Abstract
Background and aims
Keratins 8 and 18 (K8/K18) are the intermediate filaments proteins of simple-type digestive epithelia, and provide important cytoprotective function. K8/K18 variants predispose humans to chronic liver disease progression and to poor outcomes in acute acetaminophen (APAP)-related liver failure. Given that K8 G62C and R341H/R341C are common K8 variants in European and North American populations, we studied their biological significance using transgenic mice.
Methods
Mice that overexpress the human K8 variants R341H or R341C were generated and used together with previously described mice that overexpress wild-type (WT) K8 or K8 G62C. Mice were injected with 600 mg/kg APAP, or underwent bile duct ligation (BDL). Livers were evaluated by microarray analysis, quantitative RT-PCR, immunoblotting, histological and immunological staining, and biochemical assays.
Results
Under basal conditions, the K8 G62C/R341H/R341C variant-expressing mice did not show an obvious liver phenotype or altered keratin filament distribution, while K8 G62C/R341C animals had aberrant disulphide-crosslinked keratins. Animals carrying the K8 variants displayed limited gene expression changes but had lower nicotinamide N-methyl transferase (NNMT) levels and were predisposed to APAP-induced hepatotoxicity. NNMT represents a novel K8/K18-associated protein that becomes upregulated after K8/K18 transfection. The more pronounced liver damage was accompanied by increased and prolonged JNK activation; elevated APAP protein adducts; K8 hyperphosphorylation at S74/S432 with enhanced K8 solubility; and prominent pericentral keratin network disruption. No differences in APAP serum levels, glutathione or ATP levels were noted. BDL resulted in similar liver injury and biliary fibrosis in all mouse genotypes.
Conclusion
Expression of human K8 variants G62C, R341H, or R341C in mice predisposes to acute acetaminophen hepatotoxicity, thereby providing direct evidence for the importance of these variants in human acute liver failure.
Keywords: crosslinking, solubility, bile duct ligation, glutathione, MAP kinase
Introduction
Drug-induced liver injury represents a significant health problem since it constitutes the major cause of acute liver failure (ALF) in the developed world and the major adverse event leading to termination of clinical drug development program (1–2). Acetaminophen (APAP) toxicity is the predominant cause of drug-induced ALF and accounts for about 50% of ALF cases in the US and the UK (1). APAP is metabolized primarily through glucuronidation and sulfation while 5–15% is oxidized through the cytochrome P450 (CYP450) system thereby leading to production of N-acetyl-p-benzoquinone imine (NAPQI).
At therapeutic APAP doses, NAPQI is detoxified via conjugation with sulfhydryl groups that are donated by glutathione. However, after higher exposure, glutathione becomes depleted and NAPQI binds covalently to hepatocellular proteins and this proteotoxic stress leads to activation of stress kinases such as c-Jun-N-terminal kinase (JNK) and development of liver injury (3–4). While APAP is generally considered safe at therapeutic doses, nearly 30% of patients display an increase in alanine aminotransferase (ALT) levels after only five days of ingestion thereby suggesting genetic differences in APAP susceptibility (3, 5). While genes responsible for APAP metabolism are the most obvious candidates, keratin polypeptides 8 and 18 (K8/K18) are also candidate modifier genes that influence the outcome of APAP-induced ALF (3, 6). In particular, a large study suggested that the presence of biologically significant K8 variants predisposes their carriers to an adverse outcome of APAP-induced ALF (6)
Keratins represent the largest subgroup of the intermediate filament protein family, and are expressed in all epithelia cells and skin appendages (7–8). Keratins are subdivided into type I (K9–K40) and type II (K1–K8, K71–K86), and both types assemble in an equimolar ratio to form obligate noncovalent heteropolymers (7–8). Keratins function as cytoprotective proteins and their mutations are associated with at least 60 different human diseases (9). Their heteropolymeric nature and epithelial cell-specific expression are reflected by the presence of cell type-selective displays of keratin repertoires and pairings (7, 10). For example, K8/K18 are the major keratins of single-layered or glandular epithelia while the pairs K1/K10 and K5/K14 are expressed in suprabasal and basal epidermal cells, respectively (7, 10).
Adult hepatocytes are unique in that they express only K8/K18 whereas most other epithelial cells display a more complex keratin pattern (10–11), which explains why mutations in K8/K18 result in a predominantly hepatic phenotype. Animals that lack K8/K18 or overexpress the keratin filament disruptor K18 R90C develop mild liver injury under basal conditions but are markedly predisposed to a wide range of experimental liver injury models (10–12). These animal findings led to human association studies that identified K8/K18 variants as risk factors for liver fibrosis and end-stage liver disease progression in several chronic liver disorders such as hepatitis C infection and primary biliary cirrhosis (6, 10). Moreover, K8 variants predisposed their carriers to an adverse acute liver injury outcome as observed in patients with ALF (6). K8 G62C and K8 R341H are the most common heterozygous K8/K18 variants in Caucasians, and are seen in 3.2 and 1.5% of this population, respectively, while K8 R341C is rare but offers important insights into disease pathology due to the toxicity of cysteines in simple epithelial keratins (6, 12–14).
Despite the large body of evidence linking K8/K18 variants to human liver disease, little is known regarding their underlying pathomechanisms. In vitro and in vivo animal data suggest that K8/K18 variants compromise stress-induced K8/K18 hyperphosphorylation and impair keratin network reorganization under these conditions (10–11, 15–16). Similarly, livers of mice overexpressing K8 G62C stimulated with Fas ligand manifest markedly decreased K8 S74 phosphorylation and shunting of phosphorylation to other stress-activated kinase substrates with consequent enhanced liver injury as compared with mice that overexpress WT K8 (17).
To determine what hepatic stresses are modulated by the presence of K8/K18 variants, and to study the underlying mechanisms, we generated mice that overexpress human K8 R341H or R341C and (together with the previously described K8 G62C mice) subjected them to acute APAP injury or chronic biliary injury induced by bile duct ligation (BDL). Our data demonstrate that several naturally occurring K8 variants predispose to APAP toxicity via JNK activation, with increased K8 phosphorylation at S74 and S432, enhanced K8/K18 solubility and filament network disruption as well as more prominent formation of APAP-cysteine adducts.
Materials and Methods
Mouse experiments
Transgenic mice that overexpress human (h) K8 R341H/R341C were generated as described for the mice that overexpress human K8 G62C (17). First, a BamHI-SalI fragment from the wild-type (WT) 12-kb genomic sequence (18) was subcloned into pcDNA3.1 vector. The Arg-to-His/Cys mutation (CGT->CAT/TGT) was introduced using a Quickchange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and confirmed by sequencing. The mutated K8 fragment was complemented with a K8 segment excised from the original clone. The constructs were injected into pronuclei of fertilized FVB/n mouse eggs. The offspring was genotyped with primers specific for hK8 as described previously ((17); Supplementary Table 1) and maintained on FVB/n background. To confirm the presence of the described K8 variants, sequencing of K8 exons 1 and 6 was performed (Supplementary Fig. 1, Supplementary Table 1). The strains used in this study are termed as WT K8, K8 G62C, K8 R341H/C mice to highlight the K8 variant they overexpress. Notably, the sequence identity of mouse and human K8 is 88% and the K8 G62 and R341 residues are highly conserved across species. In addition, we analyzed the previously described K18-null mice (19) and their non-transgenic littermates (both are in an FVB/N background).
