Abstract
Vascular and glial involvement in the development of neurodegenerative disorders, such as Alzheimer’s disease (AD), and age-related brain vulnerabilities has been suggested. Therefore, we sought to: i) investigate which vascular and glial events are evident in ageing and/or AD, ii) to establish the temporal evolution of vascular and glial changes in AD-like and wild-type (WT) mice and iii) to relate them to amyloid-β (Aβ) accumulation. We examined immunohistochemically hippocampi and cortex from APP/PS1dE9 and WT C57BL/6 mice along ageing and disease progression (young-adulthood, middle- and old-age). Ageing resulted in the increase in receptor for advanced glycation endproducts expression, as well as the entrance of thrombin and albumin in hippocampus parenchyma. In contrast, the loss of platelet-derived growth factor receptor-β (PDGFR-β) positive cells, in both regions, was only related to AD pathogenesis. Hypovascularization was affected by both ageing and AD in the hippocampus, but resulted from the interaction between both factors in the cortex. Astrogliosis was a result of AD in hippocampus and by both factors in cortex, while microgliosis was associated with fibrillar amyloid plaques in AD-like mice and with the interaction between both factors in each of the studied regions. In sum, these data show that senile plaques precede vascular and glial alterations just in hippocampus, whereas in cortex, vascular and glial alterations, namely loss of PDGFR-β-positive cells and astrogliosis, accompanied the first senile plaques. Hence, this study points to vascular and glial events that co-exist with AD pathogenesis and age-related brain vulnerabilities.
Keywords: Alzheimer’s disease, blood-brain barrier disruption, glial activation, microvasculature, pericytes, receptor for advanced glycation endproducts
1. Introduction
Alzheimer’s disease (AD) is an age-related neurodegenerative disease characterized by intracellular neurofibrillary tangles of hyperphosphorylated tau (tauopathy), amyloid-β peptide (Aβ) extracellular aggregates (amyloidopathy), oxidative stress, inflammation and premature neuronal apoptosis (Serrano-Pozo et al., 2011). In the last few years, the relationship between the accumulation of Aβ, neuronal atrophy, glial activation and vascular dysfunction has been the target of multiple studies, as evidence grows to suggest that all of these events may possibly contribute to development of AD (Armstrong, 2013; Zlokovic, 2011). In fact, mice overexpressing amyloid precursor protein (APP) and presenting high levels of Aβ have a profound disruption of cerebrovascular autoregulation, an essential mechanism to maintain cerebral blood flow relatively constant, suggesting that cerebrovascular alterations may play a role in the mechanisms of AD (Niwa et al., 2002). Multiple studies have shown that cerebrovascular dysfunction, namely blood-brain barrier (BBB) disruption, and cerebral blood flow and capillary density alterations, may lead to faulty Aβ clearance from the brain (Deane et al., 2004a; Deane et al., 2004b), augmented influx of peripheral Aβ through the BBB (Eisele et al., 2010) and overexpression of APP (Kumar-Singh et al., 2005; Weller et al., 2008). Thus, more studies are required to understand the temporal relationship between vascular damage and glial activation, and whether neuronal dysfunction begins before or after the accumulation of Aβ and vascular damage. Furthermore, ageing is known to contribute to the compromise of BBB properties and to alterations of the microvasculature, which in turn contribute to age-related cognitive decline possibly due to their influence on neuronal and glial activity (Marques et al., 2013). Recently, a study of elderly individuals with normal cognition to mild dementia manifesting vascular disease showed that vascular brain injury was more influential than Aβ to mild cognitive dysfunction (Marchant et al., 2013), which is particularly interesting because AD is not a vascular dementia. Hence, considering that ageing is the greatest risk factor for AD (Akinyemi et al., 2013) it is important to understand which are the age-associated brain alterations, in order to modulate or prevent them.
In this study we sought to establish the glial and vascular events that are prevalent in ageing and/or AD. Moreover, we wanted to understand how glial and vascular profiles evolve during ageing and AD progression. The results presented here reveal vascular and glial alterations occurring along ageing, thus contributing to a better understanding of age-related brain vulnerabilities. Moreover, by establishing the temporal sequence of events occurring in AD and identifying the early ones this study points to potential therapeutic targets to counteract AD development and progression.
2. Results
2.1. Endothelial changes during ageing in WT and in AD-like APP/PS1 mice
Based on previous studies suggesting that ageing of healthy individuals and AD patients are both accompanied by alterations in endothelial cells (Farkas and Luiten, 2001), we investigated the temporal evolution of blood vessel density (Fig. 1) and microvascular immunoreactivity for the receptor for advanced glycation endroducts (RAGE) (Fig. 2) in hippocampus and cortex of WT and APP/PS1 mice. Analysis of the microvessel density of WT animals showed a 35% (P<0.05) and 46% (P<0.05) decrease from middle-age to old age in hippocampus (Fig. 1A,C) and cortex (Fig. 1B,D), respectively. Regarding APP/PS1 animals, no significant loss of microvessel density in hippocampus was observed along time (Fig. 1C), while in cortex a significant reduction of 40% from young adulthood to middle-age was observed (P<0.01, Fig. 1D). Therefore, the results obtained in the cortex show that the loss of microvascularization occurs earlier in AD, where a decline was observed from young adulthood to middle-age (P<0.01), than in WT, where the decrease was only observed from middle to old age mice (P<0.05). This fact supports that the biggest difference between both genotype groups was observed by middle-age, where APP/PS1 mice had significantly less (P<0.01 in hippocampus and P<0.05 in cortex) microvessel density than WT mice. Remarkably, by old age both APP/PS1 and WT mice presented similar microvessel density in both regions (Fig. 1C,D). Thus, both ageing and AD contributed to hypovascularization in hippocampus (two-way ANOVA F 6.012, P<0.05 and F 13.86, P<0.01, respectively), whereas in cortex it was a result of the interaction between ageing and AD (two-way ANOVA interaction AD × ageing F 3.733, P<0.05).
Fig. 1.
Microvascular density changes associated with Alzheimer’s disease (AD) and ageing in hippocampus and cortex. Brain sections of APP/PS1 transgenic mice, a mouse model of AD, and C57BL/6 wild-type (WT) mice at 6, 12–14 and 23–28 months (young adulthood, middle-age and old mice, respectively) were processed for immunohistochemical analysis of the endothelial marker, cluster of differentiation 31 (CD31). Representative immunohistological patterns of CD31 in hippocampus (A) and cortex (B), where arrows point to CD31 positive microvessels. Semi-quantitative analysis of the area of blood vessels per brain area in hippocampus (C) and cortex (D). *P<0.05, **P<0.01 between indicated groups; #P<0.05, ##P<0.01 vs. age-matched WT.
