Abstract
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the CNS caused by the human polyomavirus JC (JCV). JCV replication occurs only in human cells and investigation of PML has been severely hampered by the lack of an animal model. The common feature of PML is impairment of the immune system. The key to understanding PML is working out the complex mechanisms that underlie viral entry and replication within the CNS and the immunosurveillance that suppresses the virus or allows it to reactivate. Early models involved the simple inoculation of JCV into animals such as monkeys, hamsters and mice. More recently mouse models transgenic for the gene encoding the JCV early protein, T-antigen, a protein thought to be involved in the disruption of myelin seen in PML, have been employed. These animal models resulted in tumorigenesis rather than demyelination. Another approach is to use animal polyomaviruses that are closely related to JCV but able to replicate in the animal such as mouse polyomavirus and SV40. More recently, novel models have been developed that involve the engraftment of human cells into the animal. Here, we review progress that has been made to establish an animal model for PML, the advances and limitations of different models and weigh future prospects.
Keywords: Progressive multifocal leukoencephalopathy, Polyomavirus JC, Animal models
INTRODUCTION
Progressive multifocal leukoencephalopathy is a severe and often lethal demyelinating disease of the human CNS caused by the polyomavirus JC (JCV). In PML, JCV replicates productively in both astrocytes and oligodendrocytes leading to pathological multiple expanding regions of demyelination accompanied by progressive neurological deficits and death (Berger, 2011). PML was first described by Astrom et al. in 1958 and is characterized by a triad of histopathological features: demyelination, oligodendrocytes with nuclear inclusion bodies and giant “bizarre” astrocytes (Astrom et al., 1958). Symptoms reflect the location of the brain lesions and include visual deficits, cognitive impairment, speech and language disorders, motor weakness, gait disturbance, and sensory abnormalities. In almost all instances, an impairment of cell-mediated immunity is involved (Tavazzi et al., 2012). Since the inception of the AIDS pandemic, HIV infection has been the predominant predisposing condition for PML (Berger et al., 1998). More recently, exposure to certain immunomodulatory monoclonal antibodies such as natalizumab (Berger et al., 2010) has been associated with a significant risk for its development. While the molecular mechanisms of JCV reactivation are poorly understood, impaired cell-mediated immunity is thought to allow reactivated JCV to allow JCV to multiply unchecked. The persistent virus is thought to remain from an earlier non-pathological infection event that serological evidence indicates usually occurs in childhood (White and Khalili, 2011; Wollebo et al., 2015). Following primary infection, the viral replicative cycle is suppressed and the virus enters a persistent state that is not well understood. Usually, this latent/persistent viral infection remains asymptomatic but in rare circumstances involving some type of immune dysregulation, the virus escapes immunological suppression and actively replicates in glial tissue of the brain. The molecular mechanisms of JCV reactivation are complex and have been reviewed recently (White et al., 2009). They are thought to involve a number of cellular transcription factors which include NF-1 (Monaco et al., 2001), Egr1 (Romagnoli et al., 2008), NF-κB (Romagnoli et al., 2009), C/EBPβ (Romagnoli et al., 2009), NFAT4 (Wollebo et al, 2012), SpiB (Meira et al., 2014) and others. Similarly the molecular mechanism of JCV DNA replication is also complex (An et al., 2015).
Like other polyomaviruses, the genome of JCV is a covalent double-stranded DNA circle with three functional regions. The early region contains genes that are expressed during initial infection, large T-antigen (T-Ag) and small t-antigen (t-Ag), and which are involved in driving cell proliferation in order to optimize viral propagation (Khalili et al., 2006). The late region contains genes that are expressed subsequently including genes for the capsid proteins, VP1, VP2 and VP3 and a small regulatory protein called agnoprotein (Khalili et al., 2005). The noncoding regulatory region (NCCR) lies between the early and late and contains promoter/enhancer elements for transcription and the origin of viral DNA replication (DeCaprio et al., 2013).
JCV has a strict host range dictated by cellular species-specific and tissue-specific factors required for viral replication (Feigenbaum et al., 1987). Analysis of JCV/SV40 hybrid viruses has indicated that the region of the JCV genome near the origin of replication is important for determining viral tropism (Lynch and Frisque, 1990). In tissue culture studies, the virus is largely restricted to growth in primary human fetal glial cells, either astrocytes (Major and Vacante, 1989; Radhakrishnan et al., 2003) or oligodendrocytes (Darbinyan et al., 2013). These systems have been important in elucidating the basic molecular virology of JCV and testing of different antiviral compounds. However, lack of a suitable animal model for PML due to the failure of JC virus to productively replicate in non-human hosts has hampered JCV research. JCV replication is regulated by the viral early protein T-Ag, which forms a complex between the JC viral origin of DNA replication and the complex of cellular proteins that comprise the host DNA synthesis machinery to initiate viral DNA replication. This inability of JCV to replicate in non-human cells is due to a requirement for a species-specific factor, presumably a component of DNA polymerase for JCV DNA replication (Feigenbaum et al., 1987). This is a major obstacle to establishing an animal model nevertheless several groups have worked on this problem and some progress has been made.