To study APAP hepatotoxicity, two month-old mice were fasted overnight, injected intraperitoneally with APAP (600mg/kg mouse weight; Fagron GmbH&Co KG, Barsbüttel, Germany) and sacrificed 4 or 18h later by CO2 inhalation followed by blood collection. In another set of experiments, BDL was performed in three months old animals anesthetized with isoflurane. After midline laparotomy, the bile duct was ligated with 2-0 or 5-0 non-absorbable surgical silk (Perma-hand, Ethicon, Somerville, NJ) and animals were followed for 21 days.. Dissected livers were weighed and pieces were fixed using 10% formaldehyde (histological staining), snap frozen in liquid nitrogen (biochemical analysis) or placed in RNAlater stabilization reagent (Ambion, Life Technologies GmbH, Darmstadt, Germany). Serum parameters were measured in the Clinical Chemistry Department (University Hospital Ulm). Animal experiments were approved by the Institutional Animal Care Committees of the participating centers. For details on staining, mRNA isolation, RT-PCR, microarray analysis, biochemical methods, cell transfection experiments and statistical evaluation, see supplementary methods section.
Results
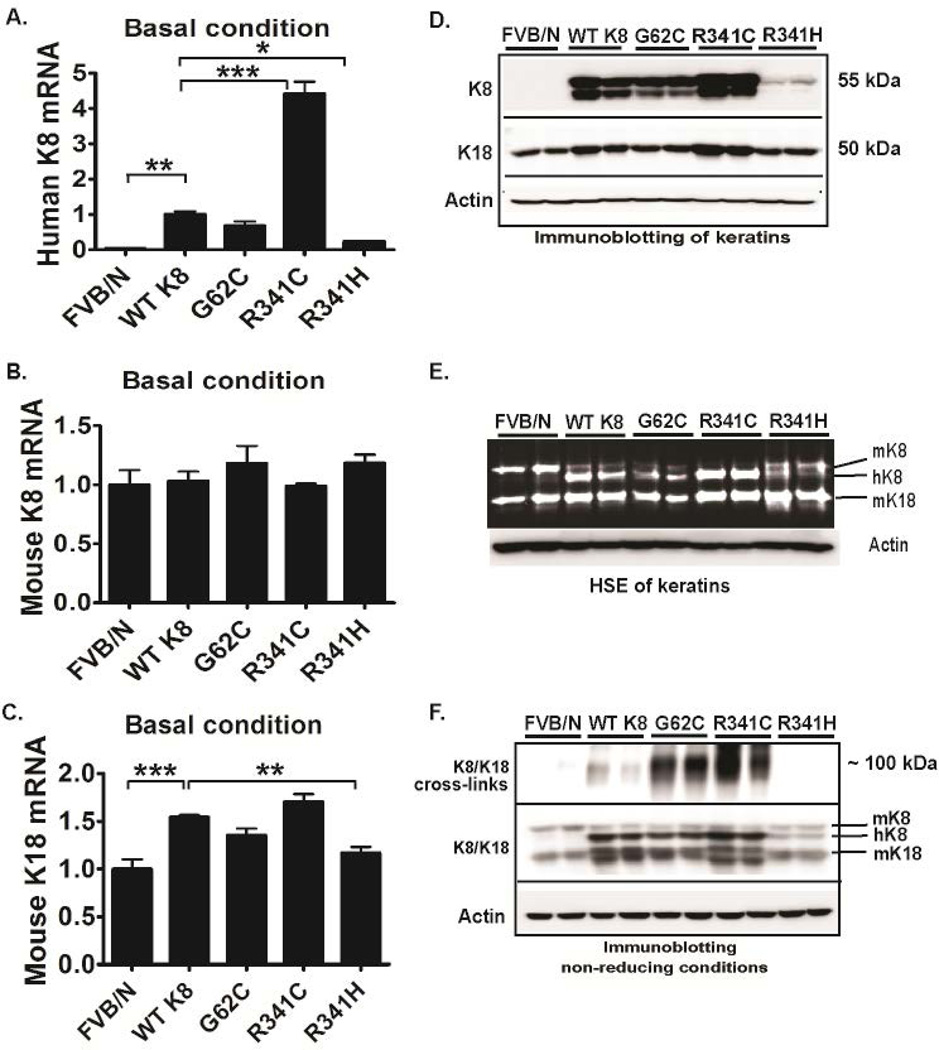
To characterize the transgenic mouse lines generated in this study, we first quantified their keratin expression levels. All transgenic lines displayed moderate hK8 mRNA overexpression with levels being K8 R341C>WT K8~K8 G62C>>K8 R341H while no changes in mouse (m) K8 mRNA were seen (Fig. 1A,B). Consistent with a previous report (20), K8 overexpression up-regulated K18 mRNA levels and consequently, the highest K18 mRNA was seen in WT K8 and K8 R341C mice (Fig. 1C). hK8 and K18 protein levels mirrored the mRNA results, whereas hK8 overexpression led to compensatory down-regulation of mK8 (Fig. 1D,E) which is in line with prior reports of WT K8 overexpression (17, 20).
Figure 1. Keratin 8 G62C/R341C variants promote keratin crosslinking under basal conditions.
Human (h) K8 (A) and mouse (m) K18/K8 (B,C) mRNA and protein levels (D) were quantified by RT-PCR and immunoblotting, respectively. (E) To directly visualize proteins, a high salt extraction with SYPRO ruby staining was performed from nontransgenic livers (FVB/N) as well as tissues overexpressing wild-type K8 (WT K8) or the highlighted K8 variants. (F) To test the formation of disulfide bridges, livers were homogenized under non-reducing conditions. After longer exposure (upper panel), significant amount of cross-linked hK8 was detected in G62C and R341C livers and the crosslinks went away under reducing conditions (not shown). L7 (mouse ribosomal protein) and actin were used as an internal/loading control for qRT-PCR and immunoblotting, respectively. At least four mice were analysed per genotype and the results are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p <0.001
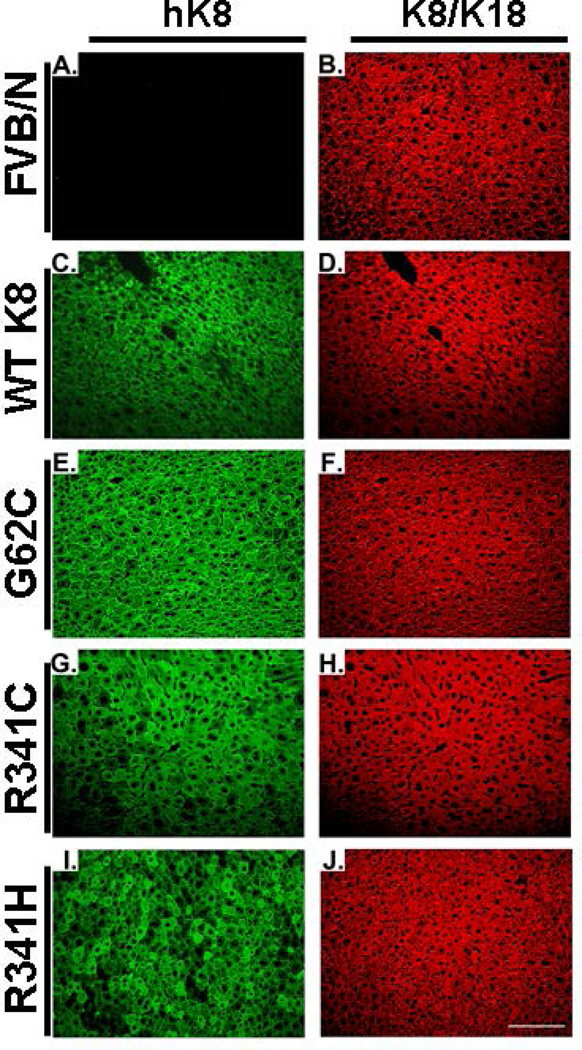
Since introduction of cysteine residues into otherwise cysteine-free hK8 may lead to formation of disulphide bridges (14, 17), we analysed K8/K18 species under non-reducing conditions. High molecular weight K8/K18 species were easily observed in K8 G62C/R341C mice but not in WT K8 and K8 R341H animals (Fig. 1F). All transgenic mouse lines displayed a non-disrupted K8/K18 filament network in the liver under basal conditions, however this network was more dense in the K8 R341C livers that carry the highest K8 expression levels (Fig. 2; Supplementary Fig. 2).