Fig. 2.
Endothelial receptor for advanced glycation end products (RAGE) changes associated with Alzheimer’s disease (AD) and ageing in hippocampus and cortex. Brain sections of APP/PS1 transgenic mice, a mouse model of AD, and C57BL/6 wild-type (WT) mice at 6, 12–14 and 23–28 months (young adulthood, middle-age and old, respectively) were processed for immunohistochemical analysis of endothelial expression of RAGE. Representative immunohistological pattern of RAGE in hippocampus (A) and cortex (B), where arrows point to RAGE positive microvessels. Semi-quantitative analysis of RAGE immunoreactivity in endothelial cells in hippocampus (C) and cortex (D). *P<0.05, **P<0.01 between indicated groups.
Analysis of RAGE expression by endothelial cells of WT animals showed an increase of the immunoreactivity per µm2 of blood vessel over time, and that this increase was already apparent at middle age in the hippocampus (24% vs. young adult, P<0.01, Fig. 2C), and only at old age in the cortex (21% vs young adult, P<0.05, Fig. 2D). The APP/PS1 animals also showed a 13% increase in RAGE immunoreactivity in hippocampus (P<0.05 young adulthood vs. middle-age, Fig. 2C) and, even though not significant, a 14% increase was observed in cortex (young adult vs. middle-age, Fig. 2D). Interestingly, the comparison of endothelial RAGE immunoreactivity between WT and APP/PS1 animals showed no difference in both regions. Accordingly, ageing was the major contributor for the alterations in RAGE immunoreactivity by endothelial cells in hippocampus (two-way ANOVA F 23.13, P<0.001) and cortex (two-way ANOVA F 9.874, P<0.001). In summary, endothelial-related changes indicate that APP/PS1 mice have a premature loss of capillary density compared to WT mice, but similar immunoreactivity of RAGE by endothelial cells with ageing.
2.2. Pericyte alterations during ageing in WT and in AD-like APP/PS1 mice
We wanted to know if ageing and/or AD affect blood vessels integrity by altering pericyte vascular coverage. For that purpose, we analyzed the widely used marker of pericytes, platelet-derived growth factor receptor-β (PDGFR-β) (Bell et al., 2010; Sá-Pereira et al., 2012) along ageing and/or AD (Fig. 3). Analysis of PDGFR-β-positive cells in hippocampus (Fig. 3A,C) and cortex (Fig 3B,D) showed that in cortex there was nearly a triple number of PDGFR-β-positive cells per field than in the hippocampus. WT mice did not show a significant alteration of the number of positive cells in either brain region with ageing, and the same was observed APP/PS1 mice (Fig. 3C,D). When WT were compared with APP/PS1 mice at each age, no significant difference was observed in the hippocampus (Fig. 3C), whereas in the cortex APP/PS1 mice had fewer immune-labeled cells than WT (Fig. 3D). In fact, by young adulthood APP/PS1 had already 32% (P<0.01) fewer PDGFR-β-positive cells in cortex than WT mice (Fig. 3D), a difference that was preserved during middle-age (P<0.01). Accordingly, the major factor contributing to the loss of PDGFR-β in hippocampus and cortex was not ageing, but AD (two-way ANOVA F<14.95, P<0.001 and F<27.31, P<0.001, respectively).
Fig. 3.
Changes in the expression of platelet-derived growth factor receptor β (PDGFRβ) by pericytes associated with Alzheimer’s disease (AD) and ageing in hippocampus and cortex. Brain sections of APP/PS1 transgenic mice, a mouse model of AD, and C57BL/6 wild-type (WT) mice at 6, 12–14 and 23–28 months (young adulthood, middle-age and old, respectively) were processed for immunohistochemical analysis of the pericyte marker PDGFRβ. Representative immunohistological pattern of PDGFRβ in hippocampus (A) and cortex (B), where arrows point to capillaries and arrowheads point to PDGFRβ-positive cells, and inserts show PDGFRβ-positive cells around microvessels. Semi-quantitative analysis of PDGFRβ-positive cells per field in hippocampus (C) and cortex (D). ##P<0.01 vs. age-matched WT. Semi-quantitative analysis of PDGFRβ-positive cells per blood vessel in hippocampus (E) and cortex (F), with results expressed as % of vessels ensheathed by no cell, by 1, or by two or more PDGFRβ positive cells. *P<0.05, **P<0.01 vs. WT young adult; $P<0.05 vs. APP/PS1 young adult; #P<0.05, ###P<0.001 vs. WT age-matched.
In addition to the total number of PDGFR-β-positive cells, we investigated the perivascular location of these cells. Even though a significant age-related loss of total pericytes was not observed in either hippocampus (Fig. 3C) or cortex (Fig. 3D), it is possible that the PDGFR-β-positive cells may have dissociated from vessels along ageing in both regions, since the number of blood vessels not associated with pericytes increased from ~20% to ~40% in hippocampus (P<0.05, from young adult to old mice) and from ~30% to ~45% in cortex (P<0.05, from middle-age to old mice) in WT mice (Fig. 3E,F respectively). In accordance, the number of blood vessels with two or more PDGFR-β-positive cells declined from ~30% to ~15% in hippocampus and from ~20% to ~10% in cortex (P<0.01, from young adult to old mice). Analysis of APP/PS1 mice showed that while the cortex does not present alterations in the perivascular PDGFR-β-positive cells along disease progression (Fig. 3F), the hippocampus (Fig. 3E) manifests a significant decrease of pericytes associated with endothelial cells during disease development, since the number of blood vessels with no pericytes increased from ~30% to ~50% (P<0.05, from young adult to middle-age and old mice). Comparison of WT with APP/PS1 animals showed a significant difference in the number of PDGFR-β-positive cells per blood vessel in cortex in young adulthood. In fact, APP/PS1 mice had ~25% (P<0.001) more blood vessels without pericytes and a decline of 15% (P<0.05) of blood vessels with two or more pericytes (Fig. 3F). In the hippocampus, APP/PS1 had ~13% less blood vessels with two or more cells, albeit, this difference did not reach statistical significance. In sum, in cortex of APP/PS1 there was both a significant loss of total pericytes and perivascular PDGFR-β-positive cells when compared to age-matched WT, whereas in the hippocampus the difference in the total number pericytes did not reach significance, although a more robust decrease of perivascular cells in APP/PS1 mice compared to WT was observed.
