Abstract
Alcoholic hepatitis (AH) frequently progresses to multiple organ failure (MOF) and death. However, the driving factors are largely unknown. At admission, patients with AH often show criteria of systemic inflammatory response syndrome (SIRS) even in the absence of an infection. We hypothesize that the presence of SIRS may predispose to MOF and death. To test this hypothesis, we studied a cohort including 162 patients with biopsy-proven AH. The presence of SIRS and infections was assessed in all patients, and multivariate analyses identified variables independently associated with MOF and 90-day mortality. At admission, 32 (19.8%) patients were diagnosed with a bacterial infection, while 75 (46.3%) fulfilled SIRS criteria; 58 patients (35.8%) developed MOF during hospitalization. Short-term mortality was significantly higher among patients who developed MOF (62.1% versus 3.8%, P <0.001). The presence of SIRS was a major predictor of MOF (odds ratio = 2.69, P=0.025) and strongly correlated with mortality. Importantly, the course of patients with SIRS with and without infection was similar in terms of MOF development and short-term mortality. Finally, we sought to identify serum markers that differentiate SIRS with and without infection. We studied serum levels of high-sensitivity C-reactive protein, procalcitonin, and lipopolysaccharide at admission. All of them predicted mortality. Procalcitonin, but not high-sensitivity C-reactive protein, serum levels identified those patients with SIRS and infection. Lipopolysaccharide serum levels predicted MOF and the response to prednisolone.
Conclusion
In the presence or absence of infections, SIRS is a major determinant of MOF and mortality in AH, and the mechanisms involved in the development of SIRS should be investigated; procalcitonin serum levels can help to identify patients with infection, and lipopolysaccharide levels may help to predict mortality and the response to steroids.
Alcoholic hepatitis (AH) is one of the most deadly conditions in hepatology. In severe cases, patients often develop multiple organ failure (MOF), and the 3-month mortality rate remains very high (25%–40%).1 Unfortunately, many patients do not respond to medical treatment, and there is an urgent need to develop new pathophysiologically oriented therapies.2 Identifying the main determinants of MOF and death in these patients could help in the development of new targeted therapies.
The mechanisms leading to early death in patients with AH are largely unknown. The degree of liver failure and the development of renal failure are associated with a poor outcome.3 In patients with acute-onchronic liver failure, mortality is not only due to endstage liver failure but often related to MOF.4 We recently found that the development of acute kidney injury (AKI) is an early prognostic factor of mortality in AH.5 In this study, we observed that the presence of systemic inflammatory response syndrome (SIRS) at admission may have an important role in the development of AKI. Moreover, SIRS has recently been associated with poor prognosis in patients with acute-onchronic liver failure.4
The prevalence of SIRS in patients with AH and its impact on the development of MOF and mortality are unknown. In the setting of AH SIRS may be related to a bacterial infection (sepsis); around 25% of patients with AH present with a bacterial infection at admission.6 However, many patients with AH show features of SIRS (e.g., leukocytosis, fever) without any identifiable bacterial infection. In these patients SIRS may be secondary to sterile inflammation, namely, an inflammatory response in the absence of pathogens.7 In the liver of patients with AH, there is a marked overexpression of proinflammatory cytokines such as interleukin-8 and chemokine (C-C motif) ligand 20.8,9 The serum levels of both cytokines correlate with short-term mortality, suggesting that inflammatory mediators produced by the injured liver play a role in the development of SIRS in patients with AH. In the current study, we tested the role of SIRS in the prognosis of patients with AH and the specific roles of SIRS with and without infection.
A critical clinical issue in patients with AH is differentiating between infection-associated SIRS and SIRS in the absence of infection. While in some patients infections can be diagnosed early based on clinical, radiological, and biochemical criteria (e.g., spontaneous bacterial peritonitis or pneumonia), other patients have a positive culture identified after 2–3 days. Administering broad-spectrum antibiotics in patients under prednisolone therapy and with SIRS without infection may be unnecessary, and recent reports have shown that broad-spectrum antibiotics can favor severe fungal infections.10 Therefore, a secondary aim of our study was to identify serum markers capable of distinguishing between SIRS with and without infection. We selected a panel of biomarkers associated with systemic inflammation and infections in patients with cirrhosis including high-sensitivity C-reactive protein (hsCRP), procalcitonin, and lipopolysaccharide (LPS).11–13
Patients and Methods
Patients and Interventions
Data on 162 consecutive patients admitted to the Liver Unit (Hospital Clinic, Barcelona, Spain) between the years 2000 and 2012 with biopsy-proven AH were analyzed retrospectively. The diagnostic criteria of AH have been described in detail.3 According to our center’s clinical protocol, all patients with clinically suspected AH underwent a liver biopsy. Only those patients with histologically confirmed AH were included in the analysis, which accounted for 76.4% of the patients with clinical suspicion of AH. In all patients, the liver biopsy was performed within the first 48 hours of hospitalization and before steroid treatment was initiated. Patients with hepatocellular carcinoma or any other potential cause of liver disease were excluded from the study. Patients with severe AH, defined as Maddrey’s discriminant function (DF) >32 and/or age, bilirubin, international normalized ratio, creatinine (ABIC) score >6.71 at admission, were treated with prednisone orally for 4 weeks, followed by a 2-week taper period, with or without treatment discontinuation in nonresponding patients assessed by the Lille model, according to the center’s guidelines enforced at the patients’ admission. All patients underwent bacterial infection screening at admission, consisting of a chest X-ray, urine and ascitic fluid analysis and cultures, and blood cultures, even in the absence of signs of infection, according to the clinical protocol of diagnosis and management of AH. In the absence of an identifiable septic focus, patients with SIRS did not systematically receive antibiotic treatment, unless the temperature was above 38°C. These last patients were considered as infected for the present study. All patients in the cohort were followed until 90 days from admission or death. The study was approved by the Ethics Committee of the Hospital Clinic, and all patients gave written informed consent.
