Abstract
Substance use disorders have significant personal, familial, and societal consequences. Despite the serious consequences of substance use, only a few therapies are effective in treating substance use disorders, thus highlighting a need for improved treatment practices. Substance use treatment response depends on multiple factors such as genetic, biological, and social. It is essential that each component is represented in treatment plans. The dopaminergic system plays a critical role in pharmacotherapy for the addictions and an understanding of the role of variation of genes involved in this system is essential for its success. This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines. A computerized literature search was conducted using PubMed and Scopus (all databases). Articles published up to April 2015 that examined the role of dopaminergic gene variation in the pharmacotherapy of alcohol, opioid, and cocaine substance use disorders were reviewed. Search terms were dopamine, gene, polymorphism, substance abuse, treatment, and response. Polymorphisms of the DRD2, ANKK1, DAT1, DBH, and DRD4 genes have been found to moderate the effects of the pharmacotherapy of alcohol, opioid, and cocaine substance use disorders. The integration of genetic information with clinical data will inform health professionals of the most efficacious pharmacotherapy intervention for substance use disorders. More studies are needed to confirm and extend these findings.
Keywords: Abuse, addiction, alcohol, cocaine, dependence, dopamine, drug, gene, polymorphism, therapy
Introduction
Current prevalence estimates of drug use highlight an urgent global health concern with few therapeutic options. Up to 324 million individuals worldwide aged 15-64 years have used an illicit drug (United Nations Office on Drugs and Crime, 2014). Within the United States alone, 24.6 million (9.4% of the population) Americans over age 12 were alcohol, tobacco, or illicit drug users in 2013 (SAMHSA, 2014). Use of addictive drugs directly impacts an individual's physical and socio-emotional health, including increased risk for comorbid drug use and addiction, emergency care, HIV infection, and family dysfunction (Baldwin et al., 2012, Macgowan et al., 1997, Meandzija et al., 1994, Wolf et al., 2004). Many mental health disorders and their symptoms also are associated with substance use: depression, suicidal ideation, suicide attempts, schizophrenia, bipolar disorder, borderline personality disorder, mood and anxiety disorders, conduct disorder, and antisocial personality disorder (Merikangas et al., 1998, Mesholam-Gately et al., 2014, Rasic et al., 2013, Trull et al., 2000, Wilens & Zulauf, 2014). In addition to the personal and familial impact, the fiscal consequences associated with addictive drug use have been estimated in the United States to be $122 billion per year in lost productivity and time (Horgan et al., 2001). The serious personal, familial, and societal costs of substance use highlights the need to advance the understanding of the mechanisms underlying treatment response and, in turn, establish more precise pharmacotherapy practices to effectively treat drug addiction.
Drug addiction is characterized by the presence of psychological (e.g., craving, unsuccessful efforts to control use) and/or physical symptoms (e.g., a need for increased amounts of the drug to achieve intoxication or a desired effect) (American Psychiatric Association, 2013, World Health Organization, 1992). As an individual develops an addiction, drug use frequency increases and the amount of the drug needed in order to maintain the drug effects increases (tolerance). Drug cessation may lead to withdrawal symptoms (e.g., nausea, anxiety) and cravings (Kreek et al., 2005b). The relief of withdrawal symptoms and cravings with subsequent drug use (relapse) perpetuates the substance abuse cycle. Thus, treatment plans that simultaneously reduce physiological and psychological symptoms related to withdrawal and craving are most effective.
Every patient responds to pharmacotherapy in an individualized manner. This response is, in part, moderated by genetic variation (Nielsen et al., 2014a). Decision-making regarding initial psychiatric medication selection, which typically does not include a patients’ genetic information, fails to reduce symptoms in 30-40% of individuals (Haile et al., 2009). Incorporating genetic information into the decision-making process provides a targeted approach that may guide the selection of psychiatric medication (Gupta et al., 2006, Malhotra et al., 2004, Murphy et al., 2003). With a genetically-guided approach, there may be an increase in compliance and positive therapeutic response and, at the same time, reduced risk for toxic side effects (Deleon et al., 2006, Malhotra et al., 2004, Murphy et al., 2003, Rogers et al., 2002). Due to its role in reward processing and substance use disorders, genetic analysis of the dopaminergic system may provide unique insights to the application of pharmacogenetics to improve treatment of substance abuse disorders. As such, herein we systematically review the current literature on variation of dopaminergic genes – i.e., dopamine receptor D2 (DRD2), ankyrin repeat and kinase domain containing 1 (ANKK1), dopamine transporter 1 (DAT1, SLC6A3), dopamine β-hydroxylase (DBH), and dopamine receptor D4 (DRD4) – and the pharmacotherapy of addiction to cocaine, alcohol, and opioids (see Table 1). This review complements our previous systematic evaluation of genetic variation of the opioidergic system in the pharmacotherapy of substance abuse (Bauer et al., 2014).
Table 1.
Dopaminergic gene variants involved in pharmacotherapy for the addictions
| Gene | Product | Variant | Addiction | Pharmacotherapy |
|---|---|---|---|---|
| DRD2 | Dopamine receptor D2 | rs6275, rs6277, rs1799978 | Cocaine, alcohol, opioids (Ament et al., 2015) | Bromocriptine, disulfiram, methadone (Doehring et al., 2009a, Volkow et al., 1990, Volkow et al., 1996, Wang et al., 1997) |
| ANKK1 | Ankyrin repeat and kinase domain-containing 1 | rs1800497 (TaqIA1) | Cocaine, alcohol, opioids (Hung et al., 2011, Lawford et al., 1995, Spellicy et al., 2013) | Disulfiram, naltrexone, methadone (Doehring et al., 2009a, Volkow et al., 1990, Volkow et al., 1996, Wang et al., 1997) |
| DAT1, SLC6A3 | Dopamine transporter 1 | exon 9 VNTR | Alcohol (Anton et al., 2012, Hung et al., 2011, Spellicy et al., 2013) | Naltrexone (Franke et al., 1999, Muramatsu & Higuchi, 1995, Sander et al., 1997) |
| DBH | Dopamine β-hydroxylase | rs1611115 (C-1021T) | Alcohol, opioid (Anton et al., 2012) | Disulfiram, cocaine vaccine (Preuss et al., 2013, Xie et al., 2013) |
| DRD4 | Dopamine receptor D4 | exon 3 VNTR | Alcohol (Kosten et al., 2013a) | Olanzapine (Hutchison et al., 2002) |
VNTR = variable number tandem repeat.
The dopaminergic system
In recent years, an empirical link has emerged between neural reward processes, the dopaminergic system, and substance use disorders (for extensive review see Nutt et al., 2015). Briefly, when there is anticipation of a reward (Schultz, 2002, Schultz, 2010) dopamine is released in the nucleus accumbens (NAc) from neurons originating in the ventral tegmental area (VTA) (Di Chiara & Imperato, 1988, Koob & Le Moal, 2001, Pfaus et al., 1990, Small et al., 2003, Wise, 1998). Dopamine is released after the use of drugs of abuse (Di Chiara & Imperato, 1988). Over time, continued drug lowers striatal dopamine receptor availability in cocaine, alcohol, and opioid dependent individuals (e.g., Martinez et al., 2007, Volkow et al., 2011, Volkow et al., 1996, Wang et al., 1997). In addition to substance use disorders being associated with lower striatal dopamine receptor availability, many are associated with blunted dopamine release (e.g., cocaine; Badiani et al., 2011). The use of addictive drugs stimulates dopamine release thereby increasing dopamine levels above typical basal levels (Volkow et al., 2011), eventually leading to craving and addiction. Notably, as highlighted by Nutt and colleagues, this is an oversimplification of the role of dopamine in substance use. Though the dopamine theory of addiction is generally accepted, Nutt and colleagues illustrate the importance of dissecting the relations between dopamine, reward, and specific drugs of abuse. Not all drugs evoke the same dopaminergic processes or effects, thus a broad theory of dopamine and addiction is incomplete (Nutt et al., 2015).