2.3. Entrance of blood-borne components into hippocampal parenchyma during ageing in WT and in AD-like APP/PS1 mice
Based on previous studies showing that pericytes play a key role in the maintenance of the BBB integrity (Bell et al., 2010; Sá-Pereira et al., 2012), we investigated if the loss of perivascular pericytes is accompanied by the entrance into the brain parenchyma of thrombin and albumin (Fig. 4), two blood-borne components widely used as indicators of BBB disruption (Bell et al., 2010; Brito et al., 2013). Interestingly, positive deposits of these proteins were only detected in the hippocampus (Fig. 4A–D), which is the region presenting the lowest number of pericytes as shown by the lower number of PDGFR-β-positive cells per field (Fig. 3 C, D). An ~18-fold increase was detected in WT mice, while a ~40-fold increase (P<0.01) was observed in APP/PS1 mice in the number of albumin-positive deposits from young adulthood to old mice (Fig. 4C). In line with these results showing that albumin-positive deposits were mainly detected in the old mice, ageing was the only factor affecting the entrance of albumin (two-way ANOVA F 13.21, P<0.001). The number of thrombin-positive deposits increased ~10-fold for WT and APP/PS1 mice from young adulthood to middle-age, further increasing in old animals (Fig. 4D). Interestingly, the thrombin-positive deposits were consistently found at an earlier time point than albumin-positive ones in both genotypes, and the increase of thrombin and albumin positive deposits in hippocampal parenchyma occurred in an age-dependent manner (two-way ANOVA F 4.28, P<0.05 for thrombin), which suggests an increase of BBB permeability with age, independent from AD.
Fig. 4.
Entrance of the blood-borne components albumin and thrombin into the hippocampal parenchyma during ageing and along Alzheimer’s disease (AD) progression. Brain sections of APP/PS1 transgenic mice, a mouse model of AD, and C57BL/6 wild-type (WT) mice at 6, 12–14 and 23–28 months (young adulthood, middle-age and old, respectively) were processed for immunohistochemical analysis of albumin and thrombin. Representative immunohistological pattern of albumin in hippocampus (A) and semi-quantitative analysis of albumin-positive deposits in that region (C). Representative immunohistological pattern of thrombin in hippocampus (B) and semi-quantitative analysis of thrombin-positive deposits in that region (D). The arrows are pointing to capillaries and arrowheads to the positive protein deposits (A and B). **P<0.01 between indicated groups.
2.4. Astrocytes and microglia related changes during ageing in WT and in AD-like APP/PS1 mice
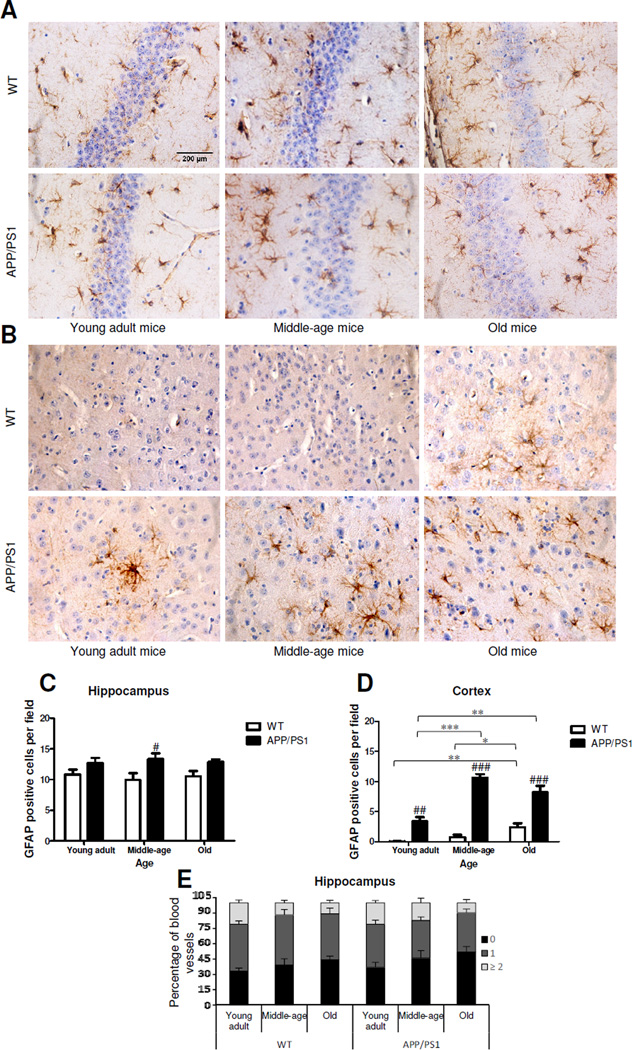
Previous work suggested that glial activation plays an important role during ageing (Jenny, 2012) and that there is an important link between neuroinflammation and AD (Pimplikar, 2014). Hence, we wanted to understand at which time point along healthy ageing and disease progression glial activation occurs. To this end, we analyzed the widely used astrocytic and microglial markers, glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (Iba-1), and measured the number of GFAP and Iba-1 positive cells per field, as well as the number of perivascular glial cells (Fig. 5,6). Interestingly, the number of GFAP-positive cells in hippocampus of WT mice was much higher than in cortex. Analysis of WT mice showed that while in hippocampus (Fig.5C) the number of GFAP-positive cells did not vary during ageing, in cortex (Fig.5D) ageing was accompanied by a ~24-fold increase of GFAP-positive cells from young adulthood to old age (P<0.01). Observation of APP/PS1 mice revealed that during disease progression there was an intense rise in astrogliosis in cortex where a ~3-fold increase from young to middle-age was observed (P<0.001, Fig. 5D), whereas the number of GFAP-positive cells did not vary significantly along time in hippocampus (Fig.5C). Even though not significant, it was interesting to observe a slight decrease from middle-age to old age, suggesting a reduction in astrocyte reactivity and/or density over time. When the WT animals were compared with APP/PS1, APP/PS1 mice had 25% more GFAP-positive cells (P<0.05) in hippocampus than WT at middle-age (Fig.5C). The difference between these two groups in cortex was much more robust as APP/PS1 mice had more than ~34-fold more GFAP-positive astrocytes at young adulthood (P<0.01), ~14-fold more at middle-age (P<0.001) and ~3-fold more (P<0.001) at old age than WT mice (Fig.5D). Thus, these results show that the increase of GFAP-positive cells is far more relevant in the cortex than in the hippocampus. Accordingly, the density of GFAP-positive cells in hippocampus (Fig. 5C) was affected only by AD (two-way ANOVA F 11.97, P<0.01), whereas in cortex (Fig.5D) it was affected equally by ageing and AD (two-way ANOVA, F 27.22, P<0.001 and F163.5, P<0.001, respectively). Furthermore, we observed the perivascular localization of these glial cells by measuring the number of GFAP-positive cells per blood vessel. This measurement was only performed in hippocampus since the low number of these cells in cortex precluded such determination. Analysis of WT and APP/PS1 animals showed that the GFAP-positive cells progressively detached from blood vessels in a very similar way (Fig.5E). From young adulthood to old age, the number of blood vessels with two or more GFAP-positive cells declined from ~20% to ~10% in WT and APP/PS1 mice (Fig.5E).
