001. (C) Western blot comparison of undifferentiated cells (first lane) and mESCs differentiated for 5 days (second lane). (D–F) Immunostaining of GIPC1 (red) and Pax6 (green, arrows). Arrows indicate cells with higher levels of GIPC1 colocalizing with Pax6+ cells. Scale bar: 50 microns.
GIPC1 is required for the development of retinal identity from embryonic stem cells
To determine whether GIPC1 is required for eye development in mice, we used a dominant-negative GIPC1 in mESCs undergoing directed differentiation to retinal cells. Disruption of the carboxylate-binding loop of the GIPC1 PDZ domain (Leu-Gly-Leu, Supplementary Figure 1) prevents the recruitment of PDZ-interacting molecules, causing a dominant negative phenotype [20, 22]. We generated stable mESC lines overexpressing a mutated GIPC1 construct (dnGIPC1) carrying three mutations in the PDZ binding pocket (LGL → AAA). We established two clonal lines through transfection and antibiotic selection (dnGIPC#2, dnGIPC#15). Similar to parental R1 cells, the resulting colonies maintained ESC-like morphology with well-defined edges and a high nucleus to cytoplasm ratio. In addition, the pluripotency markers Oct4, Nanog, Sox2 and Lin28 were not affected upon GIPC1 depletion (Supplementary Figure 2) and alkaline phosphate staining indicated that mESCs did not spontaneously differentiate after depletion of GIPC1 activity (data not shown).
We used the retinal differentiation protocol described above to test whether the dnGIPC mESC cell lines would show a reduction in EFTF expression. The eye field differentiation was assessed by RT-qPCR and immunostaining. Remarkably, upon differentiation, dnGIPC lines showed reduced levels of several EFTFs, compared to control lines (Figure 3). Pax6, Rax, Six3 and Lhx2 all showed lower expression levels (Figure 3C, G and M–P) in the dnGIPC derived cells. However, the expression of the transcription factor Otx2 was not affected in the dnGIPC1 ESC lines (Figure 3J, K and Q). Otx2 is expressed in the forebrain/midbrain region of the neural plate (Figure 3I), but not in undifferentiated stem cells (Figure 2C), suggesting that the expression of dnGIPC1 did not impair ESC differentiation into more general anterior neuronal fates. These results are very similar to the effects of Kermit2 morpholinos in Xenopus, where Xrx (Rax) and Pax6 showed reduced expression, but Otx2 was not affected [17].
Figure 3. dnGIPC1 and shRNA cell lines show reduced levels of EFTFs using 2D culturing method.
Expression of (A) Pax6, (E) Lhx2, and (I) Otx2 in embryonic day 9 embryos. Compared to control cell lines, dnGIPC1 cells and shRNA cells exhibited lower levels of (B–D) Pax6 and (F–H) Lhx2 proteins, but they showed no differences in the number of Otx2+ cells (J–L). RT-qPCR analyses showed a significant decrease in the expression of (M) Pax6, (N) Six3, (O) Lhx2, and (P) Rax but not (Q) Otx2 in both the dnGIPC1 and the shRNA cell lines. RT-qPCR data is represented as the relative change (ddCT) between undifferentiated cells and cultures differentiated for 5 days, and indicates mean ± SEM. OV: optic vesicle. Scale bars: 500 microns in panels A, E and I and 200 microns in panels B–D, F–H, J–L. P-values: * < 0.05, ** < 0.01, *** < 0.001.
To further confirm the GIPC1 loss-of-function phenotype, we generated stable ESC lines that overexpress shRNA against endogenous GIPC1. We tested 5 different shRNAs constructs for their efficiency in downregulating GIPC1 protein in HEK293T cells (Supplementary Figure 3). Three shRNAs (shRNA#1 and shRNA#3 and shRNA#5) were effective in reducing the levels of GIPC1 to 20–30%. Two shRNAs (shRNA#1 and shRNA#3) and control-scrambled shRNA were further used to generate mESC stable lines. After antibiotic selection, these cell lines were indistinguishable from the parental R1 line; the depletion of GIPC1 protein in undifferentiated cells did not affect survival, proliferation or the expression of pluripotency markers (Supplementary Figure 2).
We next tested whether knockdown of GIPC1 would affect retinal differentiation of the mESCs. We found that similar to the dnGIPC1 lines, when subjected to the differentiation protocol, the shRNA-overexpressing mESCs lines showed lower expression levels of all the EFTFs (Figure 3D, H and M–P). Also, similar to the dnGIPC1 lines, the shRNA-overexpressing mESCs also had approximately the same level of Otx2 expression as the wild type cells, consistent with the hypothesis that inhibiting GIPC1 function does not impair neuronal differentiation in general, but rather has a specific effect on eye field differentiation (Figure 3L and Q). Taken together, these data indicate that GIPC1 is necessary for the acquisition of eye fates, but not for the initial Otx2-dependent neural plate patterning.
dnGIPC1 mESC lines increase expression of telencephalic genes
Upon differentiation, dnGIPC1 cell lines did not express genes that are restricted to the eye field. To assess whether dnGIPC1 cells acquired other ANP identities, we analyzed the expression of Emx1 and Emx2, transcription factors expressed in the telencephalon, and Engrailed (En1), which is expressed in the midbrain/hindbrain junction [23]. After 5 days of differentiation, the wild type mESC did not express significant levels of these genes. By contrast, dnGIPC1 and shRNA cell lines exhibited a significant increase in Emx1 and Emx2 gene expression (Figure 4A and B). The expression of En1 was not significantly changed in any of the lines (Figure 4C). The dnGIPC1 cell lines also showed an increase in another cerebral cortical marker, Tbr2 (Figure 4D, see below). These data indicate that the cells lacking GIPC1 activity can acquire markers of telencephalic identity.
Figure 4. dnGIPC1 cell lines differentiate into telencephalic fates.
(A–D) RT-qPCR analyses of the undifferentiated mESCs and cells differentiated under the 2D protocol for 10 days. Data is represented as the relative change (ddCT) between undifferentiated samples and differentiated cultures and indicates mean ± SEM. P-values: * < 0.05, ** < 0.01, *** < 0.001. Immunolabeling experiments at 10 days showing the expression of (E, G, H, J) Otx2 (red), (F, G, I, J) Pax6 (green), (K, M, N, P) Tbr2 (green), and (L, M, O, P) Tuj1 (red).