Definitions
The presence of SIRS was evaluated early after patients’ admission, before any therapeutic procedure was performed, following the recommendations of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (Supporting Table S1).14 Infection-associated SIRS was defined as SIRS secondary to a bacterial infection (sepsis), present at the patient’s admission. Infections were diagnosed by clinical, biochemical, radiological, and/or microbiological criteria. Infections at admission were defined as those infections present at the patient’s admission or diagnosed within the first 48 hours of hospitalization. Infections diagnosed later in the patient’s hospitalization were considered in-hospital infections. In order to define single organ failure and MOF, the Sequential Organ Failure Assessment (SOFA) score15 was used. Single organ failure was defined as a score of 3 or more in a given organ according to the SOFA16 at any time during hospitalization. Minor modifications were incorporated to reflect the impact of specific AH prognostic factors such as Maddrey’s DF,17 AKI,5 and hepatic encephalopathy2 on survival in AH (Table 1). Multiple organ failure was considered when failure of two or more organs was present.
Table 1.
Definitions of Single Organ Failure
| Organ | Definition |
|---|---|
| Liver failure | SOFA ≥3 (serum bilirubin ≥6 mg/dL) or Maddrey’s DF >32 |
| Renal failure | Defined as AKI, according to the Acute Kidney Injury Network criteria |
| Nervous system failure | SOFA ≥3 (Glasgow Coma Scale ≤9) or hepatic encephalopathy grade III or IV |
| Respiratory failure | SOFA ≥3 (PaO2/FiO2 <200 and mechanically ventilated) |
| Circulatory failure | SOFA ≥3 (use of norepinephrine, epinephrine, or dopamine [dopamine >5 μg/kg/min]) |
| Coagulation failure | SOFA ≥3 (platelet count <50,000/μL) |
The response to corticosteroids was assessed at 7 days using the Lille score.18 Patients presenting a score <0.45 were considered responders to corticosteroids, while patients with a score ≥0.45 were considered nonresponders.
Determination of hsCRP, Procalcitonin, and LPS Serum Levels
Serum samples were obtained from peripheral blood at the time of the transjugular liver biopsy and stored at −80°C. The serum concentration of hsCRP was determined by particle-enhanced immunoturbidimetry using CardioPhase reagent (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY). Procalcitonin serum concentration was determined through an automated immunofluorescence assay in a Kryptor analyzer (Brahms, GmbH, Hennigsdorf, Germany). Serum levels of LPS were determined using the limulus amebocyte lysate QCL-1000 test (Lonza Walkersville, Inc., Walkersville, MA).
Statistical Analysis
Continuous variables were described by mean (95% confidence interval [CI]). Categorical variables were described by means of counts and percentages. Comparisons between groups were performed using the Student t test or Mann-Whitney U test when appropriate. Differences between categorical variables were assessed by the chi-squared test or Fisher’s exact test. The main endpoints were development of MOF during hospitalization and 90-day mortality. In the multivariate analysis of factors associated with MOF and mortality, only those that were statistically significant (P<0.05) at the univariate analysis were entered into a multivariate logistic or Cox regression. The P values for the univariate tests were not corrected for multiple testing because those tests were taken as exploratory. The results of the multivariate logistic or Cox regression analysis (odds ratio [OR] or hazard ratio) identified those variables independently associated with the main outcome (after adjusting for the contributions of other variables). A P value <0.05 was required for significance. To avoid overfitting of the models, a predefined ratio of candidate prognostic variables to the number of observed events (MOF or deaths) was set at 1:10 or less. Receiver operating characteristic curves were created and compared to assess the prognostic performance of AH prognostic scores and inflammatory biomarkers. The SPSS statistical package (version 18.0; SPSS, Inc., Chicago, IL) was used for all analyses.
Results
Patient Characteristics
Demographic, clinical, and biochemical characteristics of patients with AH are listed in Table 2. Clinical, radiological, and/or histological criteria of liver cirrhosis were present in 72.8% of the patients. A bacterial infection was identified in 19.8% of the patients at admission, while 43.8% of the patients developed in-hospital infections. A detailed description of the type of infections and their impact on mortality in the global cohort and among patients treated with steroids is provided in the Supporting Information. A summary of the outcome of the patients according to the presence of SIRS and infections is shown in Supporting Fig. S1.
Table 2.
Characteristics of the Included Patients With Alcoholic Hepatitis (n=162)
| Parameter | Mean (95% CI) or n (%) |
|---|---|
| Clinical and epidemiological variables | |
| Gender (male) | 115 (71%) |
| Age (years) | 50 (49–51) |
| Proportion of patients with cirrhosis | 118 (72.8%) |
| SIRS at admission | 75 (46.3%) |
| MOF during hospitalization | 58 (35.8%) |
| Infection at admission | 32 (19.8%) |
| Infection during hospitalization | 71 (43.8%) |
| Time to in-hospital infection (days) | 9 (7–10) |
| 90-day mortality | 40 (24.7%) |
| Biochemical parameters at admission | |
| Creatinine (mg/dL) | 1.1 (1.0–1.3) |
| Bilirubin (mg/dL) | 12 (10–13) |
| AST (IU/L) | 141 (126–157) |
| ALT (IU/L) | 54 (48–61) |
| GGT (IU/L) | 533 (430–637) |
| Albumin (g/L) | 27 (26–28) |
| INR | 1.7 (1.6–1.8) |
| Scoring systems at admission | |
| ABIC score | 7.66 (7.44–7.89) |
| ABIC class | |
| A | 39 (24.1%) |
| B | 95 (58.6%) |
| C | 28 (17.3%) |
| MELD score | 21 (20–22) |
| MELD >21 | 78 (48.1%) |
| Maddrey’s DF | 43 (38–48) |
| Maddrey’s DF >32 | 90 (55.6%) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; INR, international normalized ratio.