What features of PML should be exhibited by an animal model?
PML has many distinctive characteristics in terms of its pathology as well as the virological and immunological aspects of the disease. A good animal model should incorporate these features to the fullest extent possible (Table 1).
Table 1.
Faithful recapitulation of PML in the animal model requires
| 1) | Peripheral infection by JC virus |
| 2) | Viral latency in peripheral and/or CNS tissues before the development of the disease |
| 3) | Impaired cell-mediated immunity a prerequisite for disease expression |
| 4) | Viral replication in oligodendrocytes and astrocytes with demyelination |
| 5) | Clinical disease expression concordant with PML |
| 6) | Histopathological features to include demyelination, enlarged oligodendroglial nuclei, and bizarre astrocytes |
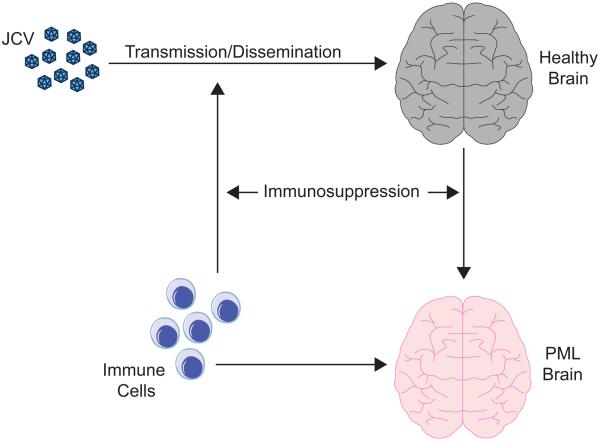
Figure 1 shows a schematic diagram of key features of the pathogenesis of PML from acquisition of JC virus and the onset of the disease. A good animal model should exhibit neurological disturbances similar to the clinical features of PML. From the pathological aspect, PML has a histopathological triad of characteristics (Astrom et al., 1958). Firstly, PML lesions contain oligodendrocytes with dense nuclear inclusion bodies. These inclusion bodies contain crystalline arrays of JC virions, which can be visualized under the electron microscope. The cytopathic effect of the virus eventually leads to oligodendrocyte lysis and the virions are released to initiate another round of infection. Recently, we observed that JCV is also able to inhibit the differentiation of oligodendrocyte precursor cells (Darbinyan et al., 2013) indicating that the virus may act to inhibit oligodendrocyte regeneration in addition to causing oligodendrocyte death. Secondly, demyelination which is responsible for the neurological symptoms and is the result of oligodendrocyte infection and death. However, if the inhibition of oligodendrocyte regeneration observed in cell culture (Darbinyan et al., 2013) also occurs in clinical PML, failure to produce new oligodendrocytes and myelin may also contribute. Thirdly, PML lesions contain giant bizarre astrocytes, which contain virions and express VP1 indicating that they are productively infected by JCV albeit to a much lesser extent than oligodendrocytes since virions in astrocytes are more often found in the cytoplasm rather than the nucleus (Mazlo et al., 2001).
Figure 1. Development of PML.
A schematic diagram of important aspects of the development of PML is shown. JCV is transmitted to the host and is disseminated, which in human is thought to occur orally followed by the hematogenous spread and in animal models may be by injection or otherwise as specified in Column 3 of Table 2. Virus enters the brain (grey) where it may go on to replicate and cause PML (red). Cells of the immune system may initially aid the spread of JCV via white blood cells, which may harbor and transport the virus and cross the blood-brain barrier conveying virus into the CNS prior to the onset of PML. On the other hand, the cellular immune system may be involved in neuroimmunoserveillance, control of the virus and inhibition of PML development by removing infected glial cells via virus-specific cytotoxic T-cells.