One factor associated with differences in the relations between drugs and dopamine, is that the specific mechanisms of action for each class of abused drugs differs. Alcohol and opioids primarily act on mesolimbic dopaminergic pathways, while cocaine blocks the action of three of the major neurotransmitter system transporters, dopamine, serotonin, and norepinephrine (Han & Gu, 2006). Albeit by different mechanisms, alcohol, opioids, and cocaine increase the release and synaptic availability of dopamine. Alcohol has been shown to promote the release of endogenous opioids (Imperato & Di Chiara, 1986, Koob, 1992, Weiss et al., 1993). Current opinion is that alcohol may increases dopamine levels via μ-opioid receptors in the mesolimbic system (Heilig et al., 2011). The binding of the endogenous opioid peptide β-endorphin disinhibits the GABAergic interneurons in the VTA stimulating the release of dopamine and increasing extracellular mesolimbic dopamine levels in the NAc (Bourdy & Barrot, 2012). Opioids, however, bind directly to the μ-opioid receptors on the GABAergic interneurons, depolarizing their membrane and inhibiting the release of GABA. This inhibition of GABA subsequently stimulates the release of dopamine in the NAc (Johnson & North, 1992). Cocaine increases the availability of dopamine in the synapse by binding to the dopamine transporter and inhibiting reuptake of dopamine from the synapses. These drug-specific dopaminergic responses underscore the utility of reviewing the connection between genetic variants of dopaminergic genes and their role in substance use disorders and pharmacotherapeutic response.
Dopaminergic genes relevant to addiction
Dopamine receptor D2 (DRD2)/ankyrin repeat and kinase domain containing (ANKK1) genes
DRD2 and ANKK1 are situated approximately 10,000 nucleotides apart on chromosome 11q22-23. DRD2 and ANKK1 polymorphisms have been found to be associated with alcohol, heroin, cocaine, and opioid substance use disorders and substance dependence (Blum et al., 1991, Lawford et al., 2000, Moyer et al., 2011, Noble, 1994). DRD2 transcripts may be alternatively spliced to code for two different protein isoforms: the long (D2L) and short (D2S, lacking exon 6) forms of the receptor protein. The single-nucleotide polymorphism (SNP) rs2283265 (in intron 5), which is a G to T transversion in an intron of the DRD2 gene, increases the ratio of D2L to D2S protein isoforms (Moyer et al., 2011). The T allele of rs2283265 is found more commonly in cocaine-addicted individuals (Meyers et al., 2013, Zhang et al., 2007). Another DRD2 variant, rs6277, is a synonymous (does not alter the amino acid coding) C to T transition in DRD2. Individuals with the T allele have greater D2 receptor availability, altered mRNA folding, and reduced mRNA stability (Duan et al., 2003, Hirvonen et al., 2009), and is carried more often in those with opioid substance dependence relative to healthy controls (Doehring et al., 2009b).
The ANKK1 gene encodes the ankyrin repeat and kinase domain containing 1 protein receptor also known as receptor interacting protein 5 (RIP5). The ANKK1 polymorphisms are one of the most examined genetic variants in connection with substance use and other psychiatric disorders (Blum et al., 1990, Comings et al., 1991), particularly alcoholism. ANKK1/DRD2 TaqIA, also known as rs1800497, is a functional SNP located in the final exon of ANKK1 that codes for a non-synonymous glutamic acid to lysine (C to T) amino acid change in the C-terminus of the ANKK1 protein. The TaqIA1 allele has been found to be associated with a reduced dopamine receptor D2 density (Jonsson et al., 1999) and an increased rate of substance dependence to cocaine, opioids, and alcohol (Volkow et al., 1990, Volkow et al., 1996, Wang et al., 1997).
The ANKK1 variant rs7118900 also may contribute to drug-related dopaminergic system changes in D2 levels. This variant codes for an alanine to threonine (Ala239Thr) substitution that creates a predicted phosphorylation site and is found to be in strong linkage disequilibrium with ANKK1/DRD2 TaqIA (Garrido et al., 2011). Cells transfected with the ANKK1 rs7118900 Thr239 variant constructs had higher expression levels than did cells containing the Ala239 variant constructs. When treated with the dopamine agonist apomorphine, the Thr239 constructs decreased expression while the Ala239 constructs increased expression. This differential response of the ANKK1 alleles highlights a potential functional link to the dopaminergic system.
Dopamine transporter (DAT1, SLC6A3) gene
The DAT1 gene codes for the dopamine transporter (DAT), a member of the sodium and chloride-dependent family of neurotransmitter transporters (Vandenbergh et al., 1992b). DAT mediates the reuptake of dopamine from synapses into the presynaptic nerve terminals (Amara & Kuhar, 1993, Giros & Caron, 1993, Iversen, 1971). The DAT1 gene contains a variable number tandem repeat (VNTR) located in the 3’-untranslated region (Sano et al., 1993, Vandenbergh et al., 1992a) that may contain 3-11 copies of the repeat. This VNTR has been shown to alter DAT1 gene expression, with the 9 allele showing a higher level expression compared to the 10 allele (Miller & Madras, 2001). Variation of this VNTR is associated with multiple disorders/diseases related to dopamine including alcoholism (Franke et al., 1999, Muramatsu & Higuchi, 1995, Sander et al., 1997), attention-deficit/hyperactivity disorder (Cook Jr et al., 1995), Parkinson's disease (Le Couteur et al., 1997, Mercier et al., 1999), and schizophrenia (Blum et al., 1997, Inada et al., 1996, Maier et al., 1996).
Dopamine β-hydroxylase (DBH) gene
Dopamine β-hydroxylase (DβH) is an enzyme located primarily within the synaptic vesicles that store the catecholamine neurotransmitters dopamine and norepinephrine where it metabolizes dopamine into norepinephrine [reviewed in (Kaufman & Friedman, 1965, Weinshilboum, 1978)]. Although the majority of DβH is membrane bound in the vesicle, some DβH is free and is concurrently released with the catecholamines during synaptic transmission from neurons and into the blood from neurosecretory cells of the adrenal medulla (Stewart & Klinman, 1988). DβH levels in serum and cerebral spinal fluid are heritable (Oxenstierna et al., 1986), having been found to be highly correlated between siblings, but not between unrelated subjects (Weinshilboum et al., 1973).
Numerous polymorphisms of the DBH gene have been shown to be associated with DβH levels, but one variant appears to best predict DβH levels: the C-1021T rs1611115 variant, also known as C-970T (Bhaduri & Mukhopadhyay, 2008, Zabetian et al., 2001, Zabetian et al., 2003). In a study that examined 11 SNPs across DBH, the C-1021T variant was determined to be the best predictor of DβH plasma levels, accounting for 35-52% of the variation (Zabetian et al., 2003). The rs1611115 variant has been associated with alcohol dependence in females (Preuss et al., 2013) and with heroin self-administration (Xie et al., 2013).
Dopamine receptor D4 (DRD4) gene
DRD4 is a relevant dopaminergic gene related to pharmacotherapy and substance use (Hutchison et al., 2002). DRD4 has a VNTR in exon 3 with three common alleles of two, four, and seven repeats (Van Tol et al., 1992). Subjects who were carriers of the seven or longer repeats had a greater “urge to drink” and lower “subjective high” following alcohol consumption compared to peers without this allele (Hutchison et al., 2002). The DRD4 seven tandem repeat allele codes for a dopaminergic receptor D4 that attenuates intracellular forskolin-stimulated cyclic AMP (cAMP) response to dopamine to a greater extent than that produced by the receptor encoded by the two or four tandem repeat alleles (Asghari et al., 1995). Thus, this VNTR appears to contribute to an individual's subjective sensitivity to drugs (e.g., increased urges).
Material and Methods
Literature Review
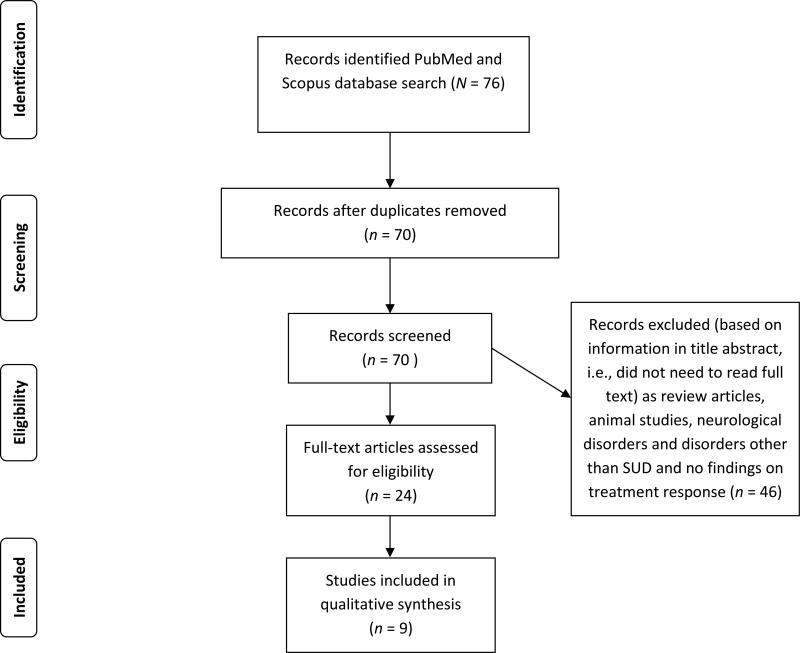
This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines (http://www.prisma-statement.org/). Peer-reviewed and published scientific papers were identified through a computerized literature search using PubMed and Scopus (all databases). Articles published up to April 2015 were reviewed. The following search terms were used: “dopamine,” “gene,” “polymorphism,” “substance abuse,” “treatment,” and “response.” Inclusion criteria were that articles have been: 1) published in English, 2) utilized human clinical population(s) with addictive disorders (e.g., substance use), 3) examined genetic polymorphisms, and 4) measured treatment response. Exclusion criteria were: 1) animal models, 2) human clinical population(s) with mood disorders, and 3) non-substance use clinical trials of psychotropic medication. Article search and selection was completed by two non-blinded reviewers (MP, DN). Articles that did not meet inclusion criteria and/or met exclusion criteria were removed (see Figure 1).