MOF: Incidence, Predictive Factors, and Impact on Mortality
Thirty-six percent of the patients developed MOF during the hospitalization. The organs most frequently involved were the liver and the kidney (70.8% and 34% of patients, respectively), while circulatory failure was present in 10.6%, coagulopathy in 9.3%, respiratory failure in 6.8%, and neurological failure in 6.2% (Supporting Fig. S2). Thirty-seven patients (22.8%) presented no organ failure, while 67 patients (41.4%) presented one organ failure, 34 patients (21%) presented two organ failure, and 24 (14.8%) presented three or more organ failure (Supporting Fig. S3A). As expected, 90-day mortality was significantly higher in patients who developed MOF (62.1% versus 3.8% in patients with and without MOF, respectively; P<0.001) (Fig. 1A). Mortality according to the number of organs failing is shown in Supporting Fig. S3B. The association of MOF with mortality was independent of the degree of liver dysfunction, as assessed by the ABIC or Model for End-Stage Liver Disease (MELD) score, Maddrey’s DF, and the Lille score. The performance of existing predictive scores in predicting MOF in patients with AH is shown in the Supporting Information.
Fig. 1.
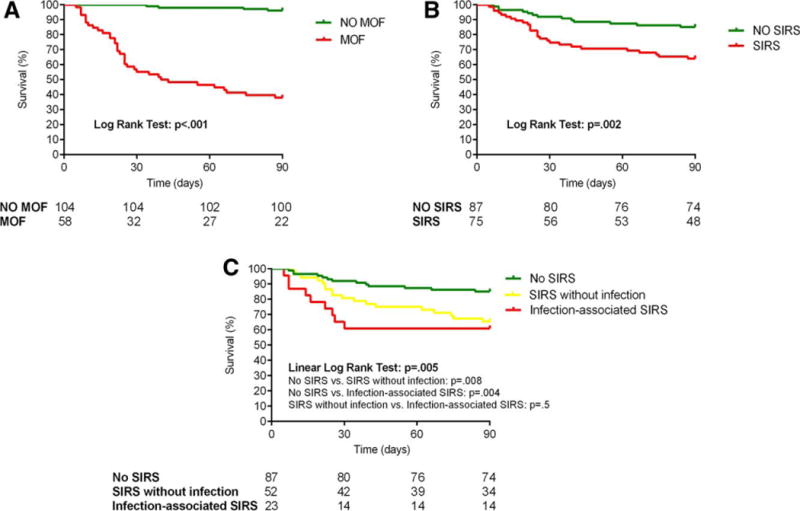
Ninety-day mortality according to (A) the presence of MOF, (B) the presence of SIRS, and (C) the SIRS-associated conditions.
Patients who developed MOF had a more severe liver dysfunction and were more likely to fulfill SIRS criteria (60.3% versus 38.5%, P=0.007) or present with a bacterial infection at admission (31% versus 13.5%, P=0.007) or during hospitalization (77.6% versus 25%, P<0.001) (Table 3). Variables associated with the development of MOF in the univariate analysis are depicted in Table 4. In a logistic regression multivariate analysis (Table 4), the variables that independently predicted MOF were the presence of SIRS at admission (OR52.687, P=0.025), serum creatinine (OR=2.508, P=0.021), and serum bilirubin (OR=1.096, P<0.001). These results strongly indicate that SIRS is a major determinant in the development of MOF in patients with AH. When included in the analysis, the presence of in-hospital infections showed a powerful association with MOF (OR=6.585, P<0.001), while SIRS and bilirubin levels at admission maintained a statistically significant association. While the presence of cirrhosis (F4) did not predict the development of MOF and death, the presence of chronic liver failure criteria of acute-onchronic liver failure4 strongly predicted these unfavorable outcomes. These results are described in detail in the Supporting Information.
Table 3.
Comparative of Patients With and Without MOF During Hospitalization
| Variable | Patients Without MOF (n=104) | Patients With MOF (n=58) | P |
|---|---|---|---|
| Gender (male), n (%) | 69 (66.3%) | 46 (79.3%) | 0.081 |
| Age (years), mean (95% CI) | 49 (48–51) | 52 (49–54) | 0.122 |
| ABIC score, mean (95% CI) | 7.10 (6.87–7.34) | 8.66 (8.32–9.00) | <0.001 |
| MELD score, mean (95% CI) | 18 (17–19) | 27 (25–29) | <0.001 |
| Maddrey’s DF, mean (95% CI) | 32 (27–38) | 62 (53–71) | <0.001 |
| Proportion of patients with cirrhosis, n (%) | 72 (69.2%) | 46 (79.3%) | 0.167 |
| SIRS at admission, n (%) | 40 (38.5%) | 35 (60.3%) | 0.007 |
| Infection at admission, n (%) | 14 (13.5%) | 18 (31.0%) | 0.007 |
| Infection during hospitalization, n (%) | 26 (25%) | 45 (77.6%) | <0.001 |
| Creatinine (mg/dL), mean (95% CI) | 0.8 (0.7–0.9) | 1.7 (1.3–2.1) | <0.001 |
| Bilirubin (mg/dL), mean (95% CI) | 8 (7–10) | 17 (15–20) | <0.001 |
| AST (IU/L), mean (95% CI) | 143 (122–165) | 137 (116–158) | 0.850 |
| ALT (IU/L), mean (95% CI) | 54 (45–63) | 54 (46–63) | 0.354 |
| GGT (IU/L), mean (95% CI) | 617 (473–761) | 384 (255–513) | 0.003 |
| Albumin (g/L), mean (95% CI) | 28 (27–29) | 25 (24–26) | 0.002 |
| INR, mean (95% CI) | 1.6 (1.5–1.7) | 2.0 (1.8–2.1) | <0.001 |
| 90-day mortality, n (%) | 4 (3.8%) | 36 (62.1%) | <0.001 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; INR, international normalized ratio.