Figure 2. Overexpression of keratin 8 variants does not alter the morphology of keratin network under basal condition.
Livers from transgenic mice overexpressing wild-type human (h) keratin 8 (WT K8), hK8 G62C or K8 R341C/R341H variant, and from nontransgenic animals (FVB/N) were stained with an antibody recognizing both mouse and human K8/K18 (antibody 8592) or an antibody specific for hK8 (M20). Only non-specific, weak hK8 staining was seen in FVB/N animals (A) while K8 R341H livers displayed a somewhat patchy hK8 distribution (I). Scale bar = 100µm.
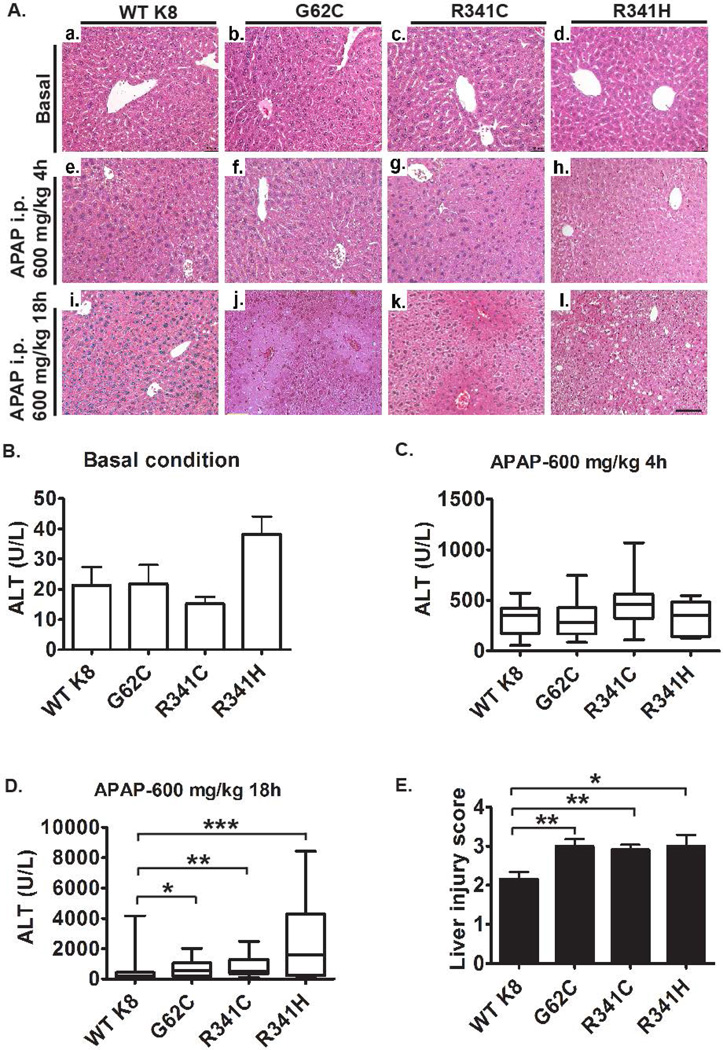
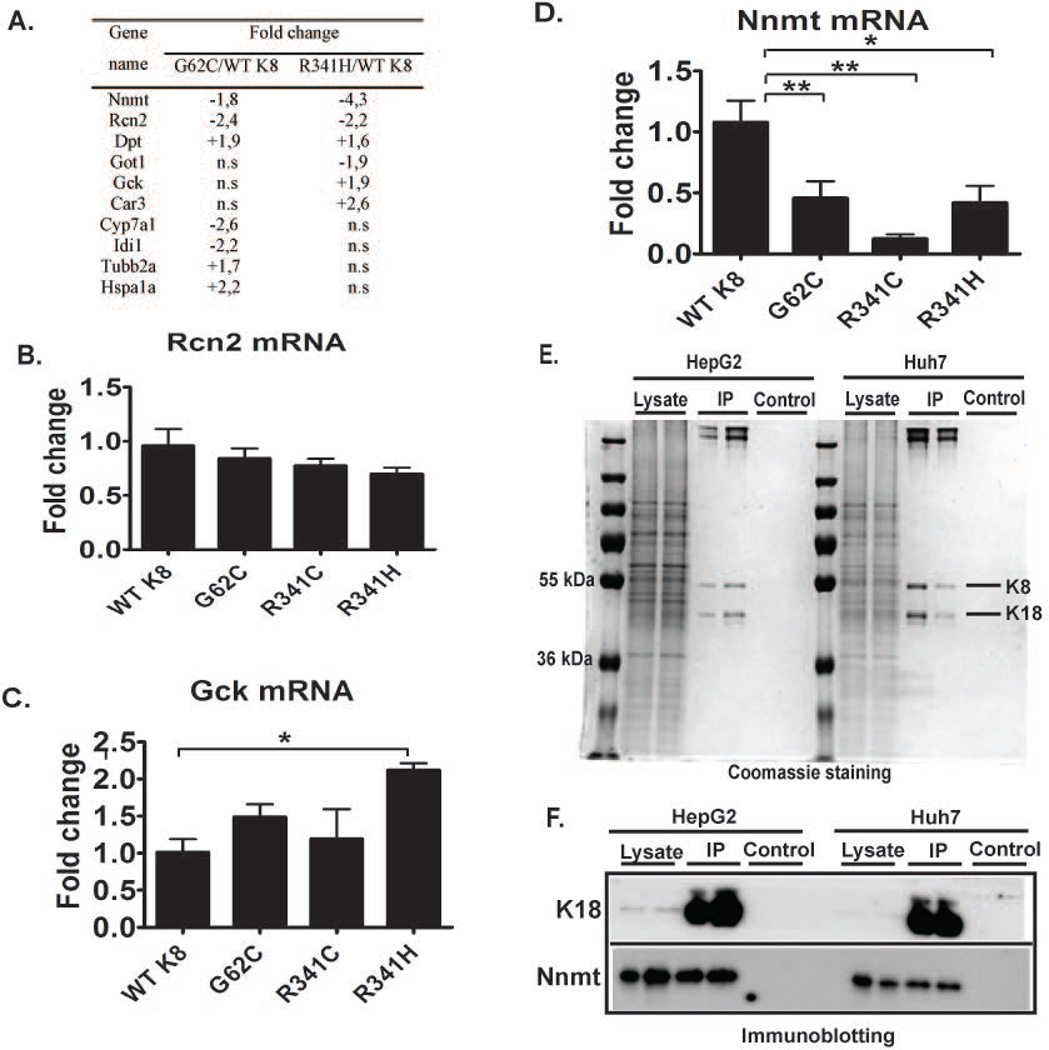
hK8 overexpression did not cause any lethality, growth defect, or organ malfunction (not shown). Under basal conditions, all transgenic lines had normal-appearing liver architecture and normal alanine aminotransferase (ALT) levels (Fig. 3Aa–d,B). To further characterize the consequences of the presence of K8 variants, we performed microarray analysis that revealed modest changes among the genotypes (Fig. 4; Supplementary Fig. 3; Supplementary Tables 2,3). Notably, nicotinamide N-methyltransferase (NNMT) was the only mRNA that was significantly altered in all mouse lines harboring K8 variants (Fig. 4D). Interestingly, K8/K18 transfection into NIH-3T3 cells resulted in NNMT upregulation, whereas K18-null livers and their normal controls displayed similar NNMT expression levels (Supplementary Fig. 4). In addition, immunoprecipitation experiments in two different hepatocellular cell lines (HepG2 and Huh7) showed coimmunoprecipitation of NNMT with K8/K18 (Fig. 4E–F).