Figure 1.
PRISMA Flowchart showing the filtering process used to select the nine studies included in the systematic review of studies investigating the relationship among dopaminergic gene polymorphisms and treatment response to pharmacotherapies for drug addiction.
Results
Dopaminergic genetics and the pharmacotherapy of drug addiction
Bromocriptine
Bromocriptine is an ergot alkaloid and dopamine receptor D2 agonist. It is commonly used to treat Parkinsonian syndrome, hyperprolactinaemia, growth hormone, and prolactin-related disorders (e.g., infertility, hypogonadism), and also has been efficacious in the treatment of alcoholism (Balldin et al., 1993, Balldin et al., 1992). Administration of bromocriptine inhibits the release of prolactin from the pituitary gland (Jarvik et al., 2000). Since alcoholics have reduced DRD2 receptor sensitivity even several years after discontinuing alcohol use (Balldin et al., 1993, Balldin et al., 1992), bromocriptine may decrease the symptoms of alcohol withdrawal by activating the DRD2 receptor. Symptom improvements following bromocriptine appear to be moderated by the presence of the TaqIA1 variant. Specifically, alcohol-addicted subjects who were TaqIA1 carriers showed the greatest reduction in self-reported alcohol cravings and anxiety following a 6-week randomized double-blind clinical trial of bromocriptine (Lawford et al., 1995). Individuals received bromocriptine (2.5 mg, three times daily) or placebo. Notably, the overwhelming majority of subjects (94%) were male and findings should not be generalized to female populations. The attrition was also highest in the placebo-treated A1 group emphasizing the positive effects of bromocriptine in treating alcohol withdrawal.
Cocaine vaccine
A specific cocaine vaccine composed of succinyl norcocaine conjugated to cholera toxin (Martellet al., 2005) was delivered through a series of vaccinations in an effort to aid in the recovery from cocaine addiction. Vaccination of patients provided therapeutic levels of anti-cocaine antibodies in approximately 40% of patients (Haney et al., 2010). It was hypothesized that if a cocaine-abstinent patient who has received this vaccine relapses and uses cocaine, the anti-cocaine antibodies will sequester cocaine in the blood reducing the ability of cocaine to enter the brain. In one study, individuals who received a higher total dose (2000 μg) versus a lower total dose (400 μg) of the vaccine had higher mean antibody titer response and greater reduction of cocaine use (Martell et al., 2005).
The DBH variant rs1611115 (−1021C>T) was tested to determine if it would moderate this response (Kosten et al., 2013a). Subjects were randomized to a double-blind placebo-controlled 16-week trial. Subjects received five vaccinations over the first 12 weeks. It was found that subjects with the low DβH level genotypes (T allele carriers) had a greater reduction in cocaine use (77% to 51% cocaine-positive urines) compared to those with the normal DβH level (CC) genotype (83% to 72% cocaine-positive urines) (Kosten et al., 2013a). Since the low expressing DβH rs1611115 variant has been found to be associated with increased cocaine-induced paranoia (Cubells et al., 2000), it is possible that this increased paranoia persuades individuals to reduce cocaine use following cocaine vaccine immunization. Similar to the study of bromocriptine, however, the majority of subjects in this study were male and are not necessarily representative of female response. Kosten and colleagues did control for population structure by including it in analyses as a covariate. Only two other studies (Kosten et al., 2013b, Spellicy et al., 2013) controlled for population structure in their analyses.
Disulfiram
Disulfiram (Antabuse, Antabus) has been used for treatment of alcoholism since the early 1950s (Chick, 1999). Following ingestion, disulfiram is oxidized to diethyldithiocarbamate, which is a copper chelator. In the treatment of alcoholism, disulfiram attenuates the conversion of acetaldehyde to acetate through the inhibition of aldehyde dehydrogenases (ALDH). This causes accumulation of acetaldehyde, triggering negative symptoms after the consumption of alcohol (e.g., nausea, vertigo, flushing). Due to the chelating properties of the disulfiram metabolite, copper-containing enzymes also are inhibited, including DβH, the enzyme that converts dopamine to norepinephrine. Treatment with disulfiram reduces DβH activity, increases the levels of dopamine, and decreases the norepinephrine to dopamine ratio in noradrenergic neurons (Bourdélat-Parks et al., 2005, Goldstein & Nakajima, 1967, Karamanakos et al., 2001, Musacchio et al., 1966).
Disulfiram has not only been demonstrated to reduce alcohol consumption, but also to decrease cocaine use (Carroll et al., 2004, Carroll et al., 1998, Petrakis et al., 2000). There are two potential mechanisms of action regarding cocaine and disulfiram: 1) inhibition of DβH and/or 2) inhibition of enzymes that systematically inactivate cocaine, plasma and microsomal carboxylesterases and plasma cholinesterase (Mccance-Katz et al., 1998). One potential site of action of disulfiram, DβH, was examined in individuals codependent on cocaine and opioids (Kosten et al., 2013b). After these subjects were stabilized on methadone for two weeks, they were randomized to receive either disulfiram (250 mg/day) or placebo. The disulfiram-treated subjects showed reduced cocaine use (measured via urine cocaine metabolites), but had no significant reduction in opioid use. When the disulfiram treatment group was separated based on the presence/absence of the DBH rs1611115 T allele, the CC genotype group with normal DβH expression reduced their cocaine use while those patients carrying a low-expressing DβH T allele did not. It was hypothesized that subjects with low DβH levels may have an upregulation of dopamine receptors and, as such, cocaine use was not decreased in response to disulfiram treatment in the subjects with low DβH expression.
Another potential site of action is the ANKK1 rs1800497 variant (TaqIA). Genetic analysis determined that the subjects that were ANKK1 rs1800497 T allele (TaqIA1) carriers showed fewer cocaine-positive urines during the disulfiram pharmacotherapy, whereas CC homozygous individuals (those carrying two copies of the C allele) showed no treatment response (Spellicy et al., 2013). Across both disulfiram studies, the majority of subjects were again male but both studies controlled for population structure to account for variance due to differences by ancestry group.
Olanzapine
Olanzapine binds to several neurotransmitter receptors including dopaminergic, adrenergic, and serotonergic (Bymaster et al., 1997). It is a second-generation dopamine D2 receptor and serontonergic 5-HT2 receptor antagonist that is typically used as psychopharmacologic treatment of schizophrenia and mania-related bipolar disorder, and has been tested in the past decade for use in treating alcohol substance dependence.
Olanzapine treatment appears to diminish the cravings related to alcohol, but only in individuals with certain dopaminergic genetic profiles. Specifically, one study examined the effects of olanzapine (5 mg) relative to a control medication (cyproheptadine, 4 mg) after subjects ingested three drinks of alcohol (Hutchison et al., 2003). The amount of alcohol was determined by taking height, weight, and gender into consideration in order to achieve peak blood alcohol levels of 0.06 g/dL. For males, each drink contained 0.15g/kg doses of ethyl alcohol and 0.11g/kg for females. Cyproheptadine was used as a control since it antagonizes 5-HT2 receptors in humans, but does not block dopamine receptors (Kapur et al., 1997). Subjects self-reported their cravings, stimulation, and sedation following consumption and those who were carriers of a seven or longer repeat of DRD4 VNTR had decreased craving following alcohol consumption. Conversely, individuals without this allele who received olanzapine did not experience reduced cravings following alcohol consumption. At baseline (i.e., before any exposure to alcohol including alcohol cues) and prior to alcohol use (i.e., after baseline and exposure to alcohol cues), olanzapine did reduce cravings in subjects with either genotype. Subjects were predominantly male and younger (undergraduate students, mean age approximately 22 years old) compared to subjects in the other reviewed studies (mean age around mid-30s to 40s). Both gender and developmental effects may limit the generalizability of the findings.