From the virological aspect, animal models should show the same properties as PML. For example, JCV has a tropism for glial cells and infects mainly oligodendrocytes and, to a lesser extent, astrocytes. Models should also show similar molecular virological aspects, such as the immunohistochemical detection of the expression of transcription factors shown to be important for the JCV life cycle, e.g., Sp1 (Henson et al., 1992), p53 (Lammie et al., 1994) and Egr-1 (Romagnoli et al., 2008). The choice of the strain of JCV, i.e., the configuration of the NCCR, is also important for the animal model. The archetypal strain (Yogo et al., 1990) is the one that is transmitted from person to person in the human population but it is always a neurotropic strain, often called “PML-type”, with a rearranged NCCR that is found in PML (White and Khalili, 2011). Neurotropic strains are likely derived from the archetype, although where and how these rearrangements occur are poorly understood. If one administers the archetype to an animal, it is not clear that the sequence can develop the essential rearrangements. If one administers a neurotropic strain, such as Mad-1 or Mad-4, then the ability of the virus to track from the uroepithelium to glial cells may be artifactually affected. Available evidence suggests that it is the neurotropic virus that enters the brain and causes PML (White and Khalili, 2011), so this would seem to be the preferable strain to use. Lastly, from an immunological aspect, PML pathogenesis is typically associated with impaired cell-mediated immunity and neuroimmunoserveillance (Beltrami and Gordon, 2014). Immune dysfunction should be a prerequisite for a PML animal model that faithfully recapitulates the disease in man.
Towards an animal model
As noted, the major problem in creating an animal model for PML is the inability of JC virus to replicate in any cell that is not human. A corollary of this is that virus becomes “stuck” in the early phase of infection in animal cells with T-Ag being expressed, but no expression of capsid proteins and no viral DNA replication, i.e., a nonpermissive infection (White and Khalili, 2004; IARC working group on the evaluation of carcinogenic risks to humans, 2012).
Rodents have been extensively used in the study of JCV and it was in newborn Syrian golden hamsters that the tumorigenic potential of JCV was first reported after virus inoculation both subcutaneously and intracerebrally (Walker et al., 1973) and further described by Zu Rhein and Varakis (1979). This was the first evidence for the oncogenic potential of JCV. In owl monkeys or squirrel monkeys inoculated with purified JCV intracerebrally, brain tumors were observed including astrocytoma and malignant mixed glial/neuronal tumors (London et al., 1978; Houff et al., 1983; London et al., 1983). Major et al., (1987) reported glioblastoma in 1 of 4 juvenile owl monkeys injected intracerebrally with the Mad-1 strain of JCV. Of note, glioblastoma cell lines were cultured from the tumors, and infectious virions were isolated from these. This study remains the only report of infectious virus recovered from the tissues or tumors of any experimental animals inoculated with JCV (IARC working group on the evaluation of carcinogenic risks to humans, 2012). Tumor formation has also been observed in mice transgenic for the JCV early region. In such mice, the role of T/t-antigens can be studied in the absence of the capsid protein genes. Small et al. (1986a) observed adrenal neuroblastomas in JCV T-Ag transgenic mice with the promoter of the JCV Mad-1 strain. Transgenic mice expressing JCV T-Ag under the control of the Mad-4 promoter also developed solid masses arising from the soft tissues surrounding the salivary gland, which histologically resembled malignant peripheral nerve sheath tumors (Shollar et al., 2004). In JCV T-Ag transgenic mice with the promoter of the JCV(CY) archetype strain, medulloblastoma or primitive neuroectodermal tumors developed (Krynska et al., 1999). Thus the phenotype of JCV T-Ag transgenic mice depends on the strain of JCV used and is also affected by the genetic background of the mouse. In conclusion, JCV T-Ag expression in the absence of robust JCV replication results in tumor formation. Later studies revealed several pathways by which T-Ag induces the progression of tumors (Del Valle et al., 2008).
Animal models exhibiting myelin deficiency in brain
Early studies created JCV T-Ag transgenic mice, in which the native JCV early promoter drove expression of T-Ag expression in the absence of the JCV late region. Some of these mice exhibited a shaking disorder and neuropathological analysis indicated dysmyelination in the central nervous system (CNS), but not the peripheral nervous system with high levels of T-Ag present in the brain (Small et al., 1986b). Immunocytochemistry and in situ hybridization of myelin-specific and JCV gene expression in these mice suggested that expression of JCV T-Ag occurred predominantly in oligodendrocytes and was the primary cause of dysmyelination with affected oligodendrocytes not properly myelinating the axons (Trapp et al., 1988). In another study, transgenic mice containing the JCV early region also developed neurological disease resulting from CNS dysmyelination and analysis of the expression of the myelin basic protein (MBP) gene at the RNA and protein level revealed great reductions in MBP levels that paralleled the severity of dysmyelination suggesting that T-Ag negatively influences transcription of the MBP gene (Haas et al., 1994). Further analysis supported a model where the effect of JCV on inducing hypomyelination occurred by interaction of JCV T-Ag and the myelin gene transcription factor, MEF-1/Purα (Tretiakova et al., 1999b). The observation that dysmyelination occurred in the absence of late gene expression, this suggests that T-Ag has an important role for T-Ag in this process (Small et al., 1986b; Trapp et al., 1988; Haas et al., 1994). Another pathway that may be involved in the demyelination observed with PML is the ability of JCV infection to suppress the differentiation of oligodendrocyte precursor cells (Darbinyan et al., 2013) precluding remyelination. In another model, oligodendrocytes from mice transgenic for mouse polyomavirus T-Ag exhibit features of immaturity and fail to myelinate axons properly (Baron-Van Evercooren et al., 1992).