Table 4.
Univariate and Multivariate Analyses of Factors Associated With MOF
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 1.030 | 0.992–1.069 | 0.124 |
| Gender (male) | 1.944 | 0.914–4.134 | 0.084 |
| Presence of cirrhosis | 1.704 | 0.797–3.642 | 0.169 |
| SIRS at admission | 2.435 | 1.261–4.701 | 0.008 |
| Infection at admission | 2.893 | 1.311–6.384 | 0.009 |
| In-hospital infection | 10.385 | 4.856–22.209 | <0.001 |
| Creatinine (mg/dL) | 4.647 | 2.109–10.240 | <0.001 |
| Bilirubin (mg/dL) | 1.121 | 1.075–1.169 | <0.001 |
| AST (IU/P) | 0.999 | 0.996–1.003 | 0.713 |
| ALT (IU/L) | 1.001 | 0.993–1.008 | 0.961 |
| GGT (IU/L) | 0.999 | 0.999–1.000 | 0.041 |
| Albumin (g/L) | 0.890 | 0.828–0.957 | 0.002 |
| INR | 5.351 | 2.414–11.860 | <0.001 |
| Multivariate model 1 (only variables at admission) | |||
| SIRS at admission | 2.687 | 1.129–6.395 | 0.025 |
| Infection at admission | 1.591 | 0.590–4.289 | 0.358 |
| Creatinine (mg/dL) | 2.508 | 1.146–5.491 | 0.021 |
| Bilirubin (mg/dL) | 1.096 | 1.042–1.153 | <0.001 |
| Albumin (g/L) | 0.956 | 0.864–1.059 | 0.393 |
| INR | 1.996 | 0.745–5.344 | 0.169 |
| Multivariate model 2 | |||
| SIRS at admission | 3.351 | 1.344–8.354 | 0.009 |
| In-hospital infection | 6.585 | 2.562–16.924 | <0.001 |
| Creatinine (mg/dL) | 1.616 | 0.739–3.532 | 0.229 |
| Bilirubin (mg/dL) | 1.095 | 1.037–1.157 | 0.001 |
| Albumin (g/L) | 0.913 | 0.814–1.025 | 0.123 |
| INR | 1.531 | 0.526–4.457 | 0.435 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; INR, international normalized ratio.
SIRS: Associated Conditions and Impact on Patient Outcome
We studied the characteristics and role of SIRS as it is a strong clinical predictor of MOF. Nearly half of the patients with AH (46.3%) fulfilled SIRS criteria at admission (Table 5). There were no differences in the prevalence of SIRS according to the severity of AH. Patients with SIRS criteria showed a higher prevalence of infection at admission (30.7% versus 10.3% with and without SIRS, respectively; P=0.001), while the incidence of inhospital infections was comparable between patients with and without SIRS (46.7% versus 41.4%, P=0.499). The presence of SIRS at admission did not predict the response to corticosteroids (Table 5). Importantly, the presence of SIRS at admission negatively impacted patients’ outcome. Overall 90-day mortality was significantly higher among patients with SIRS at admission (36% versus 14.9% in patients with and without SIRS, respectively; P=0.002) (Fig. 1B), independently of the degree of liver dysfunction and the Lille score in patients treated with corticosteroids (Supporting Table S2). The area under the receiver operating characteristic curve of SIRS for the prediction of 90-day mortality was 0.641 (95% CI 0.543–0.739), while the area under the receiver operating characteristic curve of the ABIC and MELD scores and Maddrey’s DF was 0.817 (95% CI 0.749–0.885), 0.815 (95% CI 0.744–0.886), and 0.714 (95% CI 0.623–0.805), respectively (Supporting Fig. S4). The prognostic performance of the individual SIRS criteria is described in the Supporting Information. Moreover, patients with SIRS criteria showed a higher incidence of MOF during hospitalization than patients without SIRS (46.7% versus 26.4%, P=0.007) (Table 5), also independently of the ABIC score, MELD score, Maddrey’s DF, and the Lille score (Supporting Table S2). The association of SIRS with mortality was also independent of the presence of hepatic encephalopathy, which was found to be an important prognostic factor of mortality in our study (more details are provided in the Supporting Information).
Table 5.