Figure 3. K8 G62C and R341C/H variants promote acetaminophen-induced liver injury.
Mice overexpressing wild-type K8 (WTK8) or the K8 G62C/R341H/R341C variant were injected with 600mg/kg acetaminophen (APAP) intraperitoneally (i.p.) and sacrificed after 4 and 18h, respectively. (A) H&E staining detected no obvious liver injury in untreated animals (a–d), while only a mild hepatocellular swelling that did not differ among the genotypes was seen 4h after APAP exposure (e–h). Distinct hepatocyte swelling and centrolobular necrosis became apparent 18h after APAP challenge (j–k) and the liver damage was more prominent in mice carrying K8 variants as compared to WT K8 as quantified by morphometric analysis (E). Largely normal alanine aminotransferase (ALT) levels were seen in untreated mice (B) while animals exposed with APAP for 4h displayed uniformly elevated ALTs that did not differ among genotypes (C). (D) 18h after APAP administration, mice carrying K8 variants displayed significantly higher ALT levels than WT K8 animals (medians WT K8: 221 U/L; K8 G62C 554 U/L; K8 R341C 485 U/L and K8 R341H 2165 U/L). Boxplots display median with first and third percentile, while whiskers indicate smallest and largest non-outlier observations. For APAP experiments, at least 10 mice were analysed per genotype. In (E), the results are expressed mean ± SEM. Scale bar=100µm.*p< 0.05, **p<0.01, ***p< 0.001.
Figure 4. Nicotinamide N-methyltransferase (NNMT) represents a novel K8/K18-associated protein that was discovered by a microarray analysis.
(A) List of genes that are most significantly altered in untreated mice carrying K8 G62C/R341H variant versus animals overexpressing WT K8 as determined by expression profiling analysis. RT-PCR analysis (B–D) demonstrates that the drug metabolism-associated gene nicotinamide N-methyltransferase (NNMT) is the only gene that is similarly reduced in all mouse lines carrying K8 variants. L7 (mouse ribosomal protein) gene was used as an internal control. At least four mice were analysed per genotype and the results are expressed mean ± SEM. n.s., not significant. *p<0.05, **p<0.01. (E,F) To immunoprecipitate K8/K18, 1% NP 40 lysates from HepG2 or Huh7 cells were incubated with protein G dynabeads conjugated with the anti-human K18 antibody L2A1 (IP). Non-conjugated beads were used as a control. The lysates prior incubation with the antibody were analyzed as an input. (E) Coomassie staining confirms the specificity of the immunoprecipitation, while immunoblotting for the indicated antigens (F) identifies NNMT as a K8/K18-associated protein.
Since NNMT represents a prominent N-methylating enzyme involved in xenobiotic metabolism (21) and since patients carrying K8 variants are predisposed to APAP-induced ALF (6), we examined the susceptibility of our animals to this form of injury. Four hours after APAP administration, mild hepatocellular swelling and elevated ALT levels were noted, however, both parameters did not differ significantly among the genotypes (Fig. 3Ae–h,C). More prominent hepatocyte swelling and centrolobular necrosis became apparent 18h after APAP challenge and both the liver damage and ALT levels were significantly higher in mice with the K8 variants compared to WT K8 mice (Fig. 3Ai–l,D,E). On the other hand, no obvious differences in susceptibility to APAP injury were noted between WT K8 mice and their non-transgenic littermates (not shown) or between WT and K18 KO animals (Supplementary Figure 5).
To delineate the mechanisms leading to increased APAP hepatotoxicity in mice with K8 variants, we examined APAP metabolism. Neither the APAP plasma levels (4h after APAP administration) nor the expression of APAP-metabolizing enzymes differed significantly among the transgenic mouse lines (Supplementary Fig. 6,7). Notably, 18h after APAP administration, APAP plasma levels were below the detection limit in all genotypes (not shown). Next, we evaluated glutathione content as its depletion represents a crucial event in APAP toxicity (3–4). Under basal conditions, all animals displayed similar total glutathione levels and GSSG/GSH ratios (Supplementary Fig. 8A,B). As expected, APAP treatment resulted in depletion of total glutathione and an increase in the GSSG/GSH ratio, but both parameters were similar across genotypes (Supplementary Fig. 8A,B).
APAP administration also leads to hepatic inflammation with increased plasma IL-6 and TNFα levels as well as ATP depletion (3–4). However, comparable cytokine and ATP levels were seen in all mouse lines before and after APAP exposure (Supplementary Fig. 8C–E). In addition, the apoptosis levels after APAP exposure were negligible (as expected, (4)) and did not differ significantly among genotypes (Supplementary Fig. 8F).