Fig. 5.
Astrocyte-related changes associated with Alzheimer’s disease (AD) and ageing in hippocampus and cortex. Brain sections of APP/PS1 transgenic mice, a mouse model of AD, and C57BL/6 wild-type (WT) mice at 6, 12–14 and 23–28 months (young adulthood, middle-age and old, respectively) were processed for immunohistochemical analysis of the astrocytic marker glial fibrillary acidic protein (GFAP). Representative immunohistological pattern of GFAP in hippocampus (A) and cortex (B). Semi-quantitative analysis of GFAP-positive cells in hippocampus (C) and cortex (D). Semi-quantitative analysis of GFAP-positive cells per blood vessel in hippocampus (E), with results expressed as % of vessels ensheathed by no cell, by 1, or by two or more GFAP positive cells. *P<0.05, **P<0.01 and ***P< 0.001 between indicated groups; #P<0.05, ##P<0.01, ###P<0.001 vs. age-matched WT.
Fig. 6.
Microglia related changes associated with Alzheimer’s disease (AD) and ageing in hippocampus and cortex. Brain sections of APP/PS1 transgenic mice, a mouse model of AD, and C57BL/6 wild-type (WT) mice at 6, 12–14 and 23–28 months (young adulthood, middle-age and old, respectively) were processed for immunohistochemical analysis of the microglial marker ionized calcium-binding adapter molecule 1 (Iba-1). Representative immunohistological pattern of Iba-1 in hippocampus (A) and cortex (B). Semi-quantitative analysis of Iba-1-positive cells in hippocampus (C) and cortex (D). Semi-quantitative analysis of vascular coverage by Iba-1-positive cells in hippocampus (E) and cortex (F), with results expressed as % of vessels ensheathed by no cell, by 1, or by two or more Iba-1 positive cells. *P<0.05, **P< 0.01 between indicated groups; #P<0.05, ###P<0.001 vs. age-matched WT.
Regarding Iba-1 (Fig. 6), the number of Iba-1-positive microglia/macrophage cells in WT animals were not changed in hippocampus (Fig.6A,C), but we observed an increase in cortex of 26% (P<0.05) from young adulthood to old age (Fig6B,D). In APP/PS1 mice, it was clearly seen that AD was accompanied by microgliosis not only in the cortex, as observed for astrogliosis, but also in hippocampus, where a ~2-fold increase (P<0.05 and P<0.01, respectively) in the number of Iba-1-positive cells from young adulthood to middle-age was detected in AD-like mice (Fig 6C,D). When WT and APP/PS1 animals were compared we observed that even though at young adulthood both genotypes presented a similar number of Iba-1-positive cells in hippocampus and cortex, by middle-age APP/PS1 mice had already ~2-fold more Iba-1-positive cells than WT animals (P<0.001) in both brain regions (Fig 6C,D). This difference was preserved in hippocampus at old age (P<0.001), while in the cortex it was less marked (more ~30% than age-matched WT, not significant). Considering the number of Iba-1-positive cells in both regions (Fig. 6), we determined that microgliosis was affected by AD (two-way ANOVA F 59.30, P<0.001 in hippocampus and F 32.61, P<0.001 in cortex) as well as by the interaction of AD and ageing of individuals (two-way ANOVA interaction AD × ageing F 16.75, P<0.001 in hippocampus and F 7.633, P<0.01 in cortex). Regarding the number of Iba-1-positive cells per blood vessel, analysis of the WT animals revealed that during ageing, the number of positive cells per blood vessel did not vary in hippocampus and cortex (Fig. 6E,F). Analysis of APP/PS1 mice revealed that at the peak of inflammation, there was recruitment of these cells to blood vessels, as indicated by the increase in the number of blood vessels with two or more microglia cells per blood vessel from 10% to 25% and the decrease from 50% to 35% in the number of blood vessels without any Iba-1-positive cells from young adulthood to middle-age in hippocampus (Fig. 6E). However, this effect disappears with age-associated diseases progression as the number of blood vessels with no microglia is significantly enhanced in old APP/PS1 mice as compared with age-matched WT (P<0.05) (Fig. 6E). In sum, considering the glial alterations, it seems that astrocytes react prior to microglia in cortex in APP/PS1 mice and that during ageing only cortex presents microgliosis and astrogliosis in WT mice.
3. Discussion
This study adds to the characterization of AD and ageing, by examining vascular density, BBB stability and glial activation simultaneously along healthy ageing and during disease progression in the hippocampus and cortex of WT and APP/PS1 mouse brain. We conclude that, in both regions, ageing resulted in increased RAGE immunoreactivity, as well as in the entrance of thrombin and albumin in hippocampus. On the other hand, AD was the main factor contributing to the loss of PDGFR-β positive cells, in both regions. Hypovascularization was affected by both ageing and AD in the hippocampus, but resulted from the interaction between both factors in the cortex. Regarding astrogliosis, whereas in hippocampus it was just an effect of AD, both factors contribute to it in cortex. Lastly, microgliosis was equally affected by ageing and AD in both regions.
The frontiers between ageing and AD have been greatly debated in the literature, but no consensus yet has been reached. For example, there is still no agreement on how ageing and AD affect capillary density (Brown and Thore, 2011). However, the majority of studies suggest that a decrease of capillary density in hippocampus and cortex is a hallmark of normal ageing and is even more pronounced in AD patients (Bell and Ball, 1986; Bell and Ball, 1990; Farkas and Luiten, 2001; Fischer et al., 1990), aged healthy mice (Jucker et al., 1990; Sonntag et al., 1997) and AD mouse models (Bailey et al., 2004; Paris et al., 2004), including APP/PS1 mice (Lee et al., 2005), which corroborates our results. However, the factors that lead to the hypovascularization are not entirely understood. Although it was originally hypothesized that an increase in Aβ burden and subsequent BBB disruption were related with the decreased clearance of Aβ from the brain (Shin et al., 2007), an alternative hypothesis states that the vascular dysfunction in AD occurs because amyloidogenesis promotes intense neoangiogenesis contributing to augmented hypervascularization and subsequent vascular permeability (Biron et al., 2011). It is noteworthy to mention that several factors have contributed to the lack of agreement about the effect of ageing and AD on cerebral microvasculature (Brown and Thore, 2011), including the use of different animal models, different markers for endothelial cells, differences in brain sections thickness, differences in the caliber of the blood vessel considered and differences in methodologies, especially considering the differences found between 2D and 3D methods. Based on our results, premature hypovascularization occurs in AD; hence, managing and reversing it could be a potential approach to delay the progression of the disease. Moreover, avoiding the loss of vascular density in aged individuals could also be an interesting approach to decrease the odds of developing age-related brain vulnerabilities.