Tiapride
Tiapride is a substituted benzamide related to sulpiride and is a selective dopamine receptor D2 and D3 antagonist (Costall & Naylor, 1977). It is different from other neuroleptics as it is not sedative and does not cause Parkinsonism (Jenner & Marsden, 1979). Treatment using tiapride has demonstrated reductions in psychological stress, decreased drinking, and improved reintegration into society (Peters & Faulds, 1994). Its anxiolytic effects also are used to treat symptoms of alcohol withdrawal syndrome. Similar to findings regarding dopamine receptor agonist bromocriptine, tiapride may be efficacious in treating alcohol withdrawal symptoms due to the reduced dopamine D2 receptor sensitivity that alcoholics demonstrate (Balldin et al., 1993, Balldin et al., 1992).
Tiapride may be most effective in individuals who have the A/G or G/G genotype of rs71653615 of DRD2. During a combined 9-day detoxification therapy and abstinence-focused psychotherapy, alcohol dependent individuals with the A/G or G/G genotype required lower doses of tiapride to treat alcohol withdrawal symptoms (Lucht et al., 2001). These individuals with the A/G or G/G genotype also self-reported lower anxiety and depression at admission and two weeks later (Lucht et al., 2001). The overwhelming majority of subjects were male (93%), however. Further, the interpretation of the findings cannot be completely attributed to tiapride since abstinence-focused psychotherapy was administered concurrently with tiapride.
Levodopa/Carbidopa
Levodopa (administered with carbidopa; L-dopa) increases dopamine availability, which is hypothesized to stabilize neural reward circuits (Koob, 2008). After repeated use of cocaine, dopamine stores are depleted (Volkow et al., 2011). Although direct administration of dopamine is not possible, administration of L-dopa in combination with carbidopa (a peripheral decarboxylase inhibitor) can increase levels of synaptic dopamine in the brain, and lower caudate and putamen [11C]raclopride binding potentials (Pavese et al., 2006, Tedroff et al., 1996).
In a recent 12-week randomized double-blind parallel group study, cocaine-dependent individuals were given L-dopa/carbidopa (400/100mg, twice daily) or placed in a placebo group (Liu et al., 2014). Subjects who were given L-dopa/carbidopa were genotyped for DBH rs1611115. T allele carrier subjects with low DβH expression had decreased cocaine-positive urines relative to placebo-treated subjects carrying a T allele. This DβH-moderated response to L-dopa/carbidopa was not observed in subjects with normal levels of DβH (CC genotype). The majority of subjects in this study were also male (ranged from 66-95% male in each genotype group).
Naltrexone
Naltrexone is a non-specific opioid antagonist that has been demonstrated to reduce alcohol drinking in animal models and humans (Carmen et al., 2004, Volpicelli et al., 1986), and has been approved by the U.S. Food and Drug Administration for the treatment of alcohol dependence since 1994. One of the most consistent findings regarding naltrexone has been the reduction in relapse of heavy drinking (Carmen et al., 2004). Additionally, decreases in heavy drinking days, overall drinking, and craving have been found (Garbutt, 2010).
Although naltrexone has been shown to be efficacious in the treatment of alcohol abuse (Antonet al., 2004, Anton et al., 2008), not all alcohol-dependent individuals benefit. It has been shown that A118G rs1788821 variant alters response to naltrexone treatment for alcoholism (Anton et al., 2008, Oslin et al., 2003). In particular, carriers of the less common OPRM1 118G allele responded better to naltrexone treatment. Studies have demonstrated that the 118G allele codes for a receptor that is expressed at lower levels (Kroslak et al., 2007, Wand et al., 2002, Zhang et al., 2005), but binds β-endorphins with a higher affinity (Bond et al., 1998). In a study of non-treatment-seeking individuals with alcohol dependence, subjects were randomized to naltrexone or placebo for seven days and the number of drinks consumed were measured (Anton et al., 2012). Subjects who were DAT1 9 repeat VNTR carriers and were homozygous for the OPRM1 118A allele had reduced drink consumption under natural conditions in response to naltrexone treatment. Those subjects who were DAT1 10/10 repeat homozygotes and OPRM1 118A homozygotes did not reduce drinking in response to naltrexone.
Methadone
Methadone is a long-acting synthetic μ-opioid receptor agonist metabolized in the liver by CYP3A4, CYP2D6, and CYP1A2 (Kreek et al., 2005a) and is used to treat opioid-dependent individuals (Dole et al., 1966, Faggiano et al., 2003, Mattick et al., 2009). Treatment with methadone or methadone maintenance therapy has been successful in reducing illicit opioid use, criminal behavior, and HIV risk (Marsch, 1998), as well as decreasing mortality rates due to opioid overdose (Cornish et al., 2010, Degenhardt et al., 2009, Gibson et al., 2008, Kimber et al., 2010). As a result, methadone maintenance therapy is the most common opioid maintenance therapy in the United States and Canada (Kurdyak et al., 2012).
Studies demonstrate that the effective methadone maintenance dosage may vary by the presence of DRD2 −214A>G alleles. In an analysis of heroin dependent subjects stabilized on low (<55 mg/day), medium (55-99 mg/day), or high (100-150 mg/day) methadone maintenance dose those A allele carriers were two times more likely to require a lower methadone dose than those were who were GG homozygotes (Hung et al., 2011). In addition, patients who were carriers of the CCACT, CTACT, or TCAAC haplotypes of ANKK1 2137C>T, DRD2 32+14266C>T, −214A>G, 811-83C>A, or the 939C>T allele required lower methadone doses than did noncarriers of these haplotypes. When combined in proportional odds regression analysis and controlling for liver function, height, weight, and HIV infection, DRD2, ANKK1, ABCB1, CYP2B6, and OPRM1 variants together best predicted ideal methadone dosage suggesting that methadone dose is not related only to a single gene but is polygenetic. Importantly, this was the only study to examine the potential polygenetic effect of pharmacotherapy for drug addiction. Additionally, this study was conducted with a large sample size (N = 321) that was more than three times the sample size of other studies reviewed. Similar to the other reviewed studies, the majority of subjects were male (76-81% male).
Discussion
The dopaminergic system plays a prominent role in the development and treatment of substance abuse. Since there is a clear role of dopamine, the pharmacogenetics literature examining the treatment implications and differential outcomes related to the dopaminergic system is burgeoning. The present systematic review reveals the promise, as well as complexity, of using an individual's genetic profile to guide pharmacotherapy selection.
It appears that many dopaminergic, as well as serotonergic and opioidergic genes, are involved in the specific individual responses to pharmacotherapy (Bauer et al., 2014, Nielsen et al., 2014a). This is highlighted by the results described above for methadone maintenance therapy that DRD2, ANKK1, ABCB1, CYP2B6, and OPRM1 together are related to the ideal methadone maintenance dosage. As such, pharmacotherapy for substance use may be polygeneic with multiple genes playing a role in an individual's response to pharmacotherapy or oligogenic with a few genes playing a significant role in an individual's response to pharmacotherapy. Although dopaminergic genes play a significant role in the response to pharmacotherapy, investigations of broader systems such as serotonergic and opioidergic genes may define a more comprehensive genetic profile that better predicts individualized response to pharmacotherapies.
When genes interact, they may have variants that mask certain effects that would be expressed (epistasis). Variants only may be expressed (or masked) in specific combinations in certain individuals, thus changing the effectiveness of pharmacotherapy in these patients. To statistically quantify these effects, relevant genes for substance use disorders including serotonergic (e.g., SLC6A4, TPH2; for review see Nielsen et al., 2014b), opioidergic (e.g., OPRM1, OPRD1, OPRK1; for review see Bauer et al., 2014, Nielsen et al., 2014b), dopaminergic (e.g., DRD4, ANKK1, DRD2, DBH), and other relevant genes (e.g., brain-derived neurotrophic factor) could be examined via multivariate statistical methods (e.g., structural equation modeling, hierarchical linear regression). The identification of gene patterns and other multivariate approaches would quantitatively identify the genes comprising the polygeneic or oligogenic profiles related to pharmacotherapy outcomes.
Due to the emerging field of pharmacogenetics, the current studies reviewed have limitations, including small sample sizes, low statistical power, are comprised of predominately male subjects, and have short length of treatment. Outcome variables also differed. Some studies utilized primarily subjective variables of drug use (e.g., self-reported cravings, stimulation, sedation), whereas other studies used only objective measures (e.g., cocaine positive urines). Due to publication bias (i.e., only studies with significant results are typically published), studies that failed to produce significant findings were not included. It is also important to fully disclose all analyses conducted and account for increased Type I error rates.