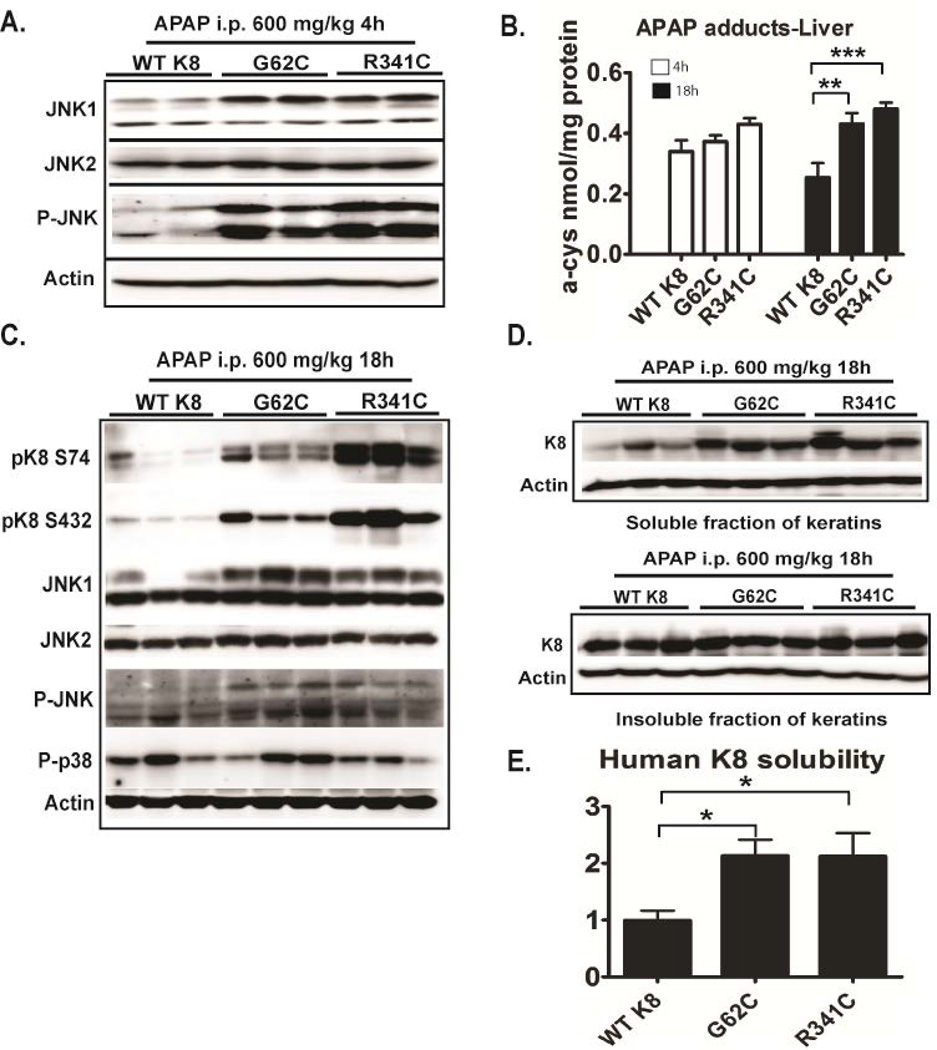
We analyzed the levels of total JNK1 and JNK2, the active pJNK and the APAP protein adducts given their prominent role in APAP hepatotoxicity (3–4). Four hours after APAP exposure, K8 G62C/R341C mice displayed moderately increased JNK1 and robustly elevated pJNK but not JNK2 levels (Fig. 5A). No differences were noted under basal conditions (not shown). Furthermore, 18h, but not 4h after APAP administration, APAP protein adducts were significantly higher in K8 G62C/K8 R341C mice compared to WT K8s (Fig. 5B).
Figure 5. Mice carrying K8 variants display activated JNK signalling, stronger formation of APAP protein adducts, K8 hyperphosphorylation and increased K8 solubility.
(A,C) Total liver lysates from APAP-exposed mice carrying either wild type human K8 (WT K8) or K8 variants were subjected to immunoblotting using antibodies to the indicated antigens. (B) The formation of APAP protein adducts was analysed 4h and 18h after APAP administration by high performance liquid chromatography with electrochemical detection (expressed as nmol of cysteine adducts per mg of protein). (D, E) Keratin solubility in 1% Triton X-containing buffer was assessed by immunoblotting and the ratio of soluble (upper panel) and insoluble (lower panel) K8 was quantified by densitometric analysis. K8 solubility in WT K8 mice was arbitrarily set as 1 and levels in the other genotypes represent a ratio. Actin was used as a loading control. At least 4 mice/genotype were analysed and the results are expressed mean ± SEM. Abbreviations: JNK, c-Jun N-Terminal Kinase; p, phospho. *p<0.05, **p<0.01, ***p< 0.001
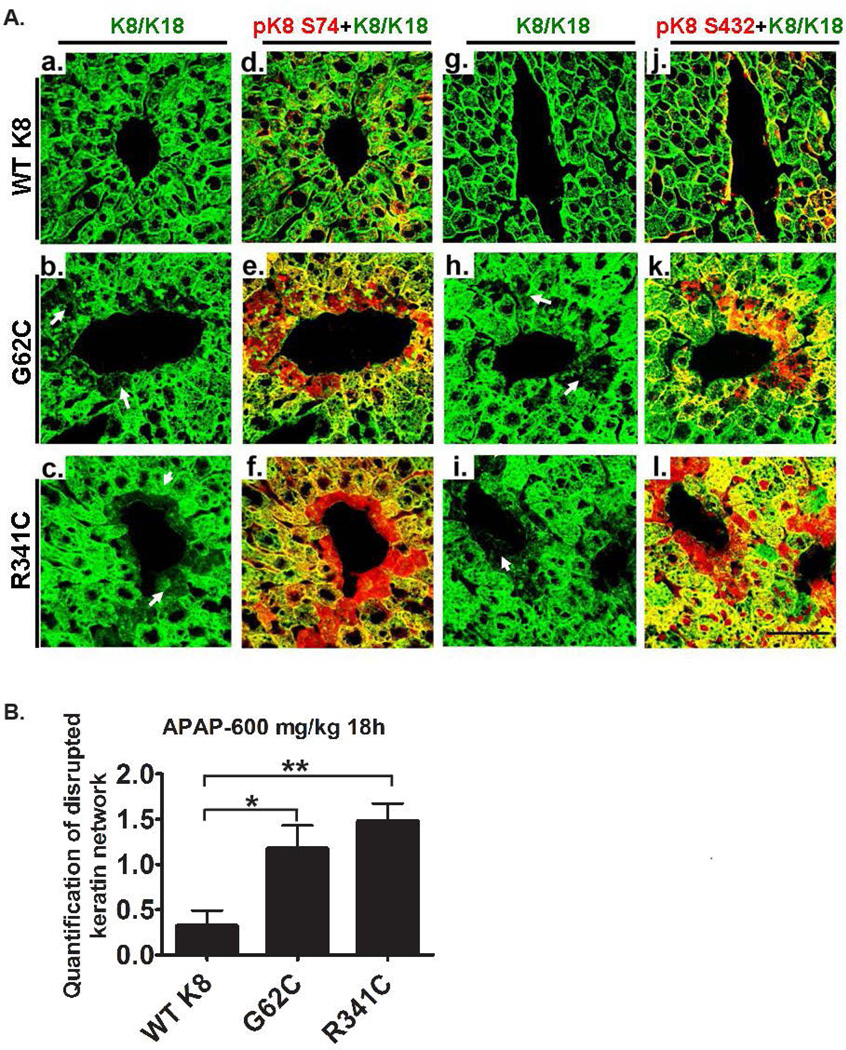
To better understand the role of K8 variants in APAP hepatotoxicity, we evaluated APAP-induced K8/K18 changes. 18 hours after APAP exposure, both K8 G62C and K8 R341C mice displayed significant K8 hyperphosphorylation at S74 and S432. As a likely mechanism, pJNK levels were increased in these animals whereas no differences were noted in phospho-p38 kinase (Fig. 5C). Consistent with the keratin hyperphosphorylation, mice carrying K8 variants displayed increased K8 solubility that was confirmed by densitometric quantification (Fig. 5D,E; (16)), while no obvious differences in solubility were noted under basal conditions (not shown). Immunofluorescence staining showed that K8 hyperphosphorylation was most prominent in pericentral areas, where it resulted in keratin network disruption (Fig. 6A; Supplementary Fig. 9). Both keratin hyperphosphorylation and keratin network disruption were more prominent in K8 G62C/R341C mice compared to WT K8 animals (Fig. 6; Supplementary Fig. 9).
Figure 6. Mice carrying K8 variants display pericentral keratin network disruption and K8 hyperphosphorylation after APAP exposure.