Comparative of Patients According to the Presence of SIRS at Admission and the Associated Conditions
| Variable | Patients Without SIRS (n=87) | Patients With SIRS (n=75) | P | Patients With SIRS Without Infection (n=52) | Patients With Infection-Associated SIRS (n=23) | P |
|---|---|---|---|---|---|---|
| Gender (male), n (%) | 60 (69.0%) | 55 (73.3%) | 0.541 | 39 (75%) | 16 (69.6%) | 0.624 |
| Age (years), mean (95% CI) | 50 (48–52) | 50 (48–52) | 0.952 | 50 (47–53) | 51 (48–54) | 0.456 |
| ABIC score, mean (95% CI) | 7.62 (7.33–7.92) | 7.71 (7.36–8.07) | 0.685 | 7.49 (7.09–7.89) | 8.22 (7.51–8.93) | 0.129 |
| MELD score, mean (95% CI) | 21 (19–23) | 22 (20–24) | 0.667 | 20 (18–22) | 25 (21–28) | 0.026 |
| Maddrey’s DF, mean (95% CI) | 41 (33–48) | 46 (38–53) | 0.210 | 41 (33–49) | 57 (42–72) | 0.048 |
| Lille model, mean (95% CI) | 0.37 (0.28–0.45) | 0.43 (0.34–0.52) | 0.361 | 0.41 (0.31–0.52) | 0.47 (0.27–0.66) | 0.380 |
| Proportion of cirrhotic patients, n (%) | 65 (74.7%) | 53 (70.7%) | 0.564 | 37 (71.2%) | 16 (69.6%) | 0.889 |
| MOF, n (%) | 23 (26.4%) | 35 (46.7%) | 0.007 | 22 (42.3%) | 13 (56.5%) | 0.255 |
| Infection at admission, n (%) | 9 (10.3%) | 23 (30.7%) | 0.001 | 0 (0%) | 23 (100%) | N/A |
| In-hospital infection, n | 36 (41.4%) | 35 (46.7%) | 0.499 | 24 (46.2%) | 11 (47.8%) | 0.894 |
| (%) Creatinine (mg/dL), mean (95% CI) | 1.0 (0.8–1.2) | 1.2 (0.9–1.5) | 0.136 | 1.1 (0.8–1.3) | 1.6 (0.9–2.4) | 0.054 |
| Bilirubin (mg/dL), mean (95% CI) | 11 (9–13) | 12 (10–14) | 0.695 | 11 (9–14) | 13 (9–18) | 0.179 |
| AST (IU/L), mean (95% CI) | 146 (123–169) | 135 (114–156) | 0.305 | 147 (119–175) | 109 (83–136) | 0.086 |
| ALT (IU/L), mean (95% CI) | 58 (47–68) | 50 (43–58) | 0.425 | 54 (44–64) | 42 (32–52) | 0.323 |
| Albumin (g/L), mean (95% CI) | 27 (26–29) | 26 (25–27) | 0.036 | 27 (25–281) | 24 (22–26) | 0.099 |
| INR, mean (95% CI) | 1.7 (1.6–1.9) | 1.7 (1.6–1.8) | 0.892 | 1.7 (1.5–1.8) | 1.9 (1.7–2.1) | 0.007 |
| 90-day mortality, n (%) | 13 (14.9%) | 27 (36.0%) | 0.002 | 18 (34.6%) | 9 (39.1%) | 0.503 |
“SIRS without infection” indicates SIRS at admission in absence of an identifiable infection at admission or within 48 hours of hospitalization; “infection-associated SIRS” indicates SIRS at admission secondary to bacterial infection, i.e., sepsis.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio.
Of the 75 patients with SIRS at admission, only 30.7% were diagnosed with an infection. Baseline characteristics of patients with SIRS with and without infection were comparable (Table 5). Mortality at 90 days was similar in patients with infection-associated SIRS and SIRS without infection (39.1% and 34.6%, respectively; P=0.503) and much higher than in patients without SIRS (14.91%, P<0.01 for both) (Fig. 1C). There was a clear trend toward an earlier death in patients with infection-associated SIRS compared to patients with SIRS without infection, with the maximum difference observed on day 30 from admission. When 30-day mortality was compared, borderline significance was found (30-day mortality 39.1% in infection-associated SIRS versus 19.2% in SIRS without infection, P = 0.057). Similarly, both causes of SIRS equally predicted MOF (42.3% in patients with SIRS without infection and 56.5% in patients with infection-associated SIRS, P = 0.255) (Table 5). These results indicate that regardless of the origin, patients with SIRS are at high risk for developing MOF and early death.
Serum Biomarkers in Patients With AH
Next, we sought to identify serum markers that could distinguish between infection-associated SIRS and SIRS without infection. For this purpose, we assessed potential serum biomarkers of infection and systemic inflammation. We first analyzed a biomarker of systemic inflammation (hsCRP) and a well-known biomarker of infection (procalcitonin) in a large subset of patients from the study cohort with AH (n = 89). The characteristics of this subset of patients with AH were similar to those of the entire cohort (Supporting Table S3). Patients with alcoholic liver cirrhosis without AH (n=14) and healthy subjects (n=10) were included as control groups. Patients with AH had higher levels of procalcitonin (Fig. 2A) and hsCRP (Supporting Fig. S5A) at admission than patients with alcoholic liver cirrhosis and healthy controls. In AH, both procalcitonin and hsCRP levels correlated with disease severity, as assessed by the ABIC and MELD scores and modified Maddrey’s DF (data not shown). Moreover, procalcitonin serum levels were higher among patients who developed MOF during hospitalization (0.75 ng/mL versus 0.31 ng/mL in patients with and without MOF, respectively; P=0.001). There was also a trend for an association of MOF with hsCRP levels (mean hsCRP 3.28 mg/dL for patients who developed MOF versus 2.40 mg/dL for patients who did not develop MOF, P=0.058). Among patients with SIRS at admission, procalcitonin levels were higher in those with infection-associated SIRS than in those with SIRS without infection (0.89 ng/mL versus 0.35 ng/mL, P=0.015) (Fig. 2B). The best cutoff value to rule out infections (negative predictive value of 78.9%) was 0.25 ng/mL. For the diagnosis of infections in AH patients with SIRS, a cutoff of 0.45 ng/mL showed the best performance (positive predictive value of 83.3% and negative predictive value of 71%). Thus, 83.3% of patients with procalcitonin levels >0.45 ng/mL were infected at admission, while only 29% of patients with procalcitonin levels <0.45 ng/mL had an infection (P = 0.012). These results suggest that procalcitonin could be a valuable biomarker to estimate the presence of infections in patients with AH and SIRS. High-sensitivity CRP was not able to discriminate between SIRS with and without infection (Supporting Fig. S5B).