To further confirm this possibility, we cultured mESC for 5 more days in vitro (day 10) and analyzed the effects of GIPC1 inhibition by immunolabeling with Pax6 and Otx2 antibodies. This combination of labels allowed us to distinguish different populations of the developing neural plate: while Otx2 and Pax6 are expressed in both the retina and the telencephalon, their expression patterns differ. In the retina, Otx2 is limited to photoreceptors and bipolar cells, while Pax6 is expressed in retinal progenitors, Retinal Ganglion cells (RGCs), amacrine cells and horizontal cells. Thus, in the retina these two transcription factors are expressed in different subsets of cell populations. As expected, as development proceeded in the control cultures, the Otx2+ and the Pax6+ populations segregated (Figure 4E–G). Conversely, in the dnGIPC1 lines, Otx2 and Pax6 did not segregate into distinct populations (Figure 4H–J).
A further way to distinguish telencephalic fates from retinal identity is with a combination of Tbr2/Eomes and Tuj1. In the telencephalon, Tbr2/Eomes is highly expressed in a population of progenitors of the ventricular zone and the subventricular zone of cerebral cortex as well as in the hippocampus and other telencephalic structures [24–26]. By contrast, in the retina, Tbr2/Eomes is expressed in a subpopulation of RGCs [27, 28]. In order to determine the identity of the small percentage of Tbr2+ cells in the control cultures, we coimmunostained these mESC-derived cells using Tbr2 and Tuj1 antibodies. The few Tbr2+ cells present in the control cultures were also Tuj1+ (Figure 4K–M, white arrows) and showed long processes indicating that these cells might be RGCs. In the dnGIPC1 mESCs, Tuj1 and Tbr2 exhibited a large up-regulation comparable to the expression pattern of these proteins in the developing cortex (Figure 4N–P). Taken together these results indicate that after GIPC1 depletion, Otx2+ neural progenitors did not differentiate into retinal progenitor cells, but instead upregulated markers for telencephalic progenitors.
GIPC1 is required for the development of retinal identity in 3D cultures
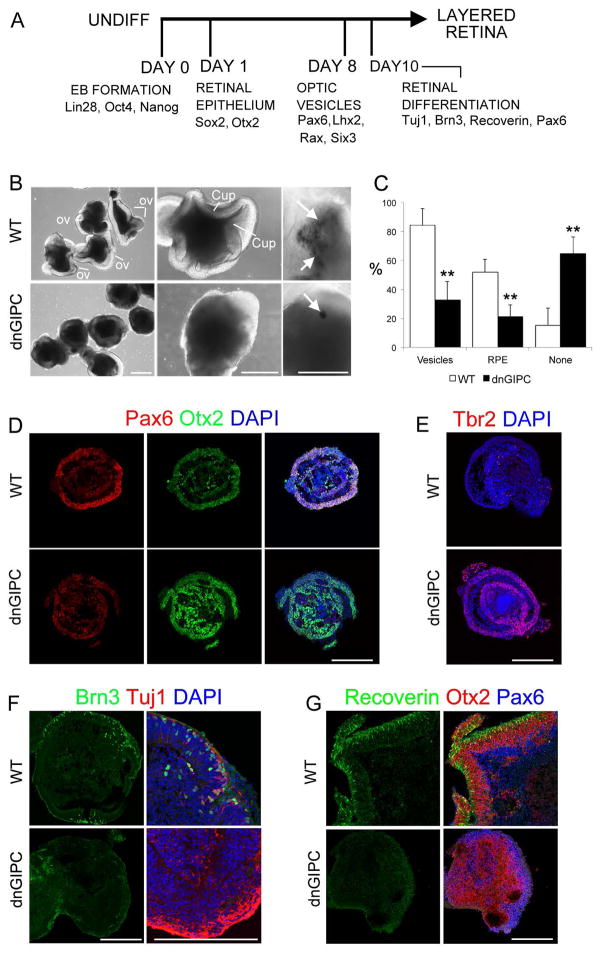
Recent protocols to differentiate ESCs into a variety of neuronal identities are based on three-dimensional culturing [12, 29–31]. We generated embryoid bodies (EBs) containing retinal neuroepithelium by dissociating mESCs and plating them in 96-well low-attachment plates (3,000 cells/well). In these conditions, cells spontaneously formed EBs, and when grown in the presence of knockout-serum replacement and Matrigel will form optic vesicle and cup-like structures (Figure 5B) [12, 29, 32]. In 13 days, pigmented RPE was observed in about 50% of the EBs and, when kept for longer periods of time, the mESCs-derived retinal progenitors generated the various layers of the retina, including ganglion cells and photoreceptors, recapitulating the normal development of the eye (Figure 5 F, G).
Figure 5. dnGIPC1 cell lines show reduced retinal differentiation in 3D culture systems.
(A) 3D differentiation protocol. (B) EBs at day 13 of differentiation. Arrows indicate pigmented RPE. OV: optic vesicle, Cup: cup-like structures. (C) Quantification of the percentage of EBs that exhibited optic vesicle-like structures, RPE or neither. Data is represented as mean ± SEM. (D) Immunostaining of EBs after 5 days of differentiation showing the expression of Pax6 (red) and Otx2 (green). EBs at day 13 showing (E) the expression of the telencephalic marker Tbr2 (red) and (F) the Retinal Ganglion Cell markers, Brn3 (green) and Tuj1 (red). (G) Stainings at day 24 of differentiation showing expression of the photoreceptors marker Recoverin (green), Otx2 (red), and Pax6 (blue). Scale bars: 200 microns. P-Values** < 0.01.