Future studies will need to improve upon these limitations by recruiting larger cohorts as practical by utilizing robust recruitment strategies. This is important when examining polymorphisms with low allele frequencies or small effect sizes. Effect sizes for the current studies reviewed ranged from small (eta squared = .012; Spellicy et al., 2013) to large (eta squared = .6268; Hung et al., 2011). To inform future research design, we conducted power analyses with using G*Power software (Faul et al., 2009) for a 2 group × 4 measurement ANOVA using small (η2 = .01), medium (η2 = .06), and large (η2 = .14) effect sizes (Cohen, 1988). Table 3 shows the corresponding sample sizes required in order to obtain a power of .80. For example, a cohort of 778 participants would be needed to obtain a power of .80 for a 2 × 4 ANOVA with a small effect size (η2 = .01). Power analyses, such as the ones conducted in Table 3, should be conducted during study design.
Table 3.
Power analysis for small (η2 = .01), medium (η2 = .06), and large (η2 = .14) effect sizes to determine adequate sample size for a 2-group, 4-measurement ANOVA with alpha = .05.
| Effect size (η2) | Power (1-β) | Total N-size |
|---|---|---|
| .01 | .80 | 778 |
| .06 | .80 | 126 |
| .14 | .80 | 52 |
| .01 | .90 | 1,044 |
| .06 | .90 | 168 |
| .14 | .90 | 68 |
| .01 | .95 | 1,290 |
| .06 | .95 | 206 |
| .14 | .95 | 82 |
Since many polymorphisms vary by ethnicity, it is also essential to control for population structure in future studies. The use of objective outcome variables (e.g., cocaine positive urines) will be necessary due to the responder bias present when self-reporting drug use. Additionally, an emerging area of investigation may find new dopaminergic genetic targets for substance abuse. For example, the dopamine D3 receptor recently was highlighted as a target for medications treating substance use disorders, particularly cocaine abuse (similar to analyses described in Kosten et al., 2013a, Kosten et al., 2013b, Spellicy et al., 2013). COMT, which metabolizes dopamine, has not been investigated in relation to cocaine, alcohol, or opioid addiction although it has been investigated in the pharmacological treatment of tobacco addiction (Keck et al., 2015). In combination with continued evidence from hypothesis- and theory-driven SNP studies, genome-wide association studies may identify important new genes from which hypotheses can be developed and tested. Further, pathway analyses may provide new computational approaches to identifying groups of genetic targets. For example, pathway analyses have already proved valuable in the identification of rare genetic variants that are associated with risk for bipolar disorder (e.g., Berrettini et al., 2007). New genetic targets and analyses for identification may be examined in future studies of the pharmacogenetics of cocaine, alcohol, and opioid addiction.
The studies reviewed herein illuminate the capability of genetics to inform a more precise, targeted approach to the pharmacotherapy of alcohol, cocaine, and opioid addiction. The heterogeneity regarding treatment response is, at least partially, moderated by the dopaminergic system and quantification of this intricate relationship is critical for establishing practices that ensure optimal treatment outcomes. Ultimately, it is hoped that genetic insights of pharmacotherapy interventions and outcomes will provide medical professionals with a patient-specific tool that ensures the best opportunity for successful remission.
Table 2.
Description of the nine studies examining the relationship between dopaminergic genes and treatment response to pharmacotherapies of drug addiction.
| Paper | Sample Characteristics N Mage±SD in years |
Gender | Addiction | Design | Outcomes | Results & Effect Size (η2) |
|---|---|---|---|---|---|---|
| Bromocriptine | ||||||
| Lawford et al., 1995 |
N = 83 43.7±1.3 |
94% male | Alcohol | 6-week, randomized, double-blind clinical trial; bromocriptine (2.5 mg three times daily) or placebo administered to A1/A1, A1/A2, A2/A2 genotypes | •Self-report craving •Self-report anxiety •Self-report depression •Attrition |
•Bromocriptine-treated A1/A1 group less craving and anxiety than any other group •Attrition highest in placebo-treated A1 group • η2 = data not available |
| Cocaine vaccine | ||||||
| Kosten et al., 2013a |
N = 114 Vaccine CT/TT: 34±9 CC: 35±10 Placebo CT/TT: 35±10 CC: 39±9 |
Vaccine CT/TT: 50% male CC: 72% male Placebo CT/TT: 60% male CC: 74% male |
Cocaine Opioids | 16-week, randomized, double-blind placebo-controlled trial; examined subjects with five vaccinations over first 12 weeks | •Cocaine-positive urines | •Vaccine group with low DβH level dropped use from 77% to 51% •Vaccine group with normal DβH level dropped from 83% to 72% • η2 = .0763-.3855 |
| Disulfiram | ||||||
| Kosten et al., 2013b |
N = 74 Disulfiram CT/TT: 37±10 CC: 38±11 Placebo CT/TT: 37±10 CC: 43±10 |
Disulfiram CT/TT: 53% male CC: 70% male Placebo CT/TT: 63% male CC: 71% male |
Cocaine Opioids | Subjects randomized into either disulfiram (250mg/d) or placebo groups for 10 weeks | •Cocaine-positive urines | •Normal DβH level dropped from 84% to 56% on disulfiram •Low DβH level showed no disulfiram effect • η2 = .1478-.1939 |
| Spellicy et al., 2013 |
N = 68 ANNK1/Disulfiram CT/TT: 39.7±9.5 CC: 37.8±11 ANNK1/Placebo CT/TT: 42.7±10.6 CC: 36.7±10.5 DRD2/Disulfiram GT/TT: 38.9±9.1 GG: 38.2±10.7 DRD2/Placebo GT/TT: 42.7±12 GG: 37.7±9.9 ANKK1/DRD2/Disulfiram CC/GG: 38.5±10.9 Others: 39.7±9.5 ANKK1/DRD2/Placebo CC/GG: 36.6±10.5 Others: 42.4±10.8 |
ANNK1/Disulfiram CT/TT: 61.5% male CC: 61.1% male ANNK1/Placebo CT/TT: 65.0% male CC: 70.6% male DRD2/Disulfiram GT/TT: 44.4% male GG: 69.5% male DRD2/Placebo GT/TT: 71.4% male GG: 68.2% male ANKK1/DRD2/Disulfiram CC/GG: 58.8% male Others: 68.4% male ANKK1/DRD2/Placebo CC/GG: 70.6% male Others: 61.5% male |
Cocaine Opioids | Subjects randomized into either disulfiram (250mg/d) or placebo groups, subjects also provided manual-based cognitive behavioral therapy | •Cocaine-positive urines | •CT or TT ANKK1 genotypes drop from 80 to 52% positive urines, no placebo effect, on disulfiram •GT/TT DRD2 genotype showed decrease positive urine on disulfiram, 67 to 48% •Carriers of at least one minor allele of DRD2 or ANKK1 responded better to disulfiram than those only carrying major alleles • η2 = .2204-.2692 |
| Olanzapine | ||||||
| Hutchison et al., 2003 |
N = 67 Olanzapine DRD4 S: 22.9±2.5 DRD4 L: 24.2±2.9 Cyproheptadine DRD4 S: 22.4±3.2 DRD4 L: 21.1±1.6 |
Olanzapine DRD4 S: 78% male DRD4 L: 50% male Cyproheptadine DRD4 S: 52% male DRD4 L: 66% male |
Alcohol | Subjects randomly assigned into either olanzapine (5 mg) or control medication (cyproheptadine, 4 mg) prior to ingesting three drinks of alcohol in one experimental session | •Self-report cravings •Self-report stimulation •Self-report sedation |
•Olanzapine reduced craving for alcohol at baseline for DRD4 S and DRD4 L •Reduced cravings after exposure to alcohol cues and drink for only DRD4 L subjects • η2 = .0665-.1403 |
| Tiapride | ||||||
| Lucht et al., 2001 |
N = 110 42.5±9.3 |
93% male | Alcohol | 9-day detoxification therapy and abstinence-focused psychotherapy | •Self-report anxiety •Self-report depression •Tiapride dosage |
•DRD2 E8 A/A genotype had increased dose of tiapride, as well as increased anxiety and depression at admission and 2 weeks later • η2 = .0336-.0405 |
| Levodopa/Carbidopa | ||||||
| Liu et al., 2014 |
N = 71 Levodopa CT/TT: 42.33±8.10 CC: 45.23±7.81 Placebo CT/TT: 41.77±6.89 CC: 49.95±41.77 |
Levodopa CT/TT: 66% male CC: 84% male Placebo CT/TT: 76% male CC: 95% male |
Cocaine | 12-week, randomized, double-blind parallel-group; levodopa/ carbidopa (400/100 mg, Sinemet CR, twice daily) or placebo groups | •Cocaine positive urines | • Low DβH level genotypes (CT/TT) odds of having positive urines significantly decreased over levodopa treatment relative with placebo subjects with CT/TT genotypes • Normal DβH level (CC) subjects showed no differences to levodopa • η2 = .1153 |
| Naltrexone | ||||||
| Anton et al., 2012 |
N = 83 Naltrexone Asn40: 31±7 Asp40: 29±10 Placebo Asn40: 23±10 Asp40: 28±10 |
Naltrexone Asn40: 68% male Asp40: 63% male Placebo Asn40: 67% male Asp40: 63% male |
Alcohol | Subjects randomized to naltrexone or placebo for 7 days | •# drinks consumed | •Subjects given naltrexone with a least one DAT1 9 VNTR and that were OPRM1 Asn40 homozygotes showed reduced alcoholic drinks consumed in natural setting • η2 = .0357 |
| Methadone | ||||||
| Hung et al., 2011 |
N = 321 36.5±18.7 |
Methadone dose Low: 78% male Medium: 81% male High: 76% male |
Opioids | Subjects received low (<55 mg/day), medium (55-99mg/day), and high dosages (100-150 mg/day) to obtain methadone maintenance dose | •Dosage of methadone required for maintenance | •A allele carriers had twofold increase chance of requiring lower methadone dose than those who were GG homozygotes • CCACT, CTACT, or TCAAC haplotypes of ANKK1 2137C>T, DRD2 32+14266C>T, −214A>G, 811-83C>A, or the 939C>T allele required lower methadone doses •ABCB1, CYP2B6, OPRM1, ANKK1, DRD2 correlated as a group with methadone dose • η2 = .0663-.6268 |
Acknowledgments
Supported by: McNair Medical Institute (MP), Pat Rutherford Jr. Chair in Psychiatry (UT Health; IB, JS), VA Rehabilitation Research & Development Center of Excellence (B6812C; DG, DN) and Career Development Award (B7496W; DG), NIH/NIDA 5 P50 DA018197-05 (DN), through MD Anderson's Cancer Center Support Grant NIH/NIDA DA026120 (DN), and the Toomim Family Fund (DN). J.C. Soares has been a speaker for Pfizer and Abbott. This material is the result of work supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX.