Fig. 2.
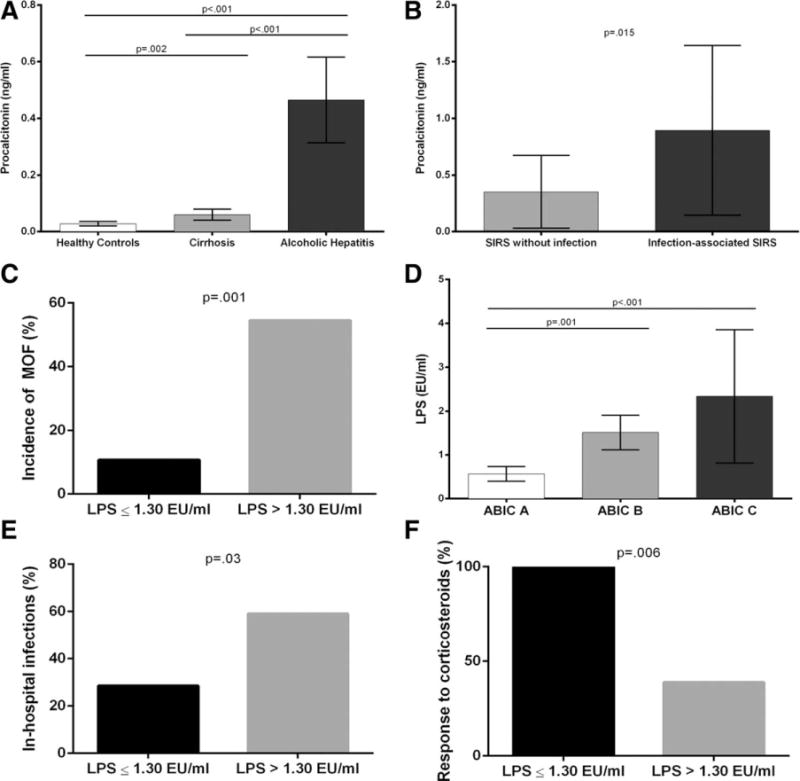
(A) Serum levels of procalcitonin according to the presence and severity of alcoholic liver disease, (B) serum levels of procalcitonin in patients with SIRS without infection versus infection-associated SIRS, (C) incidence of MOF according to serum levels of LPS, (D) serum levels of LPS according to the severity of AH assessed by ABIC score, (E) in-hospital infections, and (F) corticosteroid therapy response rate (assessed by the Lille score) according to serum levels of LPS.
We next analyzed serum levels of LPS, which is a marker of bacterial translocation and plays a role in the pathogenesis of alcoholic liver disease, in a subgroup of 50 patients with AH from the study cohort. Baseline characteristics of this subgroup were comparable with those of the entire cohort (Supporting Table S4). Levels of LPS did not discriminate between patients with or without SIRS, were similar in patients with SIRS without infection and infection-associated SIRS (data not shown), and predicted the development of MOF during hospitalization (Fig. 2C). Moreover, LPS serum levels correlated with the severity of AH (Fig. 2D) and predicted the development of inhospital infections. The incidence of in-hospital infections was 59.1% among patients with LPS >1.30 EU/mL versus 28.6% among patients with LPS ≤1.30 EU/mL (P=0.03) (Fig. 2E). Interestingly, LPS at admission predicted the response to corticosteroids, assessed by the Lille score. All patients with LPS ≤1.30 EU/mL responded to corticosteroid therapy, while 61.1% of patients with LPS >1.30 EU/mL were nonresponders (P=0.006) (Fig. 2F). These results suggest that LPS levels do not discriminate between SIRS with and without infection, but can be clinically useful to identify nonresponders to corticosteroids.
We finally investigated if the three inflammatory markers (hsCRP, procalcitonin, and LPS) predict survival in AH. A detailed description of this analysis is given in the Supporting Information. All three biomarkers significantly predicted 90-day survival. In particular, 90-day mortality was 14.9% in patients with procalcitonin <0.6 ng/mL versus 60% in patients with procalcitonin ≥0.6 ng/mL (P< 0.001) (Fig. 3A). Similarly, patients with LPS concentration ≤1.3 EU/mL also showed a very low mortality rate at 90 days (3.6%) compared to 50% mortality in patients with LPS >1.3 EU/mL (P< 0.001) (Fig. 3B).
Fig. 3.
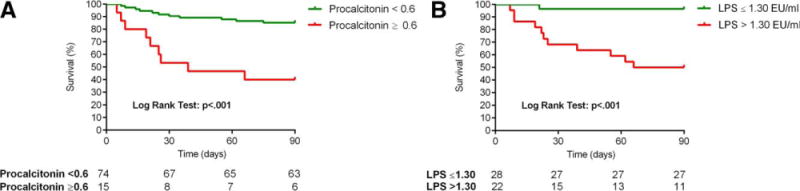
Ninety-day mortality according to the levels of (A) procalcitonin and (B) LPS.