In order to assess the effect of dnGIPC1 on the development of retina in the 3D protocol, we differentiated the dnGIPC1 mESCs using this system (Figure 5A). Compared to control mESCs, only a small fraction of dnGIPC1 EBs developed optic vesicle structures (Figure 5B, C; 32.7% vs 84.7%), which is consistent with the observation that dnGIPC1 cells express reduced levels of EFTFs. In addition, fewer of the EBs exhibited pigmented RPE cells and, when present, the patches of RPE were smaller (Figure 5B, C). Furthermore, the dnGIPC1 mESCS showed a reduction in the levels of Pax6 after 7 days of differentiation (Figure 5D) and a strong upregulation in Tbr2 at day 13 (Figure 5E) when compared with wild type R1 cells. When maintained for longer periods of time, normal mESCs differentiate into laminar structures with Recoverin+ photoreceptors, Brn3+ RGCs and a clear separation between the Otx2+ cells (bipolar cells and photoreceptors) and the Pax6+ inner retinal cells (Supplementary Figure 4); however the dnGIPC1 lines did not differentiate into Brn3+ RGCs (Figure 5F) nor Recoverin+ photoreceptors (Figure 5G). These results add further support that GIPC1 activity is necessary for the formation of eye field cells and their subsequent differentiation into mature retina. We verified that the 3D protocol was generating regions of neural retina in the wild type cells by using a newly generated Rax-GFP mESC line (Figure 6A–J) and that GIPC1 is expressed in the Rax-GFP+ regions by day 10 (Figure 6H).
Figure 6. Characterization of Rax expression during early development; mESCs derived from Rax-GFP mice differentiate into laminated retina.
Whole mount immunostaining of embryonic day 8 (A, A′) and 8.5 (B, B′), showed Rax promoter driven-GFP expression in the area of the future retina. In postnatal day 0 retinas, Rax was expressed in progenitors, some cones and Müller glia (C–C′). P0 retinas were also immunanostained with Pax6 (red) and DAPI (blue, C′). (D–J) Characterization of Rax-GFP expression in mESCs derived from Rax-GFP mice and differentiated using the 3D protocol. GFP expression was first observed after 7 days of retinal differentiation and was continually expressed throughout differentiation. Live images of EBs were taken at (D, D′) Day 7, (E, E′) Day 14, (F, F′) Day 16, and (G, G′) Day 24. (H) EBs of Rax-GFP mESCs were differentiated for 10 days and immunostained with GFP, GIPC (red), and Pax6 (white). (I) After 25 days of differentiation, GFP+ areas expressed Recoverin (red) and Pax6 (white), and were laminated. (J) At Day 28, the GFP+ regions (green) also expressed Rhodopsin (red) and S-Opsin (white). Scale bar: 50 microns.
GIPC1 participates in IGF1R signaling in developing eye field cells
IGF signaling has been linked to the eye development in Xenopus and zebrafish embryos [17, 33–35]. Ectopic expression of IGF1R in dorsal positions of the amphibian embryo induced the formation of ectopic eyes and a dominant-negative IGFR construct reduced the expression of eye field markers (e.g. Rax) [35]. Additionally, GIPC1 has been shown to interact with IGF1R [17, 36–38] and in Xenopus, Kermit2/GIPC is required for IGF1-induced ocular tissues in both embryos and animal caps [17]. We therefore hypothesized that GIPC1 could also be interacting with IGF1R during eye field induction in mouse. To test this possibility, we performed immunoprecipitation using GIPC1 and IGF1R antibodies. HEK 293T cells were transfected with GIPC1 and IGF1R plasmids and immunoprecipitated with either GIPC1, IGF1R antibodies or with an antibody against GFP as a negative control. GIPC1 protein was detected by Western blot in both the GIPC1 and the IGF1R immunoprecipitates, but not in the GFP controls, indicating that GIPC1 and IGF1R proteins can physically interact (Figure 7A, upper panel). We next tested whether GIPC1 and IGFR1 were part of a similar complex in developing retinal progenitors. We carried out immunoprecipitation using cell lysates from either mESC-derived retinal cells after 7 days of differentiation or from freshly dissected E11 mouse retinas. In both cases, we detected GIPC1 protein after immunoprecipitation with IGF1R antibodies (Figure 7A), suggesting that GIPC1 and IGF1R interact in retinal progenitors.
Figure 7. GIPC1 interacts with IGFR in retinal progenitors and inhibition of PI3K-Akt1 pathway reduces mESCs retinal differentiation.
(A) Western blot analyses of GIPC1 of cell lysates from either transfected HEK 293T cells (upper panel), mESCs differentiated for 7 days (middle panel) or E11 retinas (bottom panel). Samples were immunoprecipitated with GIPC1, IGF1R antibodies or a GFP antibody as a control. Arrows indicate GIPC1 band. (B) IGF1-dependent phosphorylation of MAPK ERK1/2 and Akt1 proteins in HEK 293T transfected with pRK5 empty vector (lanes 1–2), GIPC1 (lanes 3–4) or dnGIPC1 (lanes 5–6). Western blot analyses of phospho-ERK1/2 (upper panel), phospho-Akt1 (second panel), GIPC1 (third panel) and Actin loading control (bottom panel). Densitometric analyses of 4 independent experiments (normalized to untreated samples) showing IGF1-dependent phosphorylation of ERK1/2 and Akt1. (C) IGF1-dependent phosphorylation of IGFR was analyzed by immunoprecipitation experiments with IGF1R antibodies followed by Western blot against anti-phosphoTyrosine (4G10) of HEK 293T cells transfected with either a control empty plasmid (pRK5, first two lanes) or dnGIPC1 (lanes 3–4). (D) RT-qPCR analyses of cells differentiated for 5 days using the 2D culturing method in control conditions (light gray bars), treated with DMSO vehicle (dark gray bars), with MAPK inhibitor (blue bars) or Akt1 inhibitor (orange bars). Data is represented as the relative change (ddCT) between undifferentiated cells and cultures differentiated for 5 days and indicates mean ± SEM. P-values: * < 0.05, ** < 0.01, *** < 0.001. (E) RT-qPCR analyses of EFTFs in cells differentiated for 5 days using the 3D culturing method under control conditions (light gray bars), with DMSO vehicle (dark gray bars), with TGF-β receptor inhibitor (purple bars) and Akt inhibitor (orange bars). Data is represented as the relative change (ddCT) between undifferentiated cells and 5 days of retinal differentiation. Mean ± SEM.
