Abstract
Placebo-controlled clinical trials are useful for identifying the dose of a drug candidate that produces a meaningful clinical response in a patient population. Currently Pfizer, Inc. is enrolling a 400-person clinical trial to test the efficacy of 20 or 80 mg of tafamidis to ameliorate transthyretin (TTR)-associated cardiomyopathy using clinical endpoints. Herein, we provide guidance for how to optimize the dose of tafamidis for each WT TTR cardiomyopathy patient using its mechanism of action as the key readout, i.e., we identify the dose of tafamidis that maximally kinetically stabilizes TTR in the blood. Tetramer dissociation is rate limiting for TTR aggregation, which appears to drive the pathology in the TTR amyloidosis. Hence we measure the TTR tetramer dissociation rate (kinetic stability) in the patients’ plasma as a function of tafamidis dose to optimize the dose employed to maximize kinetic stability. Historical data tells us that a subset of patients exhibiting higher tafamidis plasma concentrations are maximally kinetically stabilized at the 20 mg tafamidis dose, whereas the patient studied herein required a 60 mg once daily dose to achieve maximum kinetic stabilization. We anticipate that establishing the dose of tafamidis that achieves maximal TTR kinetic stabilization will translate into a maximal clinical effect, but that remains to be demonstrated.
Keywords: Senile Systemic Amyloidosis, wild-type TTR amyloid cardiomyopathy
Introduction
Numerous amyloid diseases appear to be caused by the extracellular aggregation of a specific protein [1–4]. In the transthyretin (TTR) amyloidoses, genetic and pharmacologic evidence strongly support the hypothesis that mutant and/or wild-type (WT) TTR aggregation drive the degenerative phenotypes [5–12]. Wild-type TTR amyloidosis (ATTRwt), formerly called Senile Systemic Amyloidosis (SSA), is a sporadic cardiomyopathy caused by aggregation of WT TTR in ~15% of the elderly population (predominantly males and especially those with heart failure and a preserved ejection fraction, HFpEF) [13, 14]. Mutant TTR aggregation can cause either familial amyloid polyneuropathy (FAP) [9, 15] and/or familial amyloid cardiomyopathy (FAC), depending on the exact mutation [4, 16, 17].
TTR is primarily secreted by the liver into the blood, where it is responsible for transporting holo retinol-binding protein and a small quantity of thyroxine [18, 19]. TTR exists as a 55-kDa tetramer, comprising four 127-amino-acid, β-sheet-rich subunits [20]. In older adults, WT TTR can aggregate into a spectrum of aggregate structures, including cross-β-sheet amyloid fibrils. The initial and rate-limiting step in this process of TTR aggregation or amyloidogenesis is TTR tetramer dissociation [6, 12, 21]. Tetramer dissociation is followed by partial monomer denaturation, resulting in the production of a TTR conformer that can aggregate by a downhill polymerization mechanism [21–23]. Since tetramer dissociation is rate limiting for TTR aggregation, increasing the kinetic barrier for tetramer dissociation through the binding of small molecule TTR kinetic stabilizers is a promising therapeutic strategy for TTR amyloidosis [6, 24].
Tafamidis, a small molecule drug approved for the treatment of FAP [5, 7], binds to and kinetically stabilizes TTR tetramers made up of WT and/or mutant TTR subunits, slowing tetramer dissociation, and thus reducing TTR propensity for misfolding and aggregation [11]. Moreover, the mechanistically identical, structurally distinct, TTR kinetic stabilizer diflunisal also exhibited efficacy against FAP in a placebo-controlled clinical trial [8]. Based on a phase II open-label study involving ATTRwt (cardiomyopathy) and V122I FAC patients, tafamidis effectively achieved and maintained WT and V122I TTR stabilization and was well tolerated (personal communication, Matt Maurer). Moreover, phenotypic disease stabilization as measured by biochemical and echocardiographic parameters suggest that further evaluation of tafamidis in TTR amyloid cardiomyopathy is warranted. The significance of these observations will only be clear after the placebo-controlled clinical trial being enrolled by Pfizer is finished (ClinicalTrials.gov Identifier: NCT01994889). In a recent study on 6 of these ATTRwt (cardiomyopathy) patients continuing to take tafamidis, the plasma tafamidis concentration and the extent of TTR kinetic stabilization varied 4-fold between patients receiving a 20 mg once daily dose of tafamidis [25]. While the number of patients in this study is small, the variability in plasma concentration and kinetic stabilization using a single 20 mg dose of tafamidis supports the development of an approach for determining the optimal dose of tafamidis for each cardiomyopathy patient [25]. Herein, we study a single male ATTRwt patient before treatment and 6 weeks after daily doses of 20 or 40 or 60 mg of tafamidis, in a dose escalation paradigm. By measuring tafamidis plasma concentration and the extent of TTR kinetic stability as a function of tafamidis dose using our subunit exchange assay, we have determined that the optimal dose of tafamidis for maximal kinetic stabilization of WT-TTR in this patient’s plasma is 60 mg tafamidis daily.