Mice overexpressing WT K8 or K8 variants were treated with APAP for 18h and the resulting liver sections were subjected to immunofluorescence staining with K8/K18 antibody alone (a–c, g–i) or to a combination of K8/K18 (green) and the depicted phospho-specific (p) K8 antibody (red; d–f, j–l). Note that animals carrying K8 variants display a more pronounced pericentral K8 hyperphosphorylation and keratin network disruption (the latter is highlighted by arrows) as confirmed by morphometrical quantification (B). At least three mice were analysed per genotype and the results are expressed mean ± SEM. Scale bar=50µm. *p<0.05, **p<0.01
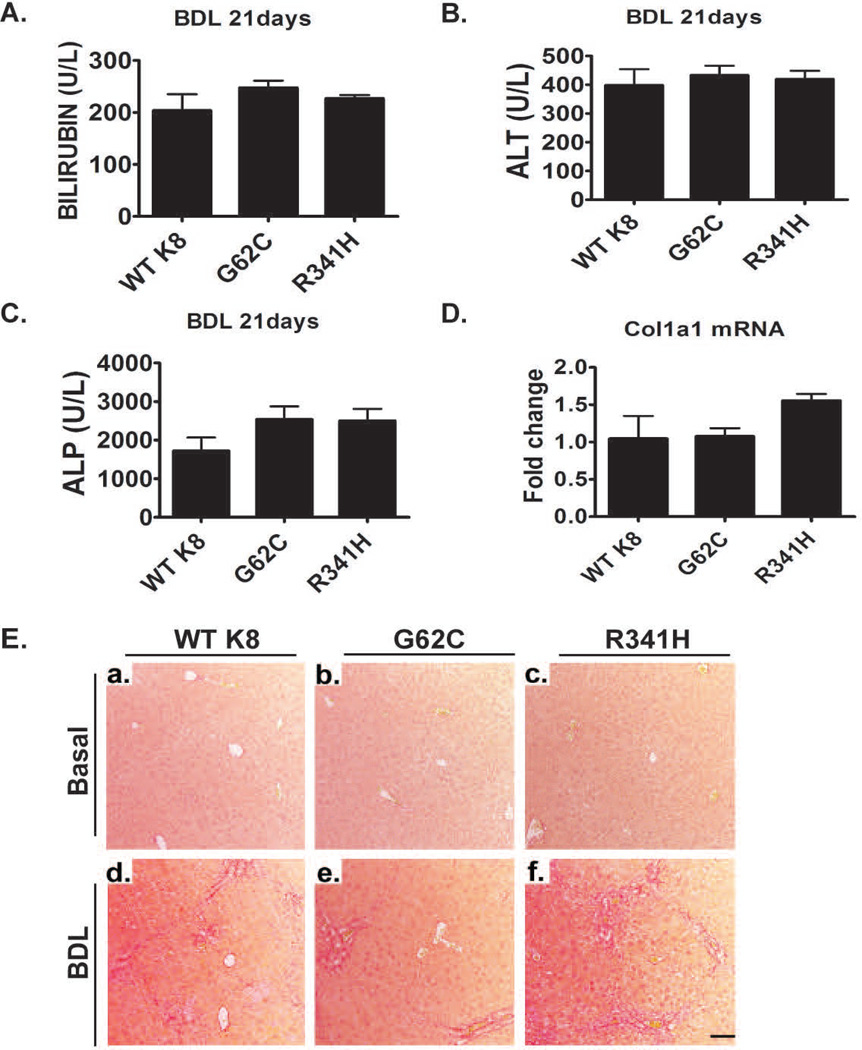
Given the reported association of K8/K18 variants with liver disease development in patients with primary biliary cirrhosis (22), we also analyzed the role of K8 variants in BDL-induced chronic cholestatic liver injury. All animals exhibited markedly elevated bilirubin, ALT and alkaline phosphatase 21d after BDL, but no differences were noted among the genotypes (Fig. 7B–D). Moreover, the overall extent of liver injury and liver fibrosis as well as collagen mRNA levels were similar in all experimental groups (Fig. 7A,E, Supplementary Fig. 10).
Figure 7. Presence of K8 variants does not affect the development of bile duct ligation (BDL)-induced liver fibrosis.
(A–C) Three weeks after BDL, mice displayed significantly elevated bilirubin, ALT and alkaline phosphatase (ALP) levels, however no significant differences were noted among the genotypes. Furthermore, In all untreated animals, bilirubin, ALT and ALP levels were within normal range. (E) The extent of liver fibrosis in untreated animals (basal) as well as mice subjected to BDL for three weeks was evaluated by picro-sirius red staining. Note that animals overexpressing WT K8 or the depicted K8 variants display a similar amount of liver scaring and similar hepatic collagen mRNA levels as determined by qRT-PCR (D). L7 (mouse ribosomal protein) gene was used as an internal control. At least four mice were analysed per genotype and the results are expressed mean ± SEM. Scale bar=100µm.
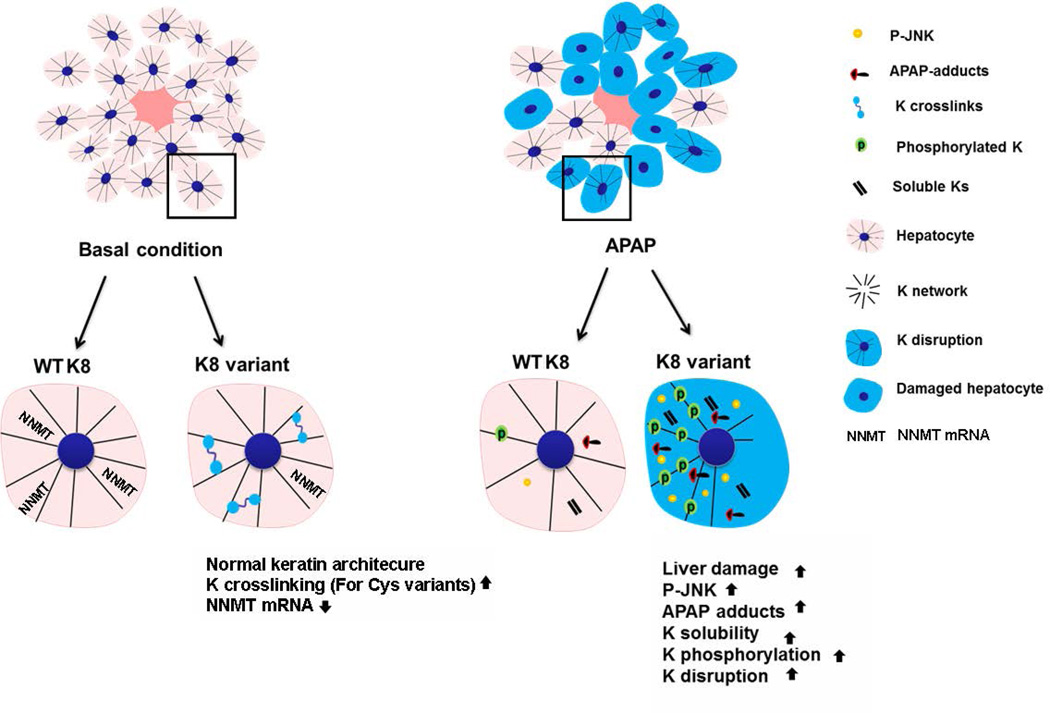
In summary, our data show that the presence of K8 variants predisposes to APAP hepatotoxicity, but not to cholestatic liver injury, in association with increased JNK activation, stronger formation of protein adducts and alterations in the K8/K18 system (Fig. 8).