In another study, a novel mouse model with a humanized immune system was created by engrafting NOD/SCID/IL-2-Rg (null) mice with human lymphocytes and thymus (Tan et al., 2013). Mice inoculated with JCV remained asymptomatic following inoculation but JCV DNA was occasionally detected in both blood and urine and mice generated both humoral and cellular immune responses against JCV concomitant with expression of the immune exhaustion marker, PD-1, on lymphocytes consistent with a response to an infection (Tan et al., 2013). This may be a useful animal model to study interactions of JCV with the immune system but it is not a model for PML.
A PML-like disease has been modeled in rhesus monkeys, which were immunosuppressed by infection with the virus SHIV-89.6P and then superinfected with the polyomavirus SV40, which is closely related to JCV but is able to replicate in rhesus monkey cells (Axthelm et al., 2004). Monkeys developed CNS pathology characterized by demyelination and meningoencephalitis. This system may be useful to shed light on the pathogenic mechanisms of primate polyomaviruses in the immunocompromised host. In another approach, stable persistence of JCV-infected human cells, either JCI cells derived from the human IMR-32.13 neuroblastoma cell line or SVG-A cells (SV40-transformed human fetal glial cell line) in the mouse brain was obtained by inoculating the virus-infected cells into nude mice brains. The JCV-infected cells persisted in the nude mouse brains for 2 weeks and JCV gene expression could be inhibited by siRNA against JCV agnoprotein with atelocollagen as a carrier (Matoba et al., 2008). Of note, none of these models has allowed JCV infection of the CNS or modeled PML pathogenesis. A summary of the various animal models for PML is given in Table 2.
Table 2.
Animal models of progressive multifocal leukoencephalopathy.
| Species | Agent | Introduction | Outcome | NCCR | Reference |
|---|---|---|---|---|---|
| Owl monkey/Squirrel monkey | Purified JCV | Intracerebral injection | Astrocytoma | Mad-1 | London et al., 1978; Houff et al., 1983; London et al., 1983 |
| Owl monkey | Purified JCV | Intracerebral injection | Astrocytoma | Mad-1 | Major et al., 1987 |
| Syrian golden hamster | Purified JCV | Intracerebral injection | Glioma | Mad-1 | Walker et al., 1973 |
| Syrian golden Hamster | Purified JCV | Intracerebral injection | Medulloblastoma | Mad-4 | Zu Rhein and Varakis, 1979 |
| Mice | JCV T-Ag | Transgenic | Adrenal Neuroblastoma | Mad-1 | Small et al., 1986a |
| Mice | JCV T-Ag | Transgenic | CNS Dysmyelination | Mad-1 | Small et al., 1986b Trapp et al., 1988 Haas et al., 1994 |
| Mice | Polyoma T-Ag | Transgenic | CNS Dysmyelination | Murine polyomavirus | Baron-Van Evercooren et al., 1992 |
| Mice | JCV T-Ag | Transgenic | Medulloblastoma/PNET | Archetype (CY) | Krynska et al., 1999 |
| Mice | JCV T-Ag | Transgenic | MPNST | Mad-4 | Shollar et al., 2004 |
| Engrafted NOD/SCID/IL-2-Rg (null) mice | Purified JCV | Intraperitoneal injection | Anti-JCV immune response | Mad-4 or CY | Tan et al., 2013 |
| SHIV infected Rhesus monkeys | SV40 | Intravenous injection | Meningoencephalitis and demyelination | SV40 | Axthelm et al., 2004 |
| Nude mice | JCV-infected human cells | Intracerebral injection | Persistence of infected cells | Mad-1 | Matoba et al., 2008 |
| Engrafted Rag2−/− Mbpshi/Shi mice | Purified JCV | Intracerebral injection | Demyelination | Mad-1 | Kondo et al., 2014 |
T-Ag – T-antigen; PNET – Primitive neuroectodermal tumor; MPNST – Malignant peripheral nerve sheath tumor
More recently, a mouse model was generated by engrafting bipotential human glial progenitor cells (GPCs) prepared from human fetal brain tissue into neonatal immunodeficient and myelin-deficient mice (Rag2−/− Mbpshi/shi). The forebrain glial populations of these mice became substantially humanized with age (Kondo et al., 2014). When injected intracerebrally with JCV, productive infection occurred and subsequent demyelination. In these mice, JCV was principally spread by infection of GPCs and astrocytes, which were infected more readily than oligodendrocytes in this system. Viral replication occurred primarily in human astrocytes and GPCs rather than oligodendrocytes. Oligodendrocytes expressed T-Ag and showed cell death by apoptosis, but this appeared to be a secondary occurrence and led to demyelination. Interestingly, sequence analysis of virus revealed mutations in the JCV VP1 capsid gene after infection, suggesting that virus may evolve during active infection. Thus in this system, the principal targets for JCV infection are astrocytes and GPCs and demyelination is a secondary occurrence, following T-Ag-triggered oligodendroglial apoptosis (Kondo et al., 2014). In this regard, previous studies have shown viral infection or viral protein expression inhibits differentiation of oligodendrocyte progenitor cells and glial cells in culture (Darbinyan et al., 2013; Tretiakova et al., 1999a).