Methods
Recombinant protein expression and purification
Recombinant dual-FLAG-tagged WT (FT2-WT) TTR was expressed in and purified from Escherichia coli as described previously [26]. Upon purification, 500 μL of 63 μM aliquots in phosphate buffer (10 mM sodium phosphate (pH 7.6), 100 mM KCl, and 1 mM EDTA) were flash frozen and stored at −80 °C until use. The molar absorptivity (ε) of FT2-WT TTR (85720 M−1 cm−1) tetramers in standard phosphate buffer was used to calculate TTR concentration.
Plasma Samples
Blood from the ATTRwt patient was collected in 10 mL BD Vacutainer tubes with EDTA (366643). Upon collection, the blood was remixed prior to centrifugation by gently inverting the tube 10–12 times and then centrifuged at room temperature (18–25 °C) in a horizontal rotor for 15 min at 1500 rcf. After centrifugation, the plasma was aspirated with a Pasteur pipet without disturbing the cell layer. Plasma was transferred to 5 cryo vials with a cap and stored at −75 °C until it was shipped. This study was approved by the Institutional Review Boards at The Scripps Research Institute and Columbia University Medical Center and the patient of focus provided written informed consent.
HPLC Analysis of Tafamidis Concentrations in Plasma
Tafamidis levels in the tafamidis-treated patient plasma samples were quantified by HPLC following the previously described method [25]. For each experiment, a standard curve was generated by adding tafamidis (in DMSO; final concentration ranging from 0–36 μM) to a healthy donor plasma sample.
Subunit Exchange Studies by Adding Recombinant TTR to Endogenous WT TTR
Subunit exchange assays were carried out as previously described [25] with the following minor modifications: (i) in order to reduce the sudden pressure jump during sample injection, and to achieve a complete separation of TTR from other plasma proteins, the first 6 min of the TTR separation on UPLC was done at a low flow rate (0.2 mL/min), and then the flow rate was increased to 0.6 mL/min for the rest of the run (total run time = 39 min); and (ii) 1 μL of a 3 mM A2 stock solution (final concentration of 300 μM) was used instead of 3 μL of a 130 μM solution (final concentration was 90 μM) to minimize the effect of DMSO and to account for high tafamidis concentrations. For analysis of TTR kinetic stability in plasma, a frozen ATTRwt plasma sample aliquot (200 μL) was thawed on ice. Subunit exchange was initiated by the addition of 1.8 μM FT2-WT TTR to 40 μL plasma samples in 4 technical replicates and the samples were incubated at 25 °C for up to 4 days. Sample incubation and analysis was done at 25 °C instead of 37 °C, which is a more physiologically relevant temperature, to keep it consistent with the previous experimental database. At each time point, a 10 μL aliquot of the exchange mixture was transferred to a UPLC sample glass vial (Waters) to which A2 (1 μL of a 3 mM solution) was added for a 3 h reaction period, followed by addition of 52 μL of 50 μM phosphate buffer (pH 7.6). Samples were flash-frozen in liquid nitrogen and stored at −20 °C until ion exchange UPLC analysis. Ion exchange chromatography is performed by thawing the samples from the entire subunit exchange time course and loading them into the UPLC autosampler. Fluorescence from the TTR•A2 conjugate allows detection of TTR tetramers. The rate of subunit exchange was quantified by the rate of appearance of the (WT TTR)3(FLAG-tagged TTR)1 heterotetramer•A2 conjugate peak (peak 2).