References
- Amara SG, Kuhar MJ. Neurotransmitter transporters: Recent progress. Annual Review of Neuroscience. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Ament SA, Szelinger S, Glusman G, Ashworth J, Hou L, Akula N, Shekhtman T, Badner JA, Brunkow ME, Mauldin DE. Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proceedings of the National Academy of Sciences. 2015;112:3576–3581. doi: 10.1073/pnas.1424958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistic Manual of Mental Disorders (DSM-5) American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: Temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: Results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of General Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: Influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcoholism: Clinical and Experimental Research. 2012;36:2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal of neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Reviews Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Christian S, Berkeljon A, Shadish WR. The effects of family therapies for adolescent delinquency and substance abuse. Journal of Marital and Family Therapy. 2012;38:281–304. doi: 10.1111/j.1752-0606.2011.00248.x. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berggren U, Lindstedt G, Sundkler A. Further neuroendocrine evidence for reduced D2 dopamine receptor function in alcoholism. Drug Alcohol Depend. 1993;32:159–162. doi: 10.1016/0376-8716(93)80008-3. [DOI] [PubMed] [Google Scholar]
- Balldin JI, Berggren UC, Lindstedt G. Neuroendocrine evidence for reduced dopamine receptor sensitivity in alcoholism. Alcoholism, clinical and experimental research. 1992;16:71–74. doi: 10.1111/j.1530-0277.1992.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Bauer IE, Soares JC, Nielsen DA. The role of opioidergic genes in the treatment outcome of drug addiction pharmacotherapy: A systematic review. The American Journal on Addictions. 2014 doi: 10.1111/ajad.12172. in press. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, Niaura R, Lerman C. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological Psychiatry. 2007;61:111–118. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Bhaduri N, Mukhopadhyay K. Correlation of plasma dopamine beta-hydroxylase activity with polymorphisms in DBH gene: A study on eastern Indian populaion. Cell Mol Neurobiol. 2008;28:343–350. doi: 10.1007/s10571-007-9256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Cull JG, Eisenberg A, Sherman M, Schnautz N, Fischer L, Mathews D, Comings DE. 109-5-Association of polymorphisms of dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes with schizoid/avoidant behaviors (SAB) and pathological violence (PV). Biological Psychiatry. 1997;42:296S. doi: 10.1038/sj.mp.4000261. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Finley O, Montgomery A, Ritchie T, Ozkaragoz T, Fitch RJ, Sadlack F, Sheffield D, et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–416. doi: 10.1016/0741-8329(91)90693-q. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–2060. [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong J, Leal S, Tischfield J, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proceedings of the National Academy of Sciences. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdélat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. Effects of dopamine β-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology. 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- Bourdy R, Barrot M. A new control center for dopaminergic systems: Pulling the VTA by the tail. Trends Neurosci. 2012;35:681–690. doi: 10.1016/j.tins.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Rasmussen K, Calligaro DO, Nelson DL, DeLapp NW, Wong DT, Moore NA. In vitro and in vivo biochemistry of olanzapine: A novel, atypical antipsychotic drug. The Journal of clinical psychiatry. 1997;58(Suppl 10):28–36. [PubMed] [Google Scholar]
- Carmen B, Angeles M, Ana M, María AJ. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction (Abingdon, England) 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: A randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction (Abingdon, England) 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Safety. 1999;20:427–435. doi: 10.2165/00002018-199920050-00003. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ.: 1988. [Google Scholar]
- Comings DE, Comings BG, Muhleman D, Dietz G, Shahbahrami B, Tast D, Knell E, Kocsis P, Baumgarten R, Kovacs BW, et al. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorders. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- Cook Jr EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, Leventhal BL. Association of attention-deficit disorder and the dopamine transporter gene. American journal of human genetics. 1995;56:993. [PMC free article] [PubMed] [Google Scholar]
- Cornish R, Macleod J, Strang J, Vickerman P, Hickman M. Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ. 2010;341:c5475. doi: 10.1136/bmj.c5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Naylor RJ. Neuropharmacological indications of an antidyskinetic potential for tiapride. Semaine des Hopitaux de Paris. 1977;58:72–76. [Google Scholar]
- Crime U.N.O.o.D.a. World Drug Report 2014. United Nations; Vienna, Austria: 2014. [Google Scholar]
- Cubells JF, Kranzler HR, McCance-Katz E, Anderson GM, Malison RT, Price LH, Gelernter J. A haplotype at the DBH locus, associated with low plasma dopamine beta-hydroxylase activity, also associates with cocaine-induced paranoia. Molecular psychiatry. 2000;5:56–63. doi: 10.1038/sj.mp.4000657. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: Risk factors and lives saved. Drug and Alcohol Dependence. 2009;105:9–15. doi: 10.1016/j.drugalcdep.2009.05.021. [DOI] [PubMed] [Google Scholar]
- deLeon J, Armstrong SC, Cozza KL. Clinical guidelines for psychiatrists for the use of pharmacogenetic testing for CYP450 and CYP450 2C19. Psychosomatics. 2006;47:75–85. doi: 10.1176/appi.psy.47.1.75. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehring A, Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G, Harder S, Lotsch J. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenetics and genomics. 2009a;19:407–414. doi: 10.1097/FPC.0b013e328320a3fd. [DOI] [PubMed] [Google Scholar]
- Doehring A, von Hentig N, Graff J, Salamat S, Schmidt M, Geisslinger G, Harder S, Lötsch J. Genetic variants altering dopamine D2 receptor expression or function modulate the risk of opiate addiction and the dosage requirements of methadone substitution. Pharmacogenetics and genomics. 2009b;19:407–414. doi: 10.1097/FPC.0b013e328320a3fd. [DOI] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Archives of Internal Medicine. 1966;118:304–309. [PubMed] [Google Scholar]
- Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, Gejman PV. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;3:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Franke P, Schwab SG, Knapp M, Gänsicke M, Delmo C, Zill P, Trixler M, Lichtermann D, Hallmayer J, Wildenauer DB. DAT1 gene polymorphism in alcoholism: a family-based association study. Biological Psychiatry. 1999;45:652–654. doi: 10.1016/s0006-3223(98)00135-8. [DOI] [PubMed] [Google Scholar]
- Garbutt J. Efficacy and tolerability of naltrexone in the management of alcohol dependence. Current Pharmaceutical Design. 2010;16:2091–2097. doi: 10.2174/138161210791516459. [DOI] [PubMed] [Google Scholar]
- Garrido E, Palomo T, Ponce G, Garcia-Consuegra I, Jimenez-Arriero MA, Hoenicka J. The ANKK1 protein associated with addictions has nuclear and cytoplasmic localization and shows a differential response of Ala239Thr to apomorphine. Neurotoxicity research. 2011;20:32–39. doi: 10.1007/s12640-010-9219-6. [DOI] [PubMed] [Google Scholar]
- Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O'Brien S. Exposure to opioid maintenance treatment reduces long- term mortality. Addiction (Abingdon, England) 2008;103:462–468. doi: 10.1111/j.1360-0443.2007.02090.x. [DOI] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends in pharmacological sciences. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Nakajima K. The effect of disulfiram on catecholamine levels in the brain. Journal of Pharmacology and Experimental Therapeutics. 1967;157:96–102. [PubMed] [Google Scholar]