We analyzed how brain microvasculature immunoreactivity of RAGE, a transmembrane receptor responsible for the influx of advanced glycation endproducts and Aβ, evolved along ageing and AD. Most studies focus on the expression of this receptor in whole brain but, as far as we know, there are no reports about RAGE expression in the microvasculature of APP/PS1, the AD-like animal model here used. Previous studies showed that normal aged brain is associated with higher expression of RAGE (Cho et al., 2009; Perry et al., 1993), and herein we further show that endothelial cells contribute to this increase. Furthermore, it was previously demonstrated that APP/PS1 mice (Liu et al., 2014) and other AD mouse model (Cho et al., 2009) have increased RAGE expression in brain homogenates. In the present study, an increased expression of RAGE was also observed in brain cells of APP/PS1 mice, as indicated by the enhanced immunoreactivity detected in the hippocampus of old mice and in the cortex of middle-age animals (Fig. 2A,B). Contrasting with these observations APP/PS1 mice do not present significant higher RAGE expression than age-matched WT in the microvasculature, which raises the hypothesis that in this AD mouse model, RAGE upregulation may occur predominantly in glial cells, where the involvement of this receptor was already demonstrated (Slowik et al., 2012). On the other hand, AD brains have higher RAGE expression than aged controls, including in endothelial cells (Jeynes and Provias, 2008; Miller et al., 2008). However, the previously reported increase of RAGE expression (Miller et al., 2008) was observed in blood vessels with 5 to 20 µm diameter, whereas the absence of variation here presented was found in capillaries (<6 µm diameter), which suggests that the upregulation occurs in other segments of the vascular tree. Moreover, the semi-quantitative analysis performed by these authors was based on the area of RAGE immunoreactivity, not taking into account the intensity of the signal per area of blood vessel, as we did in the present study. Therefore, further studies should be performed to clarify the variations in RAGE expression, as well as in the receptor’s activity in AD, inasmuch a large effort has been directed to RAGE as a potential target for modulation in AD in order to decrease Aβ accumulation in the brain (Deane et al., 2012).
We studied, for the first time, the temporal evolution of pericytes from young adulthood to old age during healthy ageing and AD progression using the commonly used pericyte marker PDGFR-β (Bell et al., 2010; Sá-Pereira et al., 2012). Previous studies reported that there is no pericyte loss during ageing in hippocampus and cortex of rodents since there was no change in coverage of capillaries based on analysis of cluster of differentiation 13 up till 9 months old (Sagare et al., 2013), and of desmin up till 16 months old (Bell et al., 2010). Herein, using transgenic mice with a different genetic background, other pericyte marker, and extending the age of the mice studied to 23–28 months old, we also did not detect significant alterations in the total number of PDGFR-β-positive cells, but detected a poorer pericyte vascular coverage along ageing in WT mice. Moreover, we found that AD-like transgenic mice have a greater loss of total and perivascular PDGFR-β-positive cells than age-matched controls, which agrees with previous reports (Bell et al., 2010; Sagare et al., 2013; Sengillo et al., 2013). Previous studies have shown that communication between pericytes and endothelial cells through endothelial platelet derived growth factor B and pericyte PDGFR-β is vital for pericyte maintenance (Bell et al., 2010). Interestingly, we found that only in cortex, where we detected a very premature and significant detachment of pericytes from blood vessels of APP/PS1 when compared with age-matched WT, there was a decrease in the number of total pericytes. The PDGFR-β pathway is not the only one to maintain pericyte attachment to endothelial cells, but it is crucial for pericyte survival. Thus, the abnormality in this pathway suggested by the reduced expression of PDGFR-β can lead to the detachment of pericytes or recruitment of these cells to other locations (Bonkowski et al., 2011), as well as to their death (Abramsson et al., 2007; Gaengel et al., 2009). On the other hand, pericytes provide important trophic support to blood vessels (Bell et al., 2010; Sá-Pereira et al., 2012). In our study, the loss of PDGFR-β-positive pericytes (observed in young adults) preceded vascular regression, which was observed here as a decrease in blood vessel area detected only at middle-age. Pericytes are also crucial for the maintenance of BBB properties (Sá-Pereira et al., 2012) and its loss contributes to BBB permeability (Bell et al., 2010), as pointed out in our study based on the detection of thrombin and albumin in brain parenchyma, which is in line with previous reports (Armulik et al., 2010; Baloyannis and Baloyannis, 2012; Daneman et al., 2010; Sengillo et al., 2013). In recent studies, it was suggested that pericyte loss in PDGFR-β−/− mice (Bell et al., 2010), lead to BBB disruption, which corroborates our results given the temporal relationship of these events and the increase of thrombin and albumin deposits. Of note in our study, the detection of these two blood-borne components in the parenchyma over time does not happen in the same magnitude or at the same time point, since thrombin was detected prior to albumin and the number of albumin deposits was twice the number of thrombin deposits. This could be due to the fact that albumin has a higher molecular weight than thrombin (67 and 37 kD, respectively), which means that a higher magnitude of BBB disruption would be required for albumin leakage into brain parenchyma as compared to the impairment that allows thrombin passage. However, we found a higher number of albumin deposits in brain parenchyma than thrombin, which may simply be due to the fact that albumin is the most common serum protein. The increase of these proteins in the brain parenchyma was a later event in our mice, suggesting that BBB disruption occurs as a result of endothelial and pericyte changes, as observed by others (Bell et al., 2010). Also interesting is the fact that leakage of blood borne proteins was observed in the hippocampus, but not in the cortex. This is in line with a recently published work reporting an increased BBB permeability in the hippocampus while other brain regions including cortical and subcortical regions remain relatively unaffected during normal ageing or ageing associated mild cognitive impairment in the living human brain (Montagne et al., 2015). Thus, these evidences point to the involvement of hippocampal BBB breakdown in cognitive impairment.