The new mouse model however shows important differences to PML in the clinic. In clinical infections, oligodendrocytes express VP1 (Jochum et al., 1997; Del Valle and Pina-Oviedo, 2006) and their nuclei contain large numbers of JC virions (inclusion bodies) indicating robust productive infection of oligodendrocytes (Del Valle and Pina-Oviedo, 2006; Major et al., 1992). While astrocytes are also infected, express VP1 (Del Valle and Pina-Oviedo, 2006; Askamit et al., 1986) and contain virions detectable by electron microscopy (Watanabe and Preskhorn, 1976; Mazlo et al., 2001), this infection occurs to a lesser extent than in oligodendrocytes. The explanation for this difference between the mouse chimerae and human clinical samples is not clear but may be related to important differences between the two situations.
Firstly, in the chimeric mice, the route of entry of virus into the brain is by direct intracerebral inoculation of high-titre (1012 – 1013 genome equivalents/ml), highly purified virus (109 – 1010 genome equivalents/injection). On the other hand, clinical PML involves indirect delivery to the brain: an asymptomatic primary infection, a period of persistence that is not well understood, migration of virus through the blood and across the blood brain barrier, infection of glial cells and reactivation. These are processes that may occur over a long period of time before the virus finally spreads lytically to cause the lesions of PML
Secondly, the nature of immunosuppression in the chimeric mouse differs from that in humans who are predisposed to PML. In the chimeric mouse, there is a homozygous loss-of-function mutation in RAG2, one of the recombination-activating genes (RAGs) that function in VDJ rearrangement of genes of immunoglobulin and T cell receptor molecules. RAG2 is essential for the generation of mature B and T cells, which are crucial components of the adaptive immune system. On the other hand, clinical PML occurs predominantly in individuals with a dysfunction or modulation of cellular immunity and immunosurveillance (Beltrami and Gordon, 2014). JCV-specific cellular immune responses in PML patients are correlated with outcome in HIV-1/AIDS (Koralnik et al., 2001) and an association between JCV-specific cytotoxic T lymphocytes and the early control of PML was demonstrated in a prospective study (Du Pasquier et al., 2004). These data suggest that it is mainly JCV-specific cellular immunity that contains PML.
Finally, perhaps the most important difference between the mouse model and the human is that while the engrafted cells in this mouse model are human, all other cells, such as the immune cells of the blood, etc., are of mouse origin and are thus not susceptible to infection by JCV. This is a significant limitation because it will preclude studies to define primary infection, establishment of latency, spread and trafficking of JCV from periphery to brain and other factors affecting disease manifestation (Haley and Atwood, 2014).
These shortcomings notwithstanding, the mouse model generated by Kondo and colleagues may serve as a starting point in the development of more sophisticated and relevant models, which more closely recapitulate pathological features of PML in the human brain. It will be interesting to see what we can learn from this new animal model and those are likely to emerge in the future, which may be more pertinent to clinical PML.