- Gupta S, Jain S, Brahmachari SK, Kukreti R. Pharmacogenomics: A path to predictive medicine for schizophrenia. Pharmacogenomics. 2006;7:31–47. doi: 10.2217/14622416.7.1.31. [DOI] [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: Cocaine, amphetamine and methamphetamine. The American journal of drug and alcohol abuse. 2009;35:161–177. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Gu H. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacology. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biological Psychiatry. 2010;67:59. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature reviews. Neuroscience. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen MM, Lumme V, Hirvonen J, Pesonen U, Nagren K, Vahlberg T, Scheinin H, Hietala J. C957T polymorphism of the human dopamine D2 receptor gene predicts extrastriatal dopamine receptor availability in vivo. Progress in neuro psychopharmacology & biological psychiatry. 2009;33:630–636. doi: 10.1016/j.pnpbp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Horgan C, Skwara KC, Strickler G, Andersen L. Substance Abuse: The Nation's Number One Health Problem. Schneider Institute for Health Policy, Brandeis University (prepared for the Robert Wood Johnson Foundation); Waltham, MA.: 2001. [Google Scholar]
- Hung C-C, Chiou M-H, Huang B-H, Hsieh Y-W, Hsieh T-J, Huang C-L, Lane H-Y. Impact of genetic polymorphisms in ABCB1, CYP2B6, OPRM1, ANKK1 and DRD2 genes on methadone therapy in Han Chinese patients. Pharmacogenomics. 2011;12:1525–1533. doi: 10.2217/pgs.11.96. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Smolen A, Bryan A, Swift RM. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health psychology : official journal of the Division of Health Psychology. American Psychological Association. 2002;21:139–146. [PubMed] [Google Scholar]
- Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, Paris L. Olanzapine reduces craving for alcohol: a DRD4 VNTR polymorphism by pharmacotherapy interaction. Neuropsychopharmacology. 2003;28:1882–1888. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. The Journal of pharmacology and experimental therapeutics. 1986;239:219–228. [PubMed] [Google Scholar]
- Inada T, Sugita T, Dobashi I, Inagaki A, Kitao Y, Matsuda G, Kato S, Takano T, Yagi G, Asai M. Dopamine transporter gene polymorphism and psychiatric symptoms seen in schizophrenic patients at their first episode. American Journal of Medical Genetics. 1996;67:406–408. doi: 10.1002/(SICI)1096-8628(19960726)67:4<406::AID-AJMG15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Iversen LL. Role of transmitter uptake mechanisms in synaptic neurotransmission. British Journal of Pharmacology. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik ME, Caskey NH, Wirshing WC, Madsen DC, Iwamoto-Schaap PN, Elins JL, Eisenberger NI, Olmstead RE. Bromocriptine reduces cigarette smoking. Addiction (Abingdon, England) 2000;95:1173–1183. doi: 10.1046/j.1360-0443.2000.95811734.x. [DOI] [PubMed] [Google Scholar]
- Jenner P, Marsden CD. The substituted benzamides-a novel class of dopamine antagonists. Life Sciences. 1979;25:479–486. doi: 10.1016/0024-3205(79)90559-9. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Jones C, Wilson AA, DaSilva JD, Houle S. Cyproheptadine: A potent in vivo serotonin antagonist. The American Journal of Psychiatry. 1997;154:884–884. doi: 10.1176/ajp.154.6.884a. [DOI] [PubMed] [Google Scholar]
- Karamanakos PN, Pappas P, Stephanou P, Marselos M. Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacology & Toxicology. 2001;88:106–110. [PubMed] [Google Scholar]
- Kaufman S, Friedman S. Dopamine-Beta-Hydroxylase. Pharmacol Rev. 1965;17:71–100. [PubMed] [Google Scholar]
- Keck TM, John WS, Czoty PW, Nader MA, Newman AH. Identifying Medication Targets for Psychostimulant Addiction: Unraveling the Dopamine D3 Receptor Hypothesis. Journal of Medicinal Chemistry. 2015 doi: 10.1021/jm501512b. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber J, Copeland L, Hickman M, Macleod J, McKenzie J, De Angelis D, Robertson JR. Survival and cessation in injecting drug users: Prospective observational study of outcomes and effect of opiate substitution treatment. BMJ. 2010;341:c3172. doi: 10.1136/bmj.c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends in pharmacological sciences. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB, Hamon SC, Nielsen DA. DBH gene as predictor of response in a cocaine vaccine clinical trial. Neurosci Lett. 2013a;541:29–33. doi: 10.1016/j.neulet.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. Pharmacogenetic randomized trial for cocaine abuse: disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013b;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005a;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005b;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kroslak T, LaForge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. Journal of neurochemistry. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- Kurdyak P, Gomes T, Yao Z, Mamdani MM, Hellings C, Fischer B, Rehm J, Bayoumi AM, Juurlink DN. Use of other opioids during methadone therapy: A population-based study. Addiction (Abingdon, England) 2012;107:776–780. doi: 10.1111/j.1360-0443.2011.03707.x. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. The D(2) dopamine receptor A(1) allele and opioid dependence: Association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96:592–598. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Rowell JA, Qualichefski J, Fletcher BH, Syndulko K, Ritchie T, Noble EP. Bromocriptine in the treatment of alcoholics with the D2 dopamine receptor A1 allele. Nature medicine. 1995;1:337–341. doi: 10.1038/nm0495-337. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, Leighton PW, McCann SJ, Pond SM. Association of a polymorphism in the dopamine-transporter gene with parkinson's disease. Movement Disorders. 1997;12:760–763. doi: 10.1002/mds.870120523. [DOI] [PubMed] [Google Scholar]
- Liu S, Green CE, Lane SD, Kosten TR, Moeller FG, Nielsen DA, Schmitz JM. The influence of dopamine β-hydroxylase gene polymorphism rs1611115 on levodopa/carbidopa treatment for cocaine dependence: a preliminary study. Pharmacogenetics and Genomics. 2014;24:370–373. doi: 10.1097/FPC.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Lucht MJ, Kuehn KU, Schroeder W, Armbruster J, Abraham G, Schattenberg A, Gaensicke M, Barnow S, Tretzel H, Herrmann FH, Freyberger HJ. Influence of the dopamine D2 receptor (DRD2) exon 8 genotype on efficacy of tiapride and clinical outcome of alcohol withdrawal. Pharmacogenetics. 2001;11:647–653. doi: 10.1097/00008571-200111000-00002. [DOI] [PubMed] [Google Scholar]
- MacGowan RJ, Fichtner RR, Swanson N, Collier C, Kroliczak A, Cole G. Factors associated with client-reported HIV infection among clients entering methadone treatment. AIDS Educ Prev. 1997;9:205–217. [PubMed] [Google Scholar]
- Maier W, Minges J, Eckstein N, Brodski C, Albus M, Lerer B, Hallmayer J, Fimmers R, Ackenheil M, Ebstein RE. Genetic relationship between dopamine transporter gene and schizophrenia: linkage and association. Schizophrenia Research. 1996;20:175–180. doi: 10.1016/0920-9964(95)00083-6. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Murphy GM, Jr., Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161:780–796. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality. Addiction (Abingdon, England) 1998;93:515–532. doi: 10.1046/j.1360-0443.1998.9345157.x. [DOI] [PubMed] [Google Scholar]
- Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang D-R, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. American Journal of Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- Meandzija B, O'Connor PG, Fitzgerald B, Rounsaville BJ, Kosten TR. HIV infection and cocaine use in methadone maintained and untreated intravenous drug users. Drug Alcohol Depend. 1994;36:109–113. doi: 10.1016/0376-8716(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Mercier G, Turpin JC, Lucotte G. Variable number tandem repeat dopamine transporter gene polymorphism and Parkinson's disease: No association found. Journal of Neurology. 1999;246:45–47. doi: 10.1007/s004150050304. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, Dewit DJ. Comorbidity of substance use disorders with mood and anxiety disorders: results of the International Consortium in Psychiatric Epidemiology. Addictive Behaviors. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Gibson LE, Seidman LJ, Green AI. Schizophrenia and co occurring substance use disorder: Reward, olfaction and clozapine. Schizophrenia Research. 2014;155:45–51. doi: 10.1016/j.schres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Meyers JL, Nyman E, Loukola A, Rose RJ, Kaprio J, Dick DM. The association between DRD2/ANKK1 and genetically informed measures of alcohol use and problems. Addiction biology. 2013;18:523–536. doi: 10.1111/j.1369-1600.2012.00490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Madras BK. Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Molecular psychiatry. 2001;7:44–55. doi: 10.1038/sj.mp.4000921. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, Sadee W. Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology. 2011;36:753–762. doi: 10.1038/npp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T, Higuchi S. Dopamine transporter gene polymorphism and alcoholism. Biochemical and biophysical research communications. 1995;211:28–32. doi: 10.1006/bbrc.1995.1773. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr., Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. Am J Psychiatry. 2003;160:1830–1835. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- Musacchio J, Goldstein M, Anagnoste B, Poch G, Kopin I. Inhibition of dopamine-beta-hydroxylase by disulfiram in vivo. Journal of Pharmacology and Experimental Therapeutics. 1966;152:56–61. [PubMed] [Google Scholar]