Figure 8.
The schematic summarizes the findings of our study. Under basal conditions, mice carrying K8 variants display normal liver architecture and keratin filament network but harbor more keratin crosslinks and have lower nicotinamide N-methyltransferase (NNMT) levels than animals overexpressing WT K8. After APAP administration, mice with K8 variants exhibit increased liver injury, JNK activation (i.e. P-JNK) APAP-adducts formation and alterations in K8/K18 filament organization.
Discussion
Given the established link between the presence of K8/K18 variants and the development of several human liver diseases (11–12), we generated transgenic animals carrying common human K8 variants seen in Caucasians from the US and Europe in order to delineate the biological significance of the variants. Under basal conditions, the K8 R341H/R341C mice did not display hepatic alterations or changes in the K8/K18 filament network which is similar to previous findings in K8 G62C mice (Fig. 8). In contrast, keratin filament disruption under basal conditions, as occurs in K18 R90C livers, results in hepatocyte fragility and spontaneous mild hepatitis (12). The effect of the K18 R90C mutation is not surprising since it is located in the most conserved regions of K18 mimicking the severely disruptive keratin mutations found in epidermal genodermatoses (23) whereas K8 G62/R341 while being highly conserved residues are situated in less conserved domains (11–12). One potential caveat of our models is the expression of the human keratins in background of endogenous mouse keratins and the potential differences in the keratin expression levels. However, human K8 appears to integrate normally into the endogenous mouse keratin filament network which is not surprising given the ~88% identity of mouse and human K8.
After APAP exposure, all mouse lines carrying K8 variants exhibited increased hepatotoxicity (Fig. 8) which supports the human findings that suggested a K8/K18 mutation-based predisposition towards adverse outcome of APAP-induced ALF (6). As a potential underlying mechanisms, the presence of K8 variants resulted in a more prominent JNK activation that is instrumental in APAP-induced cell death (24). These findings are in line with previous reports that linked activation of JNK pathway to disturbances in the keratin system. For example, prolonged JNK phosphorylation was seen in keratinocyte cell lines carrying mutations in the epidermal K5/K14 when exposed to osmotic stress (25) or in mice ectopically expressing K10 in the basal epidermal layers (26). The increased formation of APAP protein adducts also likely contributes to the increased APAP hepatotoxicity observed in mice with K8 variants. While further studies are needed to determine the exact underlying mechanisms, the decreased NNMT levels in the mutant keratin-expressing mice are likely to be important (21, 27). In that respect, our data demonstrated association between K8/K18 and NNMT, however, the precise molecular domains involved in this association and the impact of K8 variants on this process remain to be characterized.
When challenged with APAP, mice overexpressing K8 variants displayed K8 hyperphosphorylation at S74 and S432. The increased K8 pS74 levels are somewhat surprising since K8 G62C substitution blunts phosphorylation at this residue (by inducing a conformation change, (28)) in vitro using purified p38 kinase or in transgenic K8 G62C that are exposed to Fas ligand (17, 29). However, K8 S74 is phosphorylated by several kinases including p38 kinase and JNK. Therefore, the inhibitory role of the G62C substitution is likely restricted only to certain kinases. Of note, JNK is also known to phosphorylate K8 at S432 (27) and is therefore likely responsible, at least in part, for the observed increase in K8 S432 hyperphosphorylation.
The increased K8 phosphorylation in mice with K8 variants likely accounts for the higher K8 solubility as well as APAP-induced K8/K18 network reorganization thereby overcoming the impact of K8 G62C that would otherwise be expected to decrease K8 solubility via disulfide bond formation (16–17). Other posttranslational modifications might also be important since some of them affect keratin solubility as well (16)
The more prominent disruption of pericentral keratin filament network in animals with K8 variants represents another factor likely contributing to the development of APAP-induced liver injury. In that respect, keratins constitute the major mechanical stabilizers of the cell (30) and the cellular fragility resulting from keratin network disruption might be particularly detrimental in the case of APAP-induced hepatocyte swelling (3–4, 12). In agreement, keratin network disruption has been linked to hepatocellular ballooning in multiple liver disorders (31). Of note, keratin network disruption has been associated with susceptibility to oxidative stress as well as with impaired mitochondrial function that constitute important mechanisms promoting APAP toxicity (3–4, 32–33).
In contrast to APAP-mediated hepatotoxicity, the K8 variants did not affect the development of BDL-induced liver injury or fibrosis. This finding further underscores the previous observations showing that K8/K18 variant-based predisposition to liver injury is context specific. In that respect, K18 R90C mutation increases susceptibility to Fas- but not TNFα-induced apoptosis and liver damage, and to thioacetamide but not CCl4-mediated fibrogenesis (34–35). In humans, exonic K8/K18 variants segregate with poor outcome or liver disease progression in patients with ALF, primary biliary cirrhosis or chronic hepatitis C infection but do not associate with the progression of hemochromatosis-induced liver damage (6, 22, 36–37).
In summary, our findings provide insight and support for the disease relevance of human K8 variants in APAP-associated liver injury.
Supplementary Material
Acknowledgements
The expert technical assistance of Elke Preiß, Kristina Diepold, Claudia Laengle, Susanne Bobrovich, Linda Schaub, Giovanna Vella and Anke Bauer is gratefully acknowledged.
Grant support and other assistance:
This work was supported by the German Research Foundation grants STR 1095/2-1, STR 1095/4-1, the IZKF research group funding and the SFB/TRR57 (P.S. & C.T.), National Institutes of Health grant DK52951 and a Department of Veterans Affairs Merit Award (M.B.O.).
Abbreviations
- ALF
Acute liver failure
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- APAP
Acetaminophen
- BDL
Bile duct ligation
- GSH
Total glutathione
- GSSG/GSH
Oxidized/reduced glutathione
- h
human
- H&E
hematoxylin & eosin staining
- HSE
High salt extraction
- IL-6
Interleukin 6
- JNK
c-Jun amino-terminal kinase
- K
Keratin protein
- NAPQI
N-acetyl-p-benzoquinone imine
- NNMT
Nicotinamide N-methyl transferase
- P-JNK
phosphorylated JNK
- qRT-PCR
Quantitative real-time polymerase chain reaction
- TNFα
Tumor necrosis factor α
- WT
Wild type
Footnotes
Study concept and design: NG, MBO, PS
Acquisition of data: NG, QZ, OK, MR, KL, AG, RK, LPJ
Analysis and interpretation of data: NG, MBO, PS
Drafting of the manuscript: NG, PS, MBO
Editing of the manuscript: all authors
Statistical analysis: NG, PS
Funding and study supervision: PS, MBO
Technical and material support: LPJ, MBO, CT
Disclosures:
The authors declare that they do not have any conflict of interest to disclose.