Subunit Exchange Analysis
The expected relative area of tetramers 1–5 at equilibrium is predicted by a binomial distribution,
where n=4, k is the number of untagged TTR subunits in the peak of interest, and p is the probability of incorporation an untagged TTR subunit, which is the fraction of untagged TTR relative to total TTR. For each time point, the area of peak 2 is calculated relative to the total area of peak 2 at equilibrium expected from the binomial distribution and the fraction exchange (FE) is calculated as [(peak area at time t)/(peak area at equilibrium) × 100]. The rate of tetramer dissociation (kex) was calculated using the equation kex, t=t = −ln(1−FEt=t)/t and is expressed as the average of the rates obtained at subunit exchange incubation times of 48 and 96 h.
Results
Quantitative analysis of tafamidis concentration in patient plasma samples
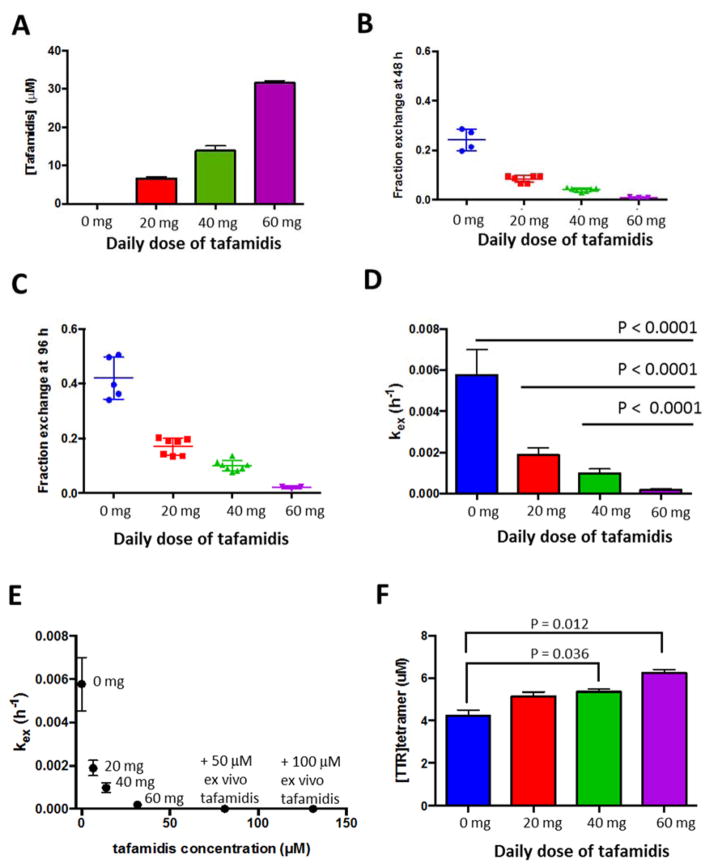
The index case is an 88-year-old Caucasian male with cardiac amyloid on endomyocardial biopsy that was confirmed to be TTR by laser capture mass spectrometry. His TTR gene sequence did not reveal any mutations. His initial BMI was 25.7 and there was no significant change during treatment period. The WT-TTRA patient was treated with once daily oral doses of tafamidis, escalating the dose from 20 mg to 40 mg and then to 60 mg tafamidis at 6 week intervals (study comprises 18 weeks of treatment). The patient’s plasma sample was collected prior to treatment and at the end of each six-week treatment period at a specific tafamidis dose. We quantified the tafamidis concentration in the patient plasma samples in triplicate using HPLC, before and after 6 weeks of dosing at 20, 40 and 60 mg of tafamidis. As expected, the plasma concentration of tafamidis rose proportionally to the daily dose of tafamidis administered. After 6 weeks of once daily dosing at 20, 40, and 60 mg of tafamidis, the tafamidis concentrations in the patient’s blood were determined to be 6.6, 13.9, and 31.6 μM, respectively, based on a standard curve generated by adding increasing concentrations of tafamidis to plasma from a healthy donor (Figure 1A and S1A).
Figure 1.
The effect of tafamidis on an ATTRwt patient’s plasma TTR tetramer stability. Plasma samples were obtained from an ATTRwt patient before treatment (0, blue), and after treatment with 20 (red), 40 (green), or 60 (purple) mg of once daily oral dose of tafamidis. (A) Plasma tafamidis concentration in the patient plasma samples was measured by HPLC. Each bar graph represents an average of three measurements, error bars represent standard deviation (SD). (B & C) Subunit exchange was quantified at 48 (B) and 96 (C) h, and the fraction exchange was calculated as described in Materials and Methods. (D) The rate of tetramer dissociation (kex) was calculated using the equation kex, t=t = −ln(1−FEt=t)/t and is expressed as the average of the rates obtained at