- Nielsen DA, Nielsen EM, Dasari T, Specllicy CJ. Pharmacogenetics of addiction therapy. In: Yan Q, editor. Pharmacogenomics in Drug Discovery and Development. Springer; New York: 2014a. pp. 590–624. [Google Scholar]
- Nielsen DA, Nielsen EM, Dasari T, Spellicy CJ. Pharmacogenomics in Drug Discovery and Development. Springer; New York: 2014b. Pharmacogenetics of addiction therapy. pp. 589–624. [DOI] [PubMed] [Google Scholar]
- Noble EP. Polymorphisms of the D2 dopamine receptor gene and alcoholism and other substance use disorders. Alcohol Alcohol Suppl. 1994;2:35–43. [PubMed] [Google Scholar]
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nature Reviews Neuroscience. 2015 doi: 10.1038/nrn3939. Advanced online publication. [DOI] [PubMed] [Google Scholar]
- Organization WH. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. World Health Organization; Geneva: 1992. [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Oxenstierna G, Edman G, Iselius L, Oreland L, Ross SB, Sedvall G. Concentrations of monoamine metabolites in the cerebrospinal fluid of twins and unrelated individuals--a genetic study. J Psychiatr Res. 1986;20:19–29. doi: 10.1016/0022-3956(86)90020-8. [DOI] [PubMed] [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, Piccini P. Clinical correlates of levodopa-induced dopamine release in Parkinson disease A PET study. Neurology. 2006;67:1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Peters DH, Faulds D. Tiapride. A review of its pharmacology and therapeutic potential in the management of alcohol dependence syndrome. Drugs. 1994;47:1010–1032. doi: 10.2165/00003495-199447060-00009. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction (Abingdon, England) 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Wurst FM, Ridinger M, Rujescu D, Fehr C, Koller G, Bondy B, Wodarz N, Soyka M, Zill P. Association of functional DBH genetic variants with alcohol dependence risk and related depression and suicide attempt phenotypes: Results from a large multicenter association study. Drug Alcohol Depend. 2013;133:459–467. doi: 10.1016/j.drugalcdep.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Quality C.f.B.H.S.a. 2013 National Survey on Drug Use and Health (NSDUH) Substance Abuse and Mental Health Service Administration; Rockville, MD.: 2014. [Google Scholar]
- Rasic D, Weerasinghe S, Asbridge M, Langille DB. Longitudinal associations of cannabis and illicit drug use with depression, suicidal ideation and suicidal attempts among Nova Scotia high school students. Drug and Alcohol Dependence. 2013;129:49–53. doi: 10.1016/j.drugalcdep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Rogers JF, Nafziger AN, Bertino JS., Jr. Pharmacogenetics affects dosing, efficacy, and toxicity of cytochrome P450-metabolized drugs. The American journal of medicine. 2002;113:746–750. doi: 10.1016/s0002-9343(02)01363-3. [DOI] [PubMed] [Google Scholar]
- Sander T, Harms H, Podschus J, Finckh U, Nickel B, Rolfs A, Rommelspacher H, Schmidt LG. Allelic association of a dopamine transporter gene polymorphism in alcohol dependence with withdrawal seizures or delirium. Biological Psychiatry. 1997;41:299–304. doi: 10.1016/s0006-3223(96)00044-3. [DOI] [PubMed] [Google Scholar]
- Sano A, Kondoh K, Kakimoto Y, Kondo I. A 40-nucleotide repeat polymorphism in the human dopamine transporter gene. Human genetics. 1993;91:405–406. doi: 10.1007/BF00217369. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Review dopamine signals for reward value and risk: basic and recent data. Behavioral and Brain Functions. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Spellicy CJ, Kosten TR, Hamon SC, Harding MJ, Nielsen DA. ANKK1 and DRD2 pharmacogenetics of disulfiram treatment for cocaine abuse. Pharmacogenetics and genomics. 2013;23:333–340. doi: 10.1097/FPC.0b013e328361c39d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LC, Klinman JP. Dopamine beta-hydroxylase of adrenal chromaffin granules: Structure and function. Annual review of biochemistry. 1988;57:551–592. doi: 10.1146/annurev.bi.57.070188.003003. [DOI] [PubMed] [Google Scholar]
- Tedroff J, Pedersen M, Aquilonius SM, Hartvig P, Jacobsson G, Långström B. Levodopa- induced changes in synaptic dopamine in patients with Parkinson's disease as measured by [11C] raclopride displacement and PET. Neurology. 1996;46:1430–1430. doi: 10.1212/wnl.46.5.1430. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Sher KJ, Minks-Brown C, Durbin J, Burr R. Borderline personality disorder and substance use disorders: A review and integration. Clinical Psychology Review. 2000;20:235–253. doi: 10.1016/s0272-7358(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, Kennedy J, Seeman P, Niznik HB, Jovanovic V. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15. 3 and displays a VNTR. Genomics. 1992a;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Uhl GR. A human dopamine transporter cDNA predicts reduced glycosylation, displays a novel repetitive element and provides racially-dimorphic TaqI RFLPs. Molecular Brain Research. 1992b;15:161–166. doi: 10.1016/0169-328x(92)90165-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F. Addiction: Beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, clinical and experimental research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Davis MA, Olgin JE. Naltrexone blocks the post-shock increase of ethanol consumption. Life Sciences. 1986;38:841–847. doi: 10.1016/0024-3205(86)90601-6. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM. Serum dopamine beta-hydroxylase. Pharmacol Rev. 1978;30:133–166. [PubMed] [Google Scholar]
- Weinshilboum RM, Raymond FA, Elveback LR, Weidman WH. Serum dopamine-beta-hydroxylase activity: Sibling-sibling correlation. Science. 1973;181:943–945. doi: 10.1126/science.181.4103.943. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. The Journal of pharmacology and experimental therapeutics. 1993;267:250–258. [PubMed] [Google Scholar]
- Wilens TE, Zulauf CA. Mental Health in Adolescents: Bipolar Disorder. Future Medicine Ltd; London: 2014. Substance use in youth with bipolar disorder. pp. 57–74. [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug and Alcohol Dependence. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wolf BC, Lavezzi WA, Sullivan LM, Flannagan LM. Methadone-related deaths in Palm Beach County. J Forensic Sci. 2004;49:375–378. [PubMed] [Google Scholar]
- Xie X, Xu L, Liu H, Chen W, Zhuang D, Zhang J, Duan S, Zhou W. Positive association between--1021TT genotype of dopamine beta hydroxylase gene and progressive behavior of injection heroin users. Neurosci Lett. 2013;541:258–262. doi: 10.1016/j.neulet.2013.02.049. [DOI] [PubMed] [Google Scholar]
- Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. American journal of human genetics. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabetian CP, Buxbaum SG, Elston RC, Kohnke MD, Anderson GM, Gelernter J, Cubells JF. The structure of linkage disequilibrium at the DBH locus strongly influences the magnitude of association between diallelic markers and plasma dopamine beta-hydroxylase activity. American journal of human genetics. 2003;72:1389–1400. doi: 10.1086/375499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadee W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. Journal of Biological Chemistry. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]