Our results are in agreement with previous reports showing that astrogliosis and microgliosis are both detected in frontal cortex of APP/PS1 mice by 15 months of age (Kamphuis et al., 2012; Wirz et al., 2013). Similar to a previous study, we detected increased microgliosis but not astrogliosis in the hippocampus of APP/PS1 mice (Wang et al., 2010). This is interesting because it contradicts the hypothesis that microgliosis could contribute in a very early phase of the disease to pathogenesis, at least in this model. It is also noteworthy to mention that at the peak of inflammation in APP/PS1 mice, when microglia are activated, these cells migrate towards blood vessels, as was made evident by the fact that the number of perivascular microglia increases. Moreover, given that neuroinflammation is a feature of AD, it is likely that this microglia recruitment is related to the immune response at the BBB. Accordingly, a recent study reported that neuroinflammation contributes to the increase of BBB permeability in an AD mouse model (Takeda et al., 2013). Thus, it is possible that the increase in perivascular microglia contributes to BBB dysfunction (Nishioku et al., 2010) instead of contributing to restore the BBB integrity, as was suggested by Willis and colleagues (Willis, 2011). Also, we observed a slight increase of perivascular microglia in cortex but not hippocampus in the aged brain of old WT mice compared with young adult WT mice. Astrocytes behave in the opposite way, since ageing of both WT and APP/PS1 mice is accompanied by a decrease of perivascular astrocytes in hippocampus compared with young adult genotype-matched animals.
Lastly, we established for the first time that while APP/PS1 mice already begin to deposit senile plaques by young adulthood in hippocampus and cortex, these regions are not affected by the same vascular and glial events simultaneously. This may indicate that these changes are not dependent upon or related to the accumulation of Aβ, as the burden of Aβ seems to be similar in these two regions. Our findings suggest that at young adulthood, Aβ accumulation in hippocampus precedes vascular and glial changes. In contrast, Aβ accumulation in cortex occurs concurrently with a reduction in PDGFR-β-positive cells and astrogliosis. In figure 7 the temporal evolution of glial and vascular events, along with the appearance of senile plaques is schematically represented.
Fig. 7.
The temporal evolution of vascular and glial changes, along with the appearance of senile plaques in cortex and hippocampus of APP/PS1 transgenic mice, a mouse model of Alzheimer’s disease, and C57BL/6 wild-type (WT) mice.
In sum, our findings suggest that it would be interesting to start targeting vascular and glial alterations in the elderly as such alterations seem to predispose the elderly to age-related brain vulnerabilities. For example, it is likely that BBB stabilizing drugs or drugs that contribute to vascular density maintenance may have beneficial effects in elderly. Our results strongly suggest that AD pathogenesis, e.g. Aβ deposition in hippocampus, further accentuates vascular and glial changes, at least in mice, which confirms that vascular and glial alterations play an important role in AD pathogenesis. This may justify further studies to evaluate the benefits of vasculature modifying drugs not only in sporadic AD, but also in familial AD patients, because even though there may be differences in the individual pathological cascades, it is clear that vascular changes contribute to disease progression.
4. Experimental Procedure
4.1. Animals
Male APPswe/PS1ΔE9 transgenic (Tg) mice on a C57BL/6J background were examined at three different ages: 6 month (young adults, n= 5), 12–14 month (middle age, n=5), and 23–28 month (old, n=4). APPswe/PS1ΔE9 Tg mice express two human genes of familial AD, the APP K594N/M595L Swedish and Presenilin 1 delta E9 (PS1ΔE9) (deletion of exon 9) under a mouse prion protein promotor (Jankowsky et al., 2004). Original Tg breeders were obtained from The Jackson Laboratory (Bar Harbor, ME; stock no. 034832) and were maintained in the Lemere Lab colony by crossing male APPswe/PS1Δ9 Tg mice with female C57BL/6J mice. All animal use was approved by the Harvard Standing Committee for Animal Use and was in compliance with all state and federal regulations. APPswe/PS1Δ9 Tg mice (henceforth, referred to as APP/PS1) mimic to some degree early-onset human AD (Xiong et al., 2011). This mouse model starts developing occasional Aβ deposits by 5–6 months of age (Jankowsky et al., 2004), presenting abundant plaques in cortex and hippocampus at 8 months, which increase along disease progression, leveling off after 18 months of age (Garcia-Alloza et al., 2006; Wirz et al., 2013), as shown in Supplementary Figure 1. Whereas there are several studies showing that cognitive deficits manifest at 6 months and are exacerbated with increasing amyloid burden (Gimbel et al., 2010; Park et al., 2006; Xiong et al., 2011), there are other studies demonstrating that these animals present impaired spatial learning starting at 12 months in the Morris water maze and, that 13 months-old APP/PS1 mice commit more errors in the same test than WT mice, unlike at 7 months wherein there is no difference (Lalonde et al., 2005). Regarding long-term potentiation, there are studies pointing to deficits as early as 3 months and not related to age from 3 to 12 months (Volianskis et al., 2010), while others reported the impairment at 8 and 15 month old mice (Gengler et al., 2010).
4.2. Tissue collection
All mice were killed by CO2 inhalation and transcardially perfused with 20 ml phosphate buffer saline (PBS). The brain of each mouse was removed and divided sagittally. As previously described, (Maier et al., 2005) the brain was fixed for 2 h in 10% buffered formalin before being processed for paraffin embedding.
4.3. Immunohistochemistry
Immunohistochemistry was performed on 10 µm paraffin sections of mouse brain as previously described (Lemere et al., 2000), and summarized in Table 1. A 0.3% hydrogen peroxide in methanol solution was used to inhibit endogenous peroxidase, for 10 minutes, and antigen recovery was achieved by heat-mediated treatment with citrate buffer. Sections were incubated overnight at 4°C with the primary antibodies, and then incubated for 30 minutes with secondary antibodies. The Vectastain Elite ABC kits was used (Vector Laboratories, Burlingame, CA), followed by development with 3,3’-diaminobenzidine tetrahydrochloride. Hematoxylin counterstaining was performed for some of the immunostainings. Negative controls with omission of primary antibodies were performed to exclude nonspecific binding or cross reactivity (Supplementary Figure 2).
Table 1.