References
- 1.Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.Fontana RJ. Pathogenesis of Idiosyncratic Drug-Induced Liver Injury and Clinical Perspectives. Gastroenterology. 2014;146:914–928. e911. doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Pickering G. Paracetamol metabolism and related genetic differences. Drug Metab Rev. 2011;43:41–52. doi: 10.3109/03602532.2010.527984. [DOI] [PubMed] [Google Scholar]
- 4.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Strnad P, Zhou Q, Hanada S, Lazzeroni LC, Zhong BH, So P, Davern TJ, et al. Keratin variants predispose to acute liver failure and adverse outcome: race and ethnic associations. Gastroenterology. 2010;139:828–835. 835, e821–e823. doi: 10.1053/j.gastro.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haines RL, Lane EB. Keratins and disease at a glance. J Cell Sci. 2012;125:3923–3928. doi: 10.1242/jcs.099655. [DOI] [PubMed] [Google Scholar]
- 8.Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol. 2013;25:47–56. doi: 10.1016/j.ceb.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeverenyi I, Cassidy AJ, Chung CW, Lee BT, Common JE, Ogg SC, Chen H, et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum Mutat. 2008;29:351–360. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 10.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strnad P, Paschke S, Jang KH, Ku NO. Keratins: markers and modulators of liver disease. Curr Opin Gastroenterol. 2012;28:209–216. doi: 10.1097/MOG.0b013e3283525cb8. [DOI] [PubMed] [Google Scholar]
- 12.Ku N, Strnad P, Zhong BH, Tao GZ, Omary MB. Keratins let liver live: Mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology. 2007;46:1639–1649. doi: 10.1002/hep.21976. [DOI] [PubMed] [Google Scholar]
- 13.Tao GZ, Strnad P, Zhou Q, Kamal A, Zhang L, Madani ND, Kugathasan S, et al. Analysis of keratin polypeptides 8 and 19 variants in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:857–864. doi: 10.1016/j.cgh.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Strnad P, Usachov V, Debes C, Grater F, Parry DA, Omary MB. Unique amino acid signatures that are evolutionarily conserved distinguish simple-type, epidermal and hair keratins. J Cell Sci. 2011;124:4221–4232. doi: 10.1242/jcs.089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ku NO, Gish R, Wright TL, Omary MB. Keratin 8 mutations in patients with cryptogenic liver disease. N Engl J Med. 2001;344:1580–1587. doi: 10.1056/NEJM200105243442103. [DOI] [PubMed] [Google Scholar]
- 16.Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15:163–177. doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku NO, Omary MB. A disease- and phosphorylation-related nonmechanical function for keratin 8. J Cell Biol. 2006;174:115–125. doi: 10.1083/jcb.200602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krauss S, Franke WW. Organization and sequence of the human gene encoding cytokeratin 8. Gene. 1990;86:241–249. doi: 10.1016/0378-1119(90)90285-y. [DOI] [PubMed] [Google Scholar]
- 19.Magin TM, Schroder R, Leitgeb S, Wanninger F, Zatloukal K, Grund C, Melton DW. Lessons from keratin 18 knockout mice: formation of novel keratin filaments, secondary loss of keratin 7 and accumulation of liver-specific keratin 8-positive aggregates. J Cell Biol. 1998;140:1441–1451. doi: 10.1083/jcb.140.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamichi I, Toivola DM, Strnad P, Michie SA, Oshima RG, Baribault H, Omary MB. Keratin 8 overexpression promotes mouse Mallory body formation. J Cell Biol. 2005;171:931–937. doi: 10.1083/jcb.200507093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269:14835–14840. [PubMed] [Google Scholar]
- 22.Zhong B, Strnad P, Selmi C, Invernizzi P, Tao GZ, Caleffi A, Chen M, et al. Keratin variants are overrepresented in primary biliary cirrhosis and associate with disease severity. Hepatology. 2009;50:546–554. doi: 10.1002/hep.23041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulombe PA, Lee CH. Defining keratin protein function in skin epithelia: epidermolysis bullosa simplex and its aftermath. J Invest Dermatol. 2012;132:763–775. doi: 10.1038/jid.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Alessandro M, Russell D, Morley SM, Davies AM, Lane EB. Keratin mutations of epidermolysis bullosa simplex alter the kinetics of stress response to osmotic shock. J Cell Sci. 2002;115:4341–4351. doi: 10.1242/jcs.00120. [DOI] [PubMed] [Google Scholar]
- 26.Santos M, Perez P, Segrelles C, Ruiz S, Jorcano JL, Paramio JM. Impaired NF-kappa B activation and increased production of tumor necrosis factor alpha in transgenic mice expressing keratin K10 in the basal layer of the epidermis. J Biol Chem. 2003;278:13422–13430. doi: 10.1074/jbc.M208170200. [DOI] [PubMed] [Google Scholar]
- 27.Nelson SD, Forte AJ, McMurtry RJ. Decreased toxicity of the N-methyl analogs of acetaminophen and phenacetin. Res Commun Chem Pathol Pharmacol. 1978;22:61–71. [PubMed] [Google Scholar]
- 28.Tao GZ, Nakamichi I, Ku NO, Wang J, Frolkis M, Gong X, Zhu W, et al. Bispecific and human disease-related anti-keratin rabbit monoclonal antibodies. Exp Cell Res. 2006;312:411–422. doi: 10.1016/j.yexcr.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Ku NO, Lim JK, Krams SM, Esquivel CO, Keeffe EB, Wright TL, Parry DA, et al. Keratins as susceptibility genes for end-stage liver disease. Gastroenterology. 2005;129:885–893. doi: 10.1053/j.gastro.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Jang KH, Kim H, Lim Y, Kim S, Yoon HN, Chung IK, et al. Predisposition to apoptosis in keratin 8-null liver is related to inactivation of NF-kappaB and SAPKs but not decreased c-Flip. Biol Open. 2013;2:695–702. doi: 10.1242/bio.20134606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lackner C, Gogg-Kamerer M, Zatloukal K, Stumptner C, Brunt EM, Denk H. Ballooned hepatocytes in steatohepatitis: The value of keratin immunohistochemistry for diagnosis. J Hepatol. 2008;48:821–828. doi: 10.1016/j.jhep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q, Ji X, Chen L, Greenberg HB, Lu SC, Omary MB. Keratin mutation primes mouse liver to oxidative injury. Hepatology. 2005;41:517–525. doi: 10.1002/hep.20578. [DOI] [PubMed] [Google Scholar]
- 33.Tao GZ, Looi KS, Toivola DM, Strnad P, Zhou Q, Liao J, Wei Y, et al. Keratins modulate the shape and function of hepatocyte mitochondria: a mechanism for protection from apoptosis. J Cell Sci. 2009;122:3851–3855. doi: 10.1242/jcs.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku NO, Soetikno RM, Omary MB. Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology. 2003;37:1006–1014. doi: 10.1053/jhep.2003.50181. [DOI] [PubMed] [Google Scholar]
- 35.Strnad P, Tao GZ, Zhou Q, Harada M, Toivola DM, Brunt EM, Omary MB. Keratin mutation predisposes to mouse liver fibrosis and unmasks differential effects of the carbon tetrachloride and thioacetamide models. Gastroenterology. 2008;134:1169–1179. doi: 10.1053/j.gastro.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strnad P, Lienau TC, Tao GZ, Ku NO, Magin TM, Omary MB. Denaturing temperature selection may underestimate keratin mutation detection by DHPLC. Hum Mutat. 2006;27:444–452. doi: 10.1002/humu.20311. [DOI] [PubMed] [Google Scholar]
- 37.Strnad P, Kucukoglu O, Lunova M, Guldiken N, Lienau TC, Stickel F, Omary MB. Non-coding keratin variants associate with liver fibrosis progression in patients with hemochromatosis. PLoS One. 2012;7:e32669. doi: 10.1371/journal.pone.0032669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.