IGF1R signals though several intracellular pathways including Ras/MAPK pathway and PI3K/Akt1 pathway. To determine which of these pathways were affected by inhibition of GIPC1, full-length wild type GIPC1 or the dnGIPC1 were transfected into HEK cells, and the serum-starved cells were treated with 25 ng/ml IGF1 for 10 min, and the IGF1-induced signaling was assayed by measuring levels of ERK1/2 and Akt1 phosphorylation. In control cells, IGF1 induces a significant phosphorylation of ERK1/2 and Akt1 (Figure 7B). By contrast, the expression of the dnGIPC1 inhibited the IGF1 induced activation of both ERK1/2 and Akt1. The inhibition of Akt1 phosphorylation is consistent with results from the Xenopus study, where a similar result was obtained with Kermit2 knockdown; however, in Xenopus, MAPK phosphorylation was not inhibited [17]. Nevertheless, there does seem to be some difference in the way in which GIPC1 interacts with these two downstream signaling pathways: transfection of full-length wild type GIPC1 was sufficient to activate the PI3K/Akt1 signaling pathway even in the absence of IGF1, but it did not affect ERK1/2 phosphorylation (Figure 7B). Nevertheless, even in the presence of dnGIPC1, IGFR is still phosphorylated (Figure 7C). Together, these results show that GIPC1 is an important component in IGF1-induced phosphorylation of ERK1/2 and Akt1 downstream of IGF1R.
Since we observed that GIPC1 was required for mESC differentiation into eye field fates and that dnGIPC1 overexpression resulted in a reduction of Akt1 and ERK1/2 phosphorylation, we assayed whether the MAPK and/or the PI3K/Akt1 pathways elicited any roles in the specification of eye field progenitors. To test this possibility, we differentiated mESCs in the 2D protocol. Wild type mESCs were maintained in differentiation media containing DMSO vehicle, a MAPK inhibitor (PD98059), or a PI3K inhibitor (LY294002). Surprisingly, a pharmacological blockade of the MAPK pathway did not have any effects on eye field differentiation at the tested dose (25 μM), and the expression of EFTFs was comparable to the controls (Figure 7D); however, the inhibition of the PI3K partially inhibited retinal specification: Pax6, Rax and Lhx2 were significantly reduced. Similar to the dnGIPC1 phenotype, the expression level of Otx2 was not affected. The expression levels of Emx1 were slightly increased by RT-qPCR, suggesting a modest increase in telencephalic fates, while the midbrain/hindbrain marker En1 was not affected in any of the conditions. Immunostaining experiments after control, PD98059 or LY294002 treatments (Supplementary Figure 5) showed consistent effects: PI3K inhibition caused a downregulation in the number of Pax6+ cells and an increase in the telencephalic marker Tbr2, compared to controls and PD98059 treatments.
GIPC1 is known to interact with other signaling pathways in addition to IGFR, such as the TGFβ receptor [39]. To test whether the effects of manipulations in GIPC1 might be mediated in part by this pathway, we differentiated the mESCs using the 3D culture system in the presence or absence of a TGFβ inhibitor, SB431542. In experiments using the Akt inhibitor, LY294002 treatment (Figure 7) resulted in the downregulation of EFTFs except for Otx2 and Pax6. By contrast, inhibition of TGFβ signaling during early stages of retinal differentiation (until day 5) resulted in a slight increase in Pax6, Lhx2, and Six3 (Figure 7E) and there was no change in Rax expression. These results support the hypothesis that the effects of GIPC1 are independent of the TGFβ signaling pathway.
Discussion
To date, much of what is known about the early steps in eye field formation have been elucidated in frog and zebrafish [40–43]. This is largely due to the accessibility of their embryos to experimental manipulations. It has been more difficult to test hypotheses concerning eye field development in mice, due to the lack of a good experimental system. Thus, we have used the directed differentiation of mESCs to eye field and retinal fates to test whether critical factors in eye field formation in frogs play a similar role in mice. We have found that the normal function of the mouse homolog to Kermit2, GIPC1, is essential for the expression of the EFTFs: overexpression of a dominant-negative form of GIPC1 (dnGIPC1), as well as the downregulation of endogenous GIPC1, is sufficient to inhibit retinal differentiation in two protocols of directed differentiation of mESCs. Moreover, these same manipulations resulted in an increase in markers of telencephalic fates in the cells, consistent with the possibility that cells fated to become eye field take on a telencephalic identity without GIPC1. Functional analyses of GIPC1 in retinal progenitors indicate that it interacts with IGFR and participates in Akt1 activation. In addition, a pharmacological blockade of Akt1 phosphorylation mimics the dnGIPC1 phenotype suggesting that the GIPC1-PI3K-Akt1 pathway plays a key role in the regional specification of the anterior CNS.
There are now several different protocols for generating eye field and retina from either mouse or human ESCs [9, 11, 12, 32, 44]. We have found eye field specification is dependent on GIPC1 in two different protocols, suggesting that this molecule regulates a key step in this developmental process; at least some of the mechanisms in the progression from ESCs to retinal fates are similar regardless of the specifics of the in vitro production method. In one protocol, the addition of anteriorizing factors, such as Dkk1, IGF1 (to antagonize the Wnt pathway) and Noggin (to inhibit BMP signaling) are used to increase the expression of EFTFs in ESC derived EBs in a synchronized manner. Alternatively, EBs maintained in Matrigel and KSR produce optic vesicles without additional factors, leading to laminated neural retinal structures. Our results show that both systems provide models for testing hypotheses about early stages of eye field formation.
The frog homolog to GIPC1, Kermit2, was shown to be important in eye formation. Experimental reductions in Kermit2 expression in Xenopus leads to absent or very small eyes in 83% of the embryos; Pax6 and Otx2 were reduced in the presumptive eye field, but not in other regions of the neural plate, and Xrx and Xath5 were strongly reduced [17]. We have utilized ESC-derived retinal cells to test the role of the PDZ domain-containing protein GIPC1, and have found that this molecule has a role in mammalian eye development similar to that reported in frog. Another parallel between the findings in Xenopus and the present study is that the loss of Kermit2/GIPC1 appears to cause a change in fate from eye field to telencephalon. We found several markers of telencephalic identity were increased in our experiments. However, it is possible that inhibition of GIPC1 causes an increase in cell death of optic fates, rather than a fate shift. We cannot rule this possibility out, though the differentiated cultures of dnGIPC1 mESCs had similar numbers of cells as those derived from wild type mESCs, so cell death was either low or compensated by growth of cells with alternate fates.