Discussion
The mortality of patients with AH has not significantly improved in the last decades. Prednisolone, the main first-line therapy, was proposed in 1971 in an attempt to reduce hepatic inflammation, a common finding in AH. However, we recently found that severe hepatic infiltration of polymorphonuclear cells is associated with a favorable prognosis, suggesting that inflammation could drive hepatic regeneration in these patients.19 Moreover, an analysis of explants from patients with AH who underwent liver transplantation20 revealed that severe AH is characterized by impaired regeneration, rather than inflammation.21 These data suggest that hepatic inflammation alone is not a good predictor of mortality in this setting. We therefore focused on the potential systemic effects of AH. This hypothesis was based on three main observations. First, patients with AH often show signs of SIRS at admission.22,23 Second, SIRS predicts the development of AKI in patients with AH, which is associated with decreased survival.5 Third, we consistently found that serum levels of powerful inflammatory mediators (e.g., interleukin-8, osteopontin, chemokine [C-C motif] ligand 20) correlate with their hepatic expression, suggesting that the liver is a source of circulating cytokines in AH.8,9,24 Our study clearly shows that the presence of SIRS is a major predictor of MOF and death in AH, suggesting that targeting systemic inflammation represents a novel therapeutic approach for these patients. In other diseases associated with sterile inflammation and SIRS, such as acute pancreatitis, the use of drugs that decrease the systemic inflammatory response has shown promising results.25
It is important to emphasize that all of the AH cases in our study were confirmed histologically. There are no reliable noninvasive methods to establish the diagnosis of AH. In our center, we have systematically performed transjugular biopsies in all patients with suspected AH; and in our experience, the diagnosis is confirmed in 75%–80% of patients. Alternative histologic diagnosis in patients with clinical suspicion of AH included decompensated alcoholic cirrhosis without superimposed AH, septic liver, ischemic hepatitis, and drug-induced liver injury. Therefore, our results may not apply to other causes of acute-on-chronic liver injury in patients with alcoholic liver disease.
The most striking result of this study is the demonstration that the presence of SIRS at admission is an important predictor of MOF and mortality in AH. This result has pathophysiological implications because it highlights the fact that extrahepatic consequences of AH are of paramount importance to a patient’s outcome. Moreover, we provide evidence that the existence of SIRS in these patients is not necessarily associated with an ongoing infection. In fact, in two-thirds of patients with SIRS criteria, an infection could not be detected by either clinical or microbiological criteria. It is difficult to accurately rule out infections in this setting because the sensibility of current culture techniques is limited. Therefore, the presence of SIRS in some of these patients could be secondary to an occult bacterial infection not identified with current diagnostic tests. However, it is also likely that SIRS in a subset of patients of this group is due to a sterile systemic inflammation. This may place AH within the growing group of liver pathologies, including acetaminophen toxicity and nonalcoholic steatohepatitis, in which sterile inflammation plays a prominent part.7,26 A key component of this is the release of extracellular damage–associated molecular pattern molecules, which increase inflammation within the liver and can also cause inflammation in additional organs, resulting in a systemic inflammatory response. A large number of damage-associated molecular pattern molecules have been identified, and many, such as DNA, are excellent therapeutic targets.27 These data are also entirely consistent with recent experimental studies which have identified a role for pathways downstream of pattern recognition receptor activation, particularly the NOD-like receptor family, pyrin domain containing-3 inflammasome and interleukin-1β production.28 Discovering the mechanisms of sterile inflammation in AH will certainly lead to new pathophysiologically oriented therapies.
An important result of the study is that infections during hospitalization, but not at admission, are a major predictor of MOF at death. These results corroborate previous studies in patients with AH or cirrhosis.6,29,30 The impact of nosocomial infections is related to a more aggressive resistance profile of bacteria as well as to a more deteriorated liver function. Clinical trials assessing the beneficial effects of intestinal decontamination in patients with severe AH are under way.
Given that the presence of SIRS in AH can be related to an infectious episode or to sterile inflammation, it seems clinically relevant to find biomarkers capable of discriminating between these two entities. For this purpose, we analyzed a panel of biomarkers. Among them, we found that procalcitonin is useful in identifying bacterial infection as the cause of SIRS. This biomarker can be clinically useful because it can help in detecting patients who would benefit from early therapy with broad-spectrum antibiotics. This finding is consistent with a recent study showing that procalcitonin can be useful as a biomarker of infections in patients with AH and SIRS.31
Another relevant result from our study is that LPS serum levels at admission predict which patients will not respond to corticosteroids. There is mounting evidence that patients with alcoholic liver disease have profound dysbacteriosis and impaired tight junctions in the intestinal mucosa, favoring the translocation of gram-negative bacterial products such as LPS to the portal vein.32 Lipopolysaccharide signals through tolllike receptor-4 located in Kupffer cells and hepatic stellate cells, leading to hepatic inflammation and fibrosis.32,33 Besides intrahepatic effects, increased circulating LPS levels could also play a role in the systemic inflammatory response and the complications of cirrhosis, such ascites, variceal bleeding, and encephalopathy34–36 Our results showing that procalcitonin and LPS predict MOF and death should be confirmed in independent cohorts. There is an ongoing pilot study assessing the beneficial effects of intestinal decontamination with rifaximin in patients with severe AH.37
In conclusion, our study demonstrates that the systemic inflammatory response, either caused by an ongoing infection or in the absence of an identifiable infection, is associated with the development of MOF and death in AH. Further studies should identify the main inflammatory mediators that cause this systemic response in order to develop targeted therapies.