Summary of the antibodies and experimental conditions used for immunohistochemical analysis.
| Markers | T/NT | Blocking | Primary antibody |
Dilution | Secondary antibody | Dilution | |
|---|---|---|---|---|---|---|---|
| CD31 | NT | 5% GS, Triton 0.05% |
Abcam, #ab28364 Rabbit Pc |
1:50 in 5% GS |
Vector, #BA-1000 Biotinylated Goat anti-Rabbit |
1:2000 in 10% GS |
|
| Albumin | NT | 5% HS | Bethyl, #A90-134A Goat Pc |
1:100 in 5% HS |
Vector, #BA-9500 Biotinylated Horse anti-Goat |
1:2000 in 10% HS |
|
| Vascular | Thrombin | T | 2% HS | Santa Cruz, #sc-23335 Goat Pc |
1:100 in 2% HS |
Vector, #BA-9500 Biotinylated Horse anti-Goat |
1:2000 in 10% HS |
| PDGFR-β | T | 2% GS | R&D, #AF1042 Goat Pc |
1:20 in 2% HS |
Vector, #BA-9500 Biotinylated Horse anti-Goat |
1:2000 in 10% HS |
|
| RAGE | T | 2% GS | NeoBioLab, #A1786 Rabbit Pc |
1:350 in 2% GS |
Vector, #BA-1000 Biotinylated Goat anti-Rabbit |
1:2000 in 10% GS |
|
| Glial | GFAP | NT | 10% GS | Dako, #Z033429-2 Rabbit Pc |
1:500 in 10% GS |
Vector, #BA-1000 Biotinylated Goat anti-Rabbit |
1:2000 in 10% GS |
| Iba-1 | T | 10% GS | Wako, #019-19741 Rabbit Pc |
1:500 in 10% GS |
Vector, #BA-1000 Biotinylated Goat anti-Rabbit |
1:2000 in 10% GS |
|
| Amyloid-β | T | 10% GS | Dr .Dennis Selkoe Lab Rabbit Pc |
1:1000 10% GS |
Vector, #BA-1000 Biotinylated Goat anti-Rabbit |
1:2000 in 10% GS | |
CD31, cluster of differentiation 31; PDGFR-β, platelet derived growth factor receptor β; RAGE, receptor for advanced glycation endproducts; GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor molecule 1. # = catalog reference; Pc, polyclonal antibody; T, pretreatment with antigen retrieval by microwave treatment using citrate buffer; NT, no pretreatment with antigen retrieval by microwave treatment using citrate buffer; GS, goat serum; HS, horse serum.
4.4. Data analysis
Photographs were acquired on bright field microscope (Zeiss, model AxioSkop HBO50) with an integrated digital camera (Leica, model DFC490). Twelve fields of the hippocampus (including CA1, CA3 and dentate gyrus) and twelve fields of frontal cortex of each animal were analyzed using the ImageJ 1.29x software (National Institutes of Health, USA), for glial and vascular indicators, as detailed below. Vascular density, receptor for RAGE expression by endothelial cells, PDGFR-β expression by pericytes and blood-borne components in brain parenchyma were determined to assess vascular alterations. For all of these vascular parameters, only capillaries (i.e., blood vessels with a diameter lower than 6 µm) were considered. For the vascular density evaluation, the area of cluster of differentiation (CD) 31-positive capillaries per field was measured (Brito et al., 2013), considering both longitudinal and transversal blood vessels, and data was expressed as total area of blood vessels per µm2 of tissue. For evaluation of RAGE immunoreactivity in endothelial cells, each longitudinal capillary was delimited, the vascular immunoreactivity was measured, and results were expressed by the mean intensity of RAGE per µm2 of blood vessel. To evaluate pericytes, the number of PDGFR-β-positive cells per field was counted. Moreover, the perivascular pericytes were evaluated by measuring the number of PDGFR-β-positive cells per longitudinal blood vessel. The blood vessels were then classified into three categories: blood vessels without or blood vessels with one or with two or more PDGFR-β-positive cells. To evaluate the entrance of thrombin and albumin into the brain parenchyma, the number of thrombin-positive and albumin-positive deposits per section was measured. Glial parameters were analyzed based on GFAP and Iba-1 for astrocytes and microglia, respectively. To evaluate the density of both glial cells, the number of GFAP- and Iba-1-positive cells per field was counted. Moreover, the number of perivascular glial cells was evaluated by counting the number of GFAP- and Iba-1-positive cells per longitudinal capillaries. The capillaries were then classified into three categories: blood vessels without, with one, or with two or more GFAP- or Iba-1-positive cells. Aβ burden in brain parenchyma was also determined by analysis of Aβ plaques per section.
4.5. Statistical analysis
Results were analyzed using GraphPad Prism® 5.0 (GraphPad Software, San Diego, CA, USA) and are expressed as mean±SEM. One-way ANOVA and the Bonferroni post hoc test were used to evaluate how the parameters evolved along time for each genotype separately, as well as to evaluate the difference between WT and APP/PS1 mice at each time point. For one-way ANOVA, the P values less than 0.05 were considered statistically significant. To determine how the parameters were affected by ageing and AD the two-way ANOVA was used. For two-way ANOVA the F values are presented.
Supplementary Material
A premature loss of capillary density occurs in APP/PS1 as compared to WT mice
A reduced pericyte vascular coverage occurs in APP/PS 1 compared with WT mice
An increase of BBB permeability occurs along ageing, independent from AD
Astrocytes react prior to microglia in the cortex of APP/PS 1 mice
During ageing only cortex presents microgliosis and astrogliosis in WT mice
Acknowledgements
This study was supported by Fundação para a Ciência e a Tecnologia (FCT - PEst-OE/SAU/UI4013/2011-2013) and by philanthropic funds.