Several lines of evidence support the hypothesis that the role of Kermit2 in eye field formation is to modulate the activity of the IGF receptor: (1) Kermit2 binds to the IGFR and (2) eye field expansion induced by IGF1 can be blocked by Kermit2 knockdown. Additional biochemical studies further demonstrated that Kermit2 is required for the maintenance of Akt signaling downstream of IGFR, but is not required for MAPK activity. It is possible that GIPC1 has a similar function in mouse eye field development, since we find that GIPC1 interacts with the IGFR in the early optic structures in mouse embryos. The mechanism by which GIPC1 facilitates Akt signaling in mESCs directed to eye fates is likely to function through its activity in signaling endosomes. One of the most well established functions for GIPC1 is its role in receptor trafficking of endocytic vesicles. In this regard, GIPC1 is important in the endosomal signaling from receptor tyrosine kinases to PI3K-AKT signaling [18, 45] as well as recycling receptors back to the cell surface [46]. Although our data support a role for GIPC1 specifically in IGF1R signaling, since GIPC proteins interact with a large number of signaling receptors, including receptor tyrosine kinases like IGF1R [36, 37], neurotransmitter receptors [47], integrins [21, 48], and TGFβ receptors [39], it is possible that other signaling molecules are also active in the eye field. However, at least for the TGFβ receptors, our results show that inhibition of this pathway does not mimic the effects of reductions in GIPC1.
The ANP, including the eye fields, is patterned with opposing gradients of Wnt/beta catenin ligands and SFRP inhibitors [49–55] [7]. In addition to the SFRPs, the non-canonical Wnt pathway provides another mechanism for regulating the Wnt activity in the ANP. Wnt11 activates the non-canonical receptor Fzd5 to promote EFTF expression in zebrafish [7], whereas Wnt4 appears to activate the non-canonical Wnt receptor, Fzd3 to promote EFTF expression in Xenopus [57]. Several studies have also shown that IGF1 signaling promotes anterior neural fates, and specifically eye field formation [35]. Although the mechanism by which IGF1 promotes EFTF expression is not well understood, Richard-Parpaillon et al. (2002) demonstrated that IGF1 can directly antagonize Wnt/beta-catenin mediated transcriptional activation in Xenopus [33]. This appears to require the Akt pathway, since Akt activation is downstream of the effects IGF1 has on eye field induction. Our results now extend this model to mammalian eye field specification and suggest that the effects of IGF1, Akt and GIPC1 on EFTF expression are conserved across vertebrates.
Experimental procedures
Animals
All experiments were done in accordance with approved protocols and the animals were housed and bred in the Department of Comparative Medicine at the University of Washington.
mESC culture
Mouse ESCs (R1) were maintained in LIF+ 2 inhibitors (LIF+2i) media: Dulbecco’s Modified Eagle’s Medium (DMEM) from Invitrogen supplemented with 20% Fetal Bovine Serum (ES qualified, Invitrogen), non-essential amino acids (Invitrogen), sodium pyruvate (Invitrogen) and 0.1 mM of β-mercaptoethanol (Sigma), 100 μl of Leukemia Inhibitory Factor (LIF, ESGRO Millipore ESG1106, 10 million units/ml), 3 μM of GSK3β inhibitor Stemolecule CHIR99021 (Stemgent) and 0.4 μM of MEK inhibitor Stemolecule PD0325901 (Stemgent). All of the cell lines were maintained in feeder-free conditions in growth factor-reduced Matrigel-coated plates.
Retinal differentiation of mESCs
Semi-confluent undifferentiated colonies were dissociated to a suspension of single cells by enzymatic treatment with TrypLE (Gibco) and gentle mechanical dissociation. Next, cells were rinsed with DMEM +10% FBS and plated in retinal differentiation media (DMEM: F12 media, supplemented with non-essential amino acids, sodium pyruvate, BSA (Invitrogen), B27, N2, and 1 ng/ml each of Dkk1, Noggin and IGF1) in low-attachment plates. Floating mESCs spontaneously formed aggregates (EBs) within few hours under these conditions. After three days, EBs were seeded onto Matrigel-coated plates in retinal differentiation media. From day 5–10, media was changed every other day. For the 3D culture method, we adapted that of Eiraku and Sasai (2012), using DMEM:F12 as above (plus retinoic acid and taurine) as the maintenance media.
qPCR
Total RNA was extracted from the cultures using Trizol (Invitrogen) followed by chloroform extraction as manufacturer’s instructions. This was followed by DNase-1 (Qiagen) treatment followed by RNA cleanup using Qiagen RNA mini cleanup kit. cDNA was reverse transcribed using Superscript III RT kit (Invitrogen) as per the manufacturer’s instructions. PCR was performed using the primers listed in the Supplementary Table and values were normalized to β-actin.
Immunohistochemistry and microscopy
Cultures were fixed in 4% paraformaldehyde (PFA), and after PBS rinse, non-specific binding was inhibited by incubation in milk powder block solution (5% dried milk powder and 0.5% Triton X-100 in PBS) for 1–2 hours (at room temperature). Cells were incubated in primary antibodies overnight at 4°C, washed in PBS and incubated with appropriate fluorescent conjugated secondary antibodies (Invitrogen) or streptavidin conjugate (Invitrogen) for 1 hour along with DAPI to counterstain nuclei. The coverslips or sections were washed and mounted for microscopy. Primary antibodies were as follows: rabbit anti-Pax6 (1:500, PRB-278P, Covance, Princeton, NJ, USA), goat anti-Otx2-biotin (1:500, BAF1979, R&D Systems, Minneapolis, MN, USA), goat anti-Sox2 (1:100, sc17320, Santa Cruz), goat anti-Lhx2 (1:250, sc-19344, Santa Cruz), Tuj1 (1:3,000, Covance), Tbr2 (1:500, ab23345, Abcam), goat anti-GIPC1 (1:100, Santa Cruz), goat anti-Brn3 (1:500, sc-6026, Santa Cruz), rabbit anti-Recoverin (1:1000, Chemicon), Lin28 (1:100, ab46020, Abcam), chicken anti-GFP (1:250, Abcam), mouse anti-Rhodopsin (1:100), goat anti-S-Opsin (1:150, Santa Cruz), goat anti-Oct3/4 (1:200, sc-8628, Santa Cruz), rabbit Nanog (1:250, ab80892, Abcam).
Whole mount in situ hybridization
E8-E9 embryos were dissected in PBS + 2mM EGTA, fixed in 4% PFA, and dehydrated in ethanol. Tissues were rehydrated, treated with Proteinase K, post-fixed with PFA 4% + 0.1% Glutaraldehyde, and incubated 1hr at 68°C in hybridization mix, followed by 0.2 mg/ml of digoxigenin (DIG)-labeled RNA probes for 18 hours. DIG-labeled probes were prepared according to the manufacturer’s protocol (Roche). After washing, tissues were incubated overnight at 4°C with anti-DIG-AP antibody (1:2000) (Roche), and developed in NBT/BCIP solution.