Future directions: development of more sophisticated and relevant animal models for PML
The life cycle of JCV and pathogenesis of PML are complex and there is much that remains to be elucidated. Producing an animal model of the disease has been challenging but some aspects of the disease have been modeled in animals. The mouse model of Tan et al. (2013) with a an engrafted humanized immune system and inoculated with JCV is a possible starting point for an animal model to study JCV/immune system interactions but it is not a model for the PML disease process. As just described, the mouse model of Kondo et al. (2014) has advantages and limitations. Perhaps, a model combining this system with aspects of the model of Tan et al. (2013) would be a next step. Importantly, virus should be injected intraperitoneally or subcutaneously or ingested orally rather than injected intracranially in large doses so that it has to cross the blood-brain barrier as it does in PML pathogenesis in humans. A major limitation of mouse models is the short (~2–3 year) life span of the mouse, so it may be necessary to modify viral delivery to get effective induction of PML in a timely fashion. Larger animals such as monkeys do not have this limitation and we have mentioned the rhesus monkey model for HIV-1/PML that is co-infected with SHIV-89.6P and SV40 and develops CNS demyelination and meningoencephalitis (Axthelm et al., 2004). While this may indeed be a useful system to study the pathogenesis of primate polyomaviruses in immunocompromised hosts, SV40 is not JCV and important differences exist between the viruses. Unfortunately, JCV cannot be used in monkeys because monkeys inoculated with JCV develop tumors and not PML (White and Khalili, 2004; IARC working group on the evaluation of carcinogenic risks to humans, 2012). Another possibility is to introduce mutations into JCV or engineer the animal genome in a way that might facilitate viral replication although this would be a complex task. Clearly, the prospect of creating new and improved animal models for PML is a difficult one and offers many challenges for future research.
ACKNOWLEDGEMENTS
We wish to thank past and present members of the Department on Neuroscience and Center for Neurovirology for their continued support and insightful discussions. We also acknowledge the intellectual contributions of the Temple University School of Medicine Comprehensive Neuroaids Center (Basic Science Cores I and II). This work was supported by grants awarded by the NIH to MKW and KK.
Contract grant sponsor: NIH/NIAID; Contract grant numbers R01AI077460 (MKW) and P30MH092177 (KK)
Footnotes
CONFLICTS OF INTEREST
None
REFERENCES
- Aksamit AJ, Sever JL, Major EO. Progressive multifocal leukoencephalopathy: JC virus detection by in situ hybridization compared with immunohistochemistry. Neurology. 1986;36:499–504. doi: 10.1212/wnl.36.4.499. [DOI] [PubMed] [Google Scholar]
- An P, Brodsky JL, Pipas JM. The conserved core enzymatic activities and the distinct dynamics of polyomavirus large T antigens. Arch Biochem Biophys. 2015;573:23–31. doi: 10.1016/j.abb.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958;81:93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Axthelm MK, Koralnik IJ, Dang X, Wüthrich C, Rohne D, Stillman IE, Letvin NL. Meningoencephalitis and demyelination are pathologic manifestations of primary polyomavirus infection in immunosuppressed rhesus monkeys. J Neuropathol Exp Neurol. 2004;63:750–758. doi: 10.1093/jnen/63.7.750. [DOI] [PubMed] [Google Scholar]
- Baron-Van Evercooren A, Jensen NA, Wyss MT, Cuzin F, Rassoulzadegan M, Brucher JM, Baron H. Transgenic mice expressing polyoma virus large T antigen in astrocytes develop severe dysmyelination of the central nervous system. Lab Invest. 1992;66:39–53. [PubMed] [Google Scholar]
- Beltrami S, Gordon J. Immune surveillance and response to JC virus infection and PML. J Neurovirol. 2014;20:137–149. doi: 10.1007/s13365-013-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4:59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- Berger JR. Progressive multifocal leukoencephalopathy and newer biological agents. Drug Saf. 2010;33:969–983. doi: 10.2165/11537510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Berger JR. The clinical features of PML. Cleve Clin J Med. 2011;78(Suppl2):S8–12. doi: 10.3949/ccjm.78.s2.03. [DOI] [PubMed] [Google Scholar]
- Darbinyan A, Kaminski R, White MK, Darbinian-Sarkissian N, Khalili K. Polyomavirus JC infection inhibits differentiation of oligodendrocyte progenitor cells. J Neurosci Res. 2013;91:116–127. doi: 10.1002/jnr.23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio JA, Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Sixth Edition Lippincott, Williams & Wilkins; Philadelphia: 2013. pp. 6133–1661. [Google Scholar]
- Del Valle L, Pina-Oviedo S. HIV disorders of the brain: pathology and pathogenesis. Front Biosci. 2006;11:718–732. doi: 10.2741/1830. [DOI] [PubMed] [Google Scholar]