Abbreviations
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- BBB
blood-brain barrier
- CD31
cluster of differentiation 31
- GFAP
glial fibrillary acidic protein
- Iba-1
ionized calcium-binding adapter molecule 1
- PDGFR-β
platelet-derived growth factor receptor-β
- RAGE
receptor for advanced glycation endproducts
- Tg
transgenic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramsson A, Kurup S, Busse M, Yamada S, Lindblom P, Schallmeiner E, Stenzel D, Sauvaget D, Ledin J, Ringvall M, Landegren U, Kjellen L, Bondjers G, Li JP, Lindahl U, Spillmann D, Betsholtz C, Gerhardt H. Defective N-sulfation of heparan sulfate proteoglycans limits PDGF-BB binding and pericyte recruitment in vascular development. Genes Dev. 2007;21:316–331. doi: 10.1101/gad.398207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer's disease and vascular dementia. Curr Alzheimer Res. 2013;10:642–653. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- Armstrong RA. What causes alzheimer's disease? Folia Neuropathol. 2013;51:169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurol Res. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- Baloyannis SJ, Baloyannis IS. The vascular factor in Alzheimer's disease: a study in Golgi technique and electron microscopy. J Neurol Sci. 2012;322:117–121. doi: 10.1016/j.jns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Bell MA, Ball MJ. The correlation of vascular capacity with the parenchymal lesions of Alzheimer's disease. Can J Neurol Sci. 1986;13:456–461. doi: 10.1017/s0317167100037124. [DOI] [PubMed] [Google Scholar]
- Bell MA, Ball MJ. Neuritic plaques and vessels of visual cortex in aging and Alzheimer's dementia. Neurobiol Aging. 1990;11:359–370. doi: 10.1016/0197-4580(90)90001-g. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron KE, Dickstein DL, Gopaul R, Jefferies WA. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer's disease. PLoS One. 2011;6:e23789. doi: 10.1371/journal.pone.0023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS. 2011;8:8. doi: 10.1186/2045-8118-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito MA, Pereira P, Barroso C, Aronica E, Brites D. New autopsy findings in different brain regions of a preterm neonate with kernicterus: neurovascular alterations and up-regulation of efflux transporters. Pediatr Neurol. 2013;49:431–438. doi: 10.1016/j.pediatrneurol.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Son SM, Jin SM, Hong HS, Shin DH, Kim SJ, Huh K, Mook-Jung I. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer's disease animal model. FASEB J. 2009;23:2639–2649. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004a;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke. 2004b;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fischer VW, Siddiqi A, Yusufaly Y. Altered angioarchitecture in selected areas of brains with Alzheimer's disease. Acta Neuropathol. 1990;79:672–679. doi: 10.1007/BF00294246. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Gengler S, Hamilton A, Holscher C. Synaptic plasticity in the hippocampus of a APP/PS1 mouse model of Alzheimer's disease is impaired in old but not young mice. PLoS One. 2010;5:e9764. doi: 10.1371/journal.pone.0009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jenny NS. Inflammation in aging: cause, effect, or both? Discov Med. 2012;13:451–460. [PubMed] [Google Scholar]
- Jeynes B, Provias J. Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Curr Alzheimer Res. 2008;5:432–437. doi: 10.2174/156720508785908937. [DOI] [PubMed] [Google Scholar]
- Jucker M, Battig K, Meier-Ruge W. Effects of aging and vincamine derivatives on pericapillary microenvironment: stereological characterization of the cerebral capillary network. Neurobiol Aging. 1990;11:39–46. doi: 10.1016/0197-4580(90)90060-d. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Mamber C, Moeton M, Kooijman L, Sluijs JA, Jansen AH, Verveer M, de Groot LR, Smith VD, Rangarajan S, Rodriguez JJ, Orre M, Hol EM. GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS One. 2012;7:e42823. doi: 10.1371/journal.pone.0042823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer's disease are centered on vessel walls. Am J Pathol. 2005;167:527–543. doi: 10.1016/S0002-9440(10)62995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Kim HD, Maxwell JA, Fukuchi K. Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+PS1/DeltaE9 mice with amyloid plaques. Neurosci Lett. 2005;390:87–92. doi: 10.1016/j.neulet.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR. Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer's disease. Brain Res Bull. 2005;65:317–322. doi: 10.1016/j.brainresbull.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, Hancock WW, Weiner HL, Selkoe DJ. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann N Y Acad Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- Liu R, Li JZ, Song JK, Zhou D, Huang C, Bai XY, Xie T, Zhang X, Li YJ, Wu CX, Zhang L, Li L, Zhang TT, Du GH. Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiol Aging. 2014;35:1275–1285. doi: 10.1016/j.neurobiolaging.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lemere CA. Modulation of the humoral and cellular immune response in Abeta immunotherapy by the adjuvants monophosphoryl lipid A (MPL), cholera toxin B subunit (CTB) and E. coli enterotoxin LT(R192G) Vaccine. 2005;23:5149–5159. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Reed BR, Sanossian N, Madison CM, Kriger S, Dhada R, Mack WJ, DeCarli C, Weiner MW, Mungas DM, Chui HC, Jagust WJ. The aging brain and cognition: contribution of vascular injury and abeta to mild cognitive dysfunction. JAMA Neurol. 2013;70:488–495. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer's disease. Mol Neurodegener. 2013;8:38. doi: 10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci. 2010;112:251–254. doi: 10.1254/jphs.09292sc. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Paris D, Patel N, DelleDonne A, Quadros A, Smeed R, Mullan M. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Park JH, Widi GA, Gimbel DA, Harel NY, Lee DH, Strittmatter SM. Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer's transgenic mice. J Neurosci. 2006;26:13279–13286. doi: 10.1523/JNEUROSCI.4504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW. Neuroinflammation in Alzheimer's disease: from pathogenesis to a therapeutic target. J Clin Immunol. 2014;34(Suppl 1):64–69. doi: 10.1007/s10875-014-0032-5. [DOI] [PubMed] [Google Scholar]
- Sá-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130:2310–2319. doi: 10.1093/brain/awm156. [DOI] [PubMed] [Google Scholar]
- Slowik A, Merres J, Elfgen A, Jansen S, Mohr F, Wruck CJ, Pufe T, Brandenburg LO. Involvement of formyl peptide receptors in receptor for advanced glycation end products (RAGE)--and amyloid beta 1-42-induced signal transduction in glial cells. Mol Neurodegener. 2012;7:55. doi: 10.1186/1750-1326-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Ikimura K, Nishino H, Rakugi H, Morishita R. Increased blood-brain barrier vulnerability to systemic inflammation in an Alzheimer disease mouse model. Neurobiol Aging. 2013;34:2064–2070. doi: 10.1016/j.neurobiolaging.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Volianskis A, Kostner R, Molgaard M, Hass S, Jensen MS. Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS 1deltaE9-deleted transgenic mice model of ss-amyloidosis. Neurobiol Aging. 2010;31:1173–1187. doi: 10.1016/j.neurobiolaging.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Gao CY, Yang M, Liu XH, Sun Y, Pollard A, Dong XY, Wu XB, Zhong JH, Zhou HD, Zhou XF. Intramuscular delivery of a single chain antibody gene prevents brain Abeta deposition and cognitive impairment in a mouse model of Alzheimer's disease. Brain Behav Immun. 2010;24:1281–1293. doi: 10.1016/j.bbi.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis CL. Glia-induced reversible disruption of blood-brain barrier integrity and neuropathological response of the neurovascular unit. Toxicol Pathol. 2011;39:172–185. doi: 10.1177/0192623310385830. [DOI] [PubMed] [Google Scholar]
- Wirz KT, Bossers K, Stargardt A, Kamphuis W, Swaab DF, Hol EM, Verhaagen J. Cortical beta amyloid protein triggers an immune response, but no synaptic changes in the APPswe/PS1dE9 Alzheimer's disease mouse model. Neurobiol Aging. 2013;34:1328–1342. doi: 10.1016/j.neurobiolaging.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Wodzinska J, Xu J, Premyslova M, Liu QY, Connelly J, Zhang W. Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer's disease. Neurosci Bull. 2011;27:221–232. doi: 10.1007/s12264-011-1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.