HEK 293T transfection
HEK 293T cells were maintained in DMEM media supplemented with 10% FBS. For transfection, the cells were cultured overnight to 40–60% confluence. Transfection was performed using Lipofectamine 2000, following the manufacturer’s instructions (Invitrogen).
Western blot and immunoprecipitation assays
HEK 293T, mESC-derived cultures or embryonic retinas were collected in lysis buffer, containing a protease inhibitor cocktail (Roche) and phosphatase inhibitors (10 mM tetra-sodium pyrophosphate, 200 μM sodium orthovanadate and 10 mM sodium fluoride). Cells were centrifuged at 13,000 rpm for 20 min and the supernatant was analyzed either by Western blot or immunoprecipitation. For immunoprecipitation experiments, antibodies were added to 500 μg of total protein of cell lysates or tissue extracts and incubated for 10 h at 4°C. As a control, a GFP antibody was used under the same conditions. Sepharose bead coupled protein G (Sigma) was then added and the suspension was incubated for 2 h at 4°C. Sepharose beads were washed with lysis buffer and the samples were analyzed by a standard Western blot protocol. Membranes were incubated first in 5% BSA in TBS and then with the indicated antibodies. Detection was performed using the ECL system from Amersham. For quantification, films were scanned and then subjected to band densitometry and quantification by ImageJ.
Lentiviral particles production, mESC viral transduction and stable cell line generation
Lentivirus was made using the four-plasmid system. HEK-293T cells were transfected with Lipofectamine 2000 (Invitrogen) and the supernatant was collected 24 and 48 hrs later. R1 mESC were transduced by overnight incubation with virus in 6-well plates on Matrigel at a confluency of 10%. 48 hours after viral transduction, the mESC were selected for with 1μg/ml Puromycin. After 7–10 days, individual colonies (<50 cells) were picked, expanded and analyzed. shRNA lentiviral constructs were obtained from Open BioSystems and tested on HEK cells co-transfected with an expression plasmid for mouse GIPC1. Of the five shRNAs tested, three (sh1, sh3, and sh5) showed substantial reduction in GIPC1 expression when analyzed by Western blotting. Catalog number sh1: RMM3981-9604607 targeted to Exon 3; sh3:RMM3981-9604609, targeted to Exon 2; sh5:RMM3981-9604611, targeted to Exon 7.
Generation of Rax-GFP mESC line
E0.5 Rax-GFP embryos were harvested from naturally mated females. Upon collection, the embryos were plated on gamma-irradiated mouse embryonic fibroblast feeders in mouse ESC culture medium. Once hatched and shortly after plating, the inner cell mass was selectively subcultured by plucking and breaking into 2 or 3 pieces onto a new feeder layer. Once colonies arose with mESC morphology, the new line was passaged using typsin-EdTA. The mouse strain used for this research project, STOCK Tg(Rax-EGFP)25Gsat/Mmcd, identification number 030564-UCD, was obtained from the Mutant Mouse Regional Resource Center, a NCRR-NIH funded strain repository, and was donated to the MMRRC by the NINDS funded GENSAT BAC transgenic project.
Supplementary Material
Supplementary 1: Clustal W (v.1.83) alignment of the Xenopus laevis orthologues in the mouse. Kermit2 GenBank accession number: NP_001082286, GIPC1 GenBank accession number: NP_061241, GIPC2 GenBank accession number: NP_058563, GIPC3 GenBank accession number: NP_683753. Identical residues have been marked with an asterisk. The PDZ domain has been colored in yellow and the PDZ carboxylate-binding pocket has been highlighted in orange.
Supplementary 2: dnGIPC1 and shRNA mESC lines.
Similar to control parental R1 mESC lines (A), dnGIPC1 (B) and shRNA cell lines (C) maintained undifferentiated morphologies under standard culture conditions. All the different cell lines expressed high levels of the pluripotency markers Nanog (red, D, F and H), Oct4 (green, E, G and I), Sox2 (red, J, L and N) and Lin28 (green, K, M and O). Scale bars: 100 microns.
Supplementary 3: shRNA inhibition of GIPC1 expression.
(A) HEK 293T cells were transfected with either an empty vector (PRK5, first lane), a full length GIPC1 vector (GIPC, second lane) or GIPC1 together with different shRNA constructs against GIPC (sh1-sh5, lanes 3–7). Two days after transfection, cells were Western blotted against GIPC1 (upper panel) and Actin as a loading control (bottom panel). (B) Densitometric analyses of 3 independent experiments (normalized to GIPC1-transfected samples) showing shRNA-dependent inhibition of GIPC1 expression.
Supplementary 4: mESCs differentiate into laminated retina. ESCs differentiated for 25 days using the 3D protocol were immunostained with Recoverin (green), Otx2 (red), and Pax6 (blue). Otx2+ bipolar cells and photoreceptors form a well defined layer separate from the Pax6+ innner retinal layer.
Supplementary 5: Inhibition of the PI3K-Akt1 pathway reduces mESCs retinal differentiation. Wild-type mESCs were differentiatiated for 5 days using the 2D method in the presence or absence of a MAPK inhibitor (PD98059) and Akt inhibitor (LY294002) and immunolabeled with Pax6 (red) or Tbr2 (red) antibodies. Scale bar: 200 microns.
SIGNIFICANCE STATEMENT.
In this study, we use the directed differentiation of embryonic stem cells (ESCs) as a model to study key regulatory steps in eye field formation. The regulatory mechanisms that define the eye field are not well understood, and most of what we know about this has been discovered in organisms with accessible embryos like zebrafish and Xenopus. Signaling pathways are difficult to manipulate in mammals at early embryonic stages. ESCs have emerged as an alternative method to study the earliest steps of mammalian ontogeny. The differentiation paradigms towards eye field progenitors and mature retinal cells from ESCs are now well established. Therefore, ESCs provide a potential model for testing hypotheses concerning forebrain patterning and eye field specification in vitro. Here, we directed mESCs towards eye field and retinal fates, to test whether a specific component of the signaling network in frog embryos, Kermit2, is important in mammalian eye development. Our results indicate that GIPC plays a key role in the segregation of the eye field from telencephalic cell fates, and likely acts through the regulation of PI3K-Akt1 pathway downstream of IGFR. Our results show the importance of IGF signaling to patterning of the CNS, and demonstrate the usefulness of ESCs in understanding basic developmental processes in organ formation.