- Del Valle L, White MK, Khalili K. Potential mechanisms of the human polyomavirus JC in neural oncogenesis. J Neuropathol Exp Neurol. 2008;67:729–40. doi: 10.1097/NEN.0b013e318180e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–1978. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- Feigenbaum L, Khalili K, Major E, Khoury G. Regulation of the host range of human papovavirus JCV. Proc Natl Acad Sci USA. 1987;84:3695–3698. doi: 10.1073/pnas.84.11.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas S, Haque NS, Beggs AH, Khalili K, Knobler RL, Small J. Expression of the myelin basic protein gene in transgenic mice expressing human neurotropic virus, JCV, early protein. Virology. 1994;202:89–96. doi: 10.1006/viro.1994.1325. [DOI] [PubMed] [Google Scholar]
- Haley SA, Atwood WJ. An animal model for progressive multifocal leukoencephalopathy. J Clin Invest. 2014;124:5103–5106. doi: 10.1172/JCI79186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J, Saffer J, Furneaux H. The transcription factor Sp1 binds to the JC virus promoter and is selectively expressed in glial cells in human brain. Ann Neurol. 1992;32:72–77. doi: 10.1002/ana.410320112. [DOI] [PubMed] [Google Scholar]
- Houff SA, London WT, Zu Rhein GM, Padgett BL, Walker DL, Sever JL. New world primates as a model of viral-induced astrocytomas. Prog Clin Biol Res. 1983;105:223–226. [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 104. WHO Press; Geneva, Switzerland: 2012. Malaria and some polyomaviruses (SV40, BK, JC, and Merkel cell viruses) pp. 1–406. [PMC free article] [PubMed] [Google Scholar]
- Jochum W, Weber T, Frye S, Hunsmann G, Lüke W, Aguzzi A. Detection of JC virus by anti-VP1 immunohistochemistry in brains with progressive multifocal leukoencephalopathy. Acta Neuropathol. 1997;94:226–231. doi: 10.1007/s004010050697. [DOI] [PubMed] [Google Scholar]
- Khalili K, Gordon J, White MK. The polyomavirus, JCV and its involvement in human disease. Adv Exp Med Biol. 2006;577:274–287. doi: 10.1007/0-387-32957-9_20. [DOI] [PubMed] [Google Scholar]
- Khalili K, White MK, Sawa H, Nagashima K, Safak M. The agnoprotein of polyomaviruses: a multifunctional auxiliary protein. J Cell Physiol. 2005;204:1–7. doi: 10.1002/jcp.20266. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Windrem MS, Zou L, Chandler-Militello D, Schanz SJ, Auvergne RM, Betstadt SJ, Harrington AR, Johnson M, Kazarov A, Gorelik L, Goldman SA. Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest. 2014;124:5323–5336. doi: 10.1172/JCI76629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik IJ, Du Pasquier RA, Letvin NL. JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol. 2001;75:3483–3487. doi: 10.1128/JVI.75.7.3483-3487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV(CY) T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999;18:39–46. doi: 10.1038/sj.onc.1202278. [DOI] [PubMed] [Google Scholar]
- Lammie GA, Beckett A, Courtney R, Scaravilli F. An immunohistochemical study of p53 and proliferating cell nuclear antigen expression in progressive multifocal leukoencephalopathy. Acta Neuropathol. 1994;88:465–471. doi: 10.1007/BF00389500. [DOI] [PubMed] [Google Scholar]
- London WT, Houff SA, Madden DL, Fuccillo DA, Gravell M, Wallen WC, Palmer AE, Sever JL, Padgett BL, Walker DL, ZuRhein GM, Ohashi T. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus) Science. 1978;201:1246–1249. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- London WT, Houff SA, McKeever PE, Wallen WC, Sever JL, Padgett BL, Walker DL. Viral-induced astrocytomas in squirrel monkeys. Prog Clin Biol Res. 1983;105:227–237. [PubMed] [Google Scholar]
- Lynch KJ, Frisque RJ. Identification of critical elements within the JC virus DNA replication origin. J Virol. 1990;64:5812–5822. doi: 10.1128/jvi.64.12.5812-5822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major EO, Vacante DA, Traub RG, London WT, Sever JL. Owl monkey astrocytoma cells in culture spontaneously produce infectious JC virus which demonstrates altered biological properties. J Virol. 1987;61:1435–1441. doi: 10.1128/jvi.61.5.1435-1441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5:49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major EO, Vacante DA, Traub RG, London WT, Sever JL. Owl monkey astrocytoma cells in culture spontaneously produce infectious JC virus which demonstrates altered biological properties. J Virol. 1987;61:1435–1441. doi: 10.1128/jvi.61.5.1435-1441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major EO, Vacante DA. Human fetal astrocytes in culture support the growth of the neurotropic human polyomavirus, JCV. J Neuropathol Exp Neurol. 1989;48:425–436. doi: 10.1097/00005072-198907000-00004. [DOI] [PubMed] [Google Scholar]