Supplementary Material
Acknowledgments
Supported by grants from Fondo de Investigación Sanitaria (FIS PI041538 and FIS PI042380, to R.B. and J.C., respectively) and by National Institute on Alcohol Abuse and Alcoholism grants 1U01AA021908, 1U01AA020821, and P60-AA011605. J.M. was enrolled in the Master on Research in Liver Diseases of the Universitat de Barcelona and received a Formación del Profesorado Universitario grant of the Spanish Education Ministry. J.A. received a grant from Fundación Banco Bilbao Vizcaya Argentaria and was enrolled in the Master on Research in Liver Diseases of the Universitat de Barcelona. S.A. received a grant from Institut d’Investigacions Biomèdiques August-Pi-Sunyer. O.M.-I. received a grant from la Generalitat de Catalunya for Research Stays Abroad (BE-DGR 2012). P.S.-B. is funded by ICSIII, Miguel Servet (CP11/00071) and cofinanced by Fondo Europeo de Desarrollo Europeo, Unión Europea, “Una manera de hacer Europa.”
Abbreviations
- ABIC
age, bilirubin, international normalized ratio, creatinine
- AH
alcoholic hepatitis
- AKI
acute kidney injury
- CI
confidence interval
- DF
discriminant function
- hsCRP
high-sensitivity C-reactive protein
- LPS
lipopolysaccharide
- MELD
Model for End-Stage Liver Disease
- MOF
multiple organ failure
- OR
odds ratio
- SIRS
systemic inflammatory response syndrome
- SOFA
Sequential Organ Failure Assessment
Footnotes
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.27779/suppinfo
Potential conflict of interest: Nothing to report.
Author names in bold designate shared co-first authorship.
References
- 1.Lucey M, Mathurin P, Morgan T. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 2.Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 4.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Altamirano J, Fagundes C, Dominguez M, García E, Michelena J, Cárdenas A, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–548. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 7.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–1650. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Affò S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782–1792. doi: 10.1136/gutjnl-2013-306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustot T, Maillart E, Bocci M, Surin R, Trépo E, Degré D, et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J Hepatol. 2014;60:267–274. doi: 10.1016/j.jhep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, et al. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299–1304. doi: 10.1016/j.jhep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, et al. Acute phase proteins for the diagnosis of bacterial infections and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599–607. [PubMed] [Google Scholar]
- 13.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. HEPATOLOGY. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. Crit Care Med. 1992;20:864–874. [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. ; on behalf of the Working Group on Sepsis-related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter prospective study. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 18.Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. HEPATOLOGY. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 19.Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–1239. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 21.Dubuquoy L, Louvet A, Lassailly G, Artru F, Truant S, Maggiotto F, et al. Alcoholic hepatitis resistant to medical therapy is characterized by an altered differentiation of hepatic progenitors under the influence of extracellular matrix [Abstract] J Hepatol. 2014;60(Suppl):S59. [Google Scholar]
- 22.Katoonizadeh A, Laleman W, Verslype C, Wilmer A, Maleux G, Roskams T, et al. Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut. 2010;59:1561–1569. doi: 10.1136/gut.2009.189639. [DOI] [PubMed] [Google Scholar]
- 23.Mookerjee RP, Lackner C, Stauber R, Stadlbauer V, Deheragoda M, Aigelsreiter A, et al. The role of liver biopsy in the diagnosis and prognosis of patients with acute deterioration of alcoholic cirrhosis. J Hepatol. 2011;55:1103–1111. doi: 10.1016/j.jhep.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Ibanez O, Dominguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. HEPATOLOGY. 2013;58:1742–1756. doi: 10.1002/hep.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda CJ, Babu BI, Siriwardena AK. Recombinant human activated protein C as a disease modifier in severe acute pancreatitis: systematic review of current evidence. Pancreatology. 2012;12:119–123. doi: 10.1016/j.pan.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Hoque R, Farooq A, Mehal WZ. Sterile inflammation in the liver and pancreas. J Gastroenterol Hepatol. 2013;28(Suppl):61–67. doi: 10.1111/jgh.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Olson JC, Subramanian RM, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. HEPATOLOGY. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barreto R, Fagundes C, Guevara M, Solà E, Pereira G, Rodríguez E, et al. Type-1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. HEPATOLOGY. 2014;59:1505–1513. doi: 10.1002/hep.26687. [DOI] [PubMed] [Google Scholar]
- 31.Kumar K, Mohindra S, Raj M, Choudhuri G. Procalcitonin as a marker of sepsis in alcoholic hepatitis. Hepatol Int. 2014;8:436–442. doi: 10.1007/s12072-014-9540-x. [DOI] [PubMed] [Google Scholar]
- 32.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. HEPATOLOGY. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 34.Mehta G, Gustot T, Mookerjee RP, Garcia-Pagan JC, Fallon MB, Shah VH, et al. Inflammation and portal hypertension—the undiscovered country. J Hepatol. 2014;61:155–163. doi: 10.1016/j.jhep.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G, et al. Acute-on chronic liver failure. J Hepatol. 2012;57:1336–1348. doi: 10.1016/j.jhep.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. Effects of rifaximin in patients with acute alcoholic hepatitis (RIFA-AAH) :116556. https://clinicaltrials.gov/ct2/show/NCT02.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