Acknowledgments
The authors acknowledge the help and advice of the members of the Reh lab and Bermingham-McDonogh lab members, and NIH 1 PO1 GM081619 and the imaging core of the Vision Core Grant to the University of Washington, P30EY01730.
Footnotes
The authors declare no potential conflicts of interest.
Author Contributions: Anna La Torre: Conception and Design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, revisions of manuscript, final approval of manuscript. Akina Hoshino: Collection and/or assembly of data, data analysis and interpretation, manuscript writing, revisions of manuscript, final approval of manuscript. Chris Cavanaugh: key reagent; Carol B. Ware: key reagent. Thomas A. Reh: Conception and Design, financial support, analysis and interpretation, manuscript writing, revisions of manuscript, final approval of manuscript.
Citations
- 1.Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brulet P. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121:3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- 2.Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167. doi: 10.1242/dev.00723. [DOI] [PubMed] [Google Scholar]
- 3.Chuang JC, Raymond PA. Embryonic origin of the eyes in teleost fish. Bioessays. 2002;24:519–529. doi: 10.1002/bies.10097. [DOI] [PubMed] [Google Scholar]
- 4.Stigloher C, Ninkovic J, Laplante M, Geling A, Tannhauser B, Topp S, Kikuta H, Becker TS, Houart C, Bally-Cuif L. Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development. 2006;133:2925–2935. doi: 10.1242/dev.02450. [DOI] [PubMed] [Google Scholar]
- 5.Niehrs C. Developmental biology. Solving a sticky problem. Nature. 2001;413:787–788. doi: 10.1038/35101682. [DOI] [PubMed] [Google Scholar]
- 6.Bielen H, Houart C. BMP signaling protects telencephalic fate by repressing eye identity and its Cxcr4-dependent morphogenesis. Dev Cell. 2012;23:812–822. doi: 10.1016/j.devcel.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- 9.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.La Torre A, Lamba DA, Jayabalu A, Reh TA. Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol Biol. 2012;884:229–246. doi: 10.1007/978-1-61779-848-1_16. [DOI] [PubMed] [Google Scholar]
- 12.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amirpour N, Karamali F, Rabiee F, Rezaei L, Esfandiari E, Razavi S, Dehghani A, Razmju H, Nasr-Esfahani MH, Baharvand H. Differentiation of human embryonic stem cell-derived retinal progenitors into retinal cells by Sonic hedgehog and/or retinal pigmented epithelium and transplantation into the subretinal space of sodium iodate-injected rabbits. Stem Cells Dev. 2012;21:42–53. doi: 10.1089/scd.2011.0073. [DOI] [PubMed] [Google Scholar]
- 15.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30:673–686. doi: 10.1002/stem.1037. [DOI] [PubMed] [Google Scholar]
- 16.Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu J, O’Donnell M, Gitler AD, Klein PS. Kermit 2/XGIPC, an IGF1 receptor interacting protein, is required for IGF signaling in Xenopus eye development. Development. 2006;133:3651–3660. doi: 10.1242/dev.02547. [DOI] [PubMed] [Google Scholar]
- 18.Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, Testa JR, Yates JR, 3rd, Farquhar MG. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeanneteau F, Guillin O, Diaz J, Griffon N, Sokoloff P. GIPC recruits GAIP (RGS19) to attenuate dopamine D2 receptor signaling. Mol Biol Cell. 2004;15:4926–4937. doi: 10.1091/mbc.E04-04-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spicer E, Suckert C, Al-Attar H, Marsden M. Integrin alpha5beta1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation. PLoS One. 2010;5:e10665. doi: 10.1371/journal.pone.0010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, et al. Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katoh M. Functional proteomics, human genetics and cancer biology of GIPC family members. Exp Mol Med. 2013;45:e26. doi: 10.1038/emm.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis CA, Joyner AL. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988;2:1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- 24.Hodge RD, Nelson BR, Kahoud RJ, Yang R, Mussar KE, Reiner SL, Hevner RF. Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci. 2012;32:6275–6287. doi: 10.1523/JNEUROSCI.0532-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnar Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao CA, Kiyama T, Pan P, Furuta Y, Hadjantonakis AK, Klein WH. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development. 2008;135:271–280. doi: 10.1242/dev.009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweeney NT, Tierney H, Feldheim DA. Tbr2 is required to generate a neural circuit mediating the pupillary light reflex. J Neurosci. 2014;34:5447–5453. doi: 10.1523/JNEUROSCI.0035-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eiraku M, Sasai Y. Mouse embryonic stem cell culture for generation of three-dimensional retinal and cortical tissues. Nat Protoc. 2012;7:69–79. doi: 10.1038/nprot.2011.429. [DOI] [PubMed] [Google Scholar]
- 30.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koehler KR, Hashino E. 3D mouse embryonic stem cell culture for generating inner ear organoids. Nat Protoc. 2014;9:1229–1244. doi: 10.1038/nprot.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard-Parpaillon L, Heligon C, Chesnel F, Boujard D, Philpott A. The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev Biol. 2002;244:407–417. doi: 10.1006/dbio.2002.0605. [DOI] [PubMed] [Google Scholar]
- 34.Eivers E, McCarthy K, Glynn C, Nolan CM, Byrnes L. Insulin-like growth factor (IGF) signalling is required for early dorso-anterior development of the zebrafish embryo. Int J Dev Biol. 2004;48:1131–1140. doi: 10.1387/ijdb.041913ee. [DOI] [PubMed] [Google Scholar]
- 35.Pera EM, Wessely O, Li SY, De Robertis EM. Neural and head induction by insulin-like growth factor signals. Dev Cell. 2001;1:655–665. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 36.Ligensa T, Krauss S, Demuth D, Schumacher R, Camonis J, Jaques G, Weidner KM. A PDZ domain protein interacts with the C-terminal tail of the insulin-like growth factor-1 receptor but not with the insulin receptor. J Biol Chem. 2001;276:33419–33427. doi: 10.1074/jbc.M104509200. [DOI] [PubMed] [Google Scholar]