- Matoba T, Orba Y, Suzuki T, Makino Y, Shichinohe H, Kuroda S, Ochiya T, Itoh H, Tanaka S, Nagashima K, Sawa H. An siRNA against JC virus (JCV) agnoprotein inhibits JCV infection in JCV-producing cells inoculated in nude mice. Neuropathology. 2008;28:286–294. doi: 10.1111/j.1440-1789.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- Mazlo M, Ressetar HG, Stoner GL. The neuropathology and pathogenesis of progressive multifocal leukoencephalopathy. In: Khalili K, Stoner GL, editors. Human polyomaviruses: molecular and clinical perspectives. John Wiley & Sons, Inc; New York: 2001. pp. p257–336. [Google Scholar]
- Meira M, Sievers C, Hoffmann F, Derfuss T, Kuhle J, Kappos L, Lindberg RL. MiR-126: a novel route for natalizumab action? Mult Scler. 2014;20:1363–1370. doi: 10.1177/1352458514524998. [DOI] [PubMed] [Google Scholar]
- Monaco MC, Sabath BF, Durham LC, Major EO. JC virus multiplication in human hematopoietic progenitor cells requires the NF-1 class D transcription factor. J Virol. 2001;75:9687–9695. doi: 10.1128/JVI.75.20.9687-9695.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S, Otte J, Enam S, Del Valle L, Khalili K, Gordon J. JC virus-induced changes in cellular gene expression in primary human astrocytes. J Virol. 2003;77:10638–10644. doi: 10.1128/JVI.77.19.10638-10644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli L, Sariyer IK, Tung J, Feliciano M, Sawaya BE, Del Valle L, Ferrante P, Khalili K, Safak M, White MK. Early growth response-1 protein is induced by JC virus infection and binds and regulates the JC virus promoter. Virology. 2008;375:331–341. doi: 10.1016/j.virol.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPbeta. Virus Res. 2009;146:97–106. doi: 10.1016/j.virusres.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shollar D, Del Valle L, Khalili K, Otte J, Gordon J. JCV T-antigen interacts with the neurofibromatosis type 2 gene product in a transgenic mouse model of malignant peripheral nerve sheath tumors. Oncogene. 2004;23:5459–5467. doi: 10.1038/sj.onc.1207728. [DOI] [PubMed] [Google Scholar]
- Small JA, Khoury G, Jay G, Howley PM, Scangos GA. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc Natl Acad Sci USA. 1986a;83:8288–8292. doi: 10.1073/pnas.83.21.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JA, Scangos GA, Cork L, Jay G, Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986b;46:13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- Tan CS, Broge TA, Seung E, Vrbanac V, Viscidi R, Gordon J, Tager AM, Koralnik IJ. Detection of JC virus-specific immune responses in a novel humanized mouse model. PLoS One. 2013;8:e64313. doi: 10.1371/journal.pone.0064313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev Med Virol. 2012;22:18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Small JA, Pulley M, Khoury G, Scangos GA. Dysmyelination in transgenic mice containing JC virus early region. Ann Neurol. 1988;23:38–48. doi: 10.1002/ana.410230108. [DOI] [PubMed] [Google Scholar]
- Tretiakova A, Krynska B, Gordon J, Khalili K. Human neurotropic JC virus early protein deregulates glial cell cycle pathway and impairs cell differentiation. J Neurosci Res. 1999a;55:588–599. doi: 10.1002/(SICI)1097-4547(19990301)55:5<588::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tretiakova A, Otte J, Croul SE, Kim JH, Johnson EM, Amini S, Khalili K. Association of JC virus large T antigen with myelin basic protein transcription factor (MEF-1/Puralpha) in hypomyelinated brains of mice transgenically expressing T antigen. J Virol. 1999b;73:6076–6084. doi: 10.1128/jvi.73.7.6076-6084.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973;181:674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Preskorn SH. Virus-cell interaction in oligodendroglia, astroglia and phagocyte in progressive multifocal leukoencephalopathy. An electron microscopic study. Acta Neuropathol. 1976;36:101–115. doi: 10.1007/BF00685273. [DOI] [PubMed] [Google Scholar]
- White MK, Khalili K. Pathogenesis of progressive multifocal leukoencephalopathy--revisited. J Infect Dis. 2011;203:578–586. doi: 10.1093/infdis/jiq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Khalili K. Polyomaviruses and human cancer: molecular mechanisms underlying patterns of tumorigenesis. Virology. 2004;324:1–16. doi: 10.1016/j.virol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol. 2009;83:10846–10856. doi: 10.1128/JVI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollebo HS, Melis S, Khalili K, Safak M, White MK. Cooperative roles of NF-κB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology. 2012;432:146–154. doi: 10.1016/j.virol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollebo HS, White MK, Gordon J, Berger JR, Khalili K. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol. 2015;77:560–570. doi: 10.1002/ana.24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990;64:3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Rhein GM, Varakis JN. Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC) Natl Cancer Inst Monogr. 1979;51:205–208. [PubMed] [Google Scholar]