- 37.Booth RA, Cummings C, Tiberi M, Liu XJ. GIPC participates in G protein signaling downstream of insulin-like growth factor 1 receptor. J Biol Chem. 2002;277:6719–6725. doi: 10.1074/jbc.M108033200. [DOI] [PubMed] [Google Scholar]
- 38.Paek AR, You HJ. GAIP-interacting protein, C-terminus is involved in the induction of zinc-finger protein 143 in response to insulin-like growth factor-1 in colon cancer cells. Mol Cells. 2011;32:415–419. doi: 10.1007/s10059-011-0078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blobe GC, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor beta (TGF-beta) signaling. Functional modulation of type III TGF-beta receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- 40.Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol. 2010;93:61–84. doi: 10.1016/B978-0-12-385044-7.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 42.Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuber ME. Eye field specification in Xenopus laevis. Curr Top Dev Biol. 2010;93:29–60. doi: 10.1016/B978-0-12-385044-7.00002-3. [DOI] [PubMed] [Google Scholar]
- 44.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Varsano T, Taupin V, Guo L, Baterina OY, Jr, Farquhar MG. The PDZ protein GIPC regulates trafficking of the LPA1 receptor from APPL signaling endosomes and attenuates the cell’s response to LPA. PLoS One. 2012;7:e49227. doi: 10.1371/journal.pone.0049227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muders MH, Vohra PK, Dutta SK, Wang E, Ikeda Y, Wang L, Udugamasooriya DG, Memic A, Rupasinghe CN, Baretton GB, et al. Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth. Clin Cancer Res. 2009;15:4095–4103. doi: 10.1158/1078-0432.CCR-08-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi Z, Petralia RS, Fu Z, Swanwick CC, Wang YX, Prybylowski K, Sans N, Vicini S, Wenthold RJ. The role of the PDZ protein GIPC in regulating NMDA receptor trafficking. J Neurosci. 2007;27:11663–11675. doi: 10.1523/JNEUROSCI.3252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tani TT, Mercurio AM. PDZ interaction sites in integrin alpha subunits. T14853, TIP/GIPC binds to a type I recognition sequence in alpha 6A/alpha 5 and a novel sequence in alpha 6B. J Biol Chem. 2001;276:36535–36542. doi: 10.1074/jbc.M105785200. [DOI] [PubMed] [Google Scholar]
- 49.Kim SH, Shin J, Park HC, Yeo SY, Hong SK, Han S, Rhee M, Kim CH, Chitnis AB, Huh TL. Specification of an anterior neuroectoderm patterning by Frizzled8a-mediated Wnt8b signalling during late gastrulation in zebrafish. Development. 2002;129:4443–4455. doi: 10.1242/dev.129.19.4443. [DOI] [PubMed] [Google Scholar]
- 50.Kim HS, Shin J, Kim SH, Chun HS, Kim JD, Kim YS, Kim MJ, Rhee M, Yeo SY, Huh TL. Eye field requires the function of Sfrp1 as a Wnt antagonist. Neurosci Lett. 2007;414:26–29. doi: 10.1016/j.neulet.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 51.Esteve P, Lopez-Rios J, Bovolenta P. SFRP1 is required for the proper establishment of the eye field in the medaka fish. Mech Dev. 2004;121:687–701. doi: 10.1016/j.mod.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Houart C, Westerfield M, Wilson SW. A small population of anterior cells patterns the forebrain during zebrafish gastrulation. Nature. 1998;391:788–792. doi: 10.1038/35853. [DOI] [PubMed] [Google Scholar]
- 53.Buckles GR, Thorpe CJ, Ramel MC, Lekven AC. Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech Dev. 2004;121:437–447. doi: 10.1016/j.mod.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 54.Chapman SC, Brown R, Lees L, Schoenwolf GC, Lumsden A. Expression analysis of chick Wnt and frizzled genes and selected inhibitors in early chick patterning. Dev Dyn. 2004;229:668–676. doi: 10.1002/dvdy.10491. [DOI] [PubMed] [Google Scholar]
- 55.Staudt N, Houart C. The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol. 2007;5:e69. doi: 10.1371/journal.pbio.0050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- 57.Maurus D, Heligon C, Burger-Schwarzler A, Brandli AW, Kuhl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. Embo J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1: Clustal W (v.1.83) alignment of the Xenopus laevis orthologues in the mouse. Kermit2 GenBank accession number: NP_001082286, GIPC1 GenBank accession number: NP_061241, GIPC2 GenBank accession number: NP_058563, GIPC3 GenBank accession number: NP_683753. Identical residues have been marked with an asterisk. The PDZ domain has been colored in yellow and the PDZ carboxylate-binding pocket has been highlighted in orange.
Supplementary 2: dnGIPC1 and shRNA mESC lines.
Similar to control parental R1 mESC lines (A), dnGIPC1 (B) and shRNA cell lines (C) maintained undifferentiated morphologies under standard culture conditions. All the different cell lines expressed high levels of the pluripotency markers Nanog (red, D, F and H), Oct4 (green, E, G and I), Sox2 (red, J, L and N) and Lin28 (green, K, M and O). Scale bars: 100 microns.
Supplementary 3: shRNA inhibition of GIPC1 expression.
(A) HEK 293T cells were transfected with either an empty vector (PRK5, first lane), a full length GIPC1 vector (GIPC, second lane) or GIPC1 together with different shRNA constructs against GIPC (sh1-sh5, lanes 3–7). Two days after transfection, cells were Western blotted against GIPC1 (upper panel) and Actin as a loading control (bottom panel). (B) Densitometric analyses of 3 independent experiments (normalized to GIPC1-transfected samples) showing shRNA-dependent inhibition of GIPC1 expression.
Supplementary 4: mESCs differentiate into laminated retina. ESCs differentiated for 25 days using the 3D protocol were immunostained with Recoverin (green), Otx2 (red), and Pax6 (blue). Otx2+ bipolar cells and photoreceptors form a well defined layer separate from the Pax6+ innner retinal layer.
Supplementary 5: Inhibition of the PI3K-Akt1 pathway reduces mESCs retinal differentiation. Wild-type mESCs were differentiatiated for 5 days using the 2D method in the presence or absence of a MAPK inhibitor (PD98059) and Akt inhibitor (LY294002) and immunolabeled with Pax6 (red) or Tbr2 (red) antibodies. Scale bar: 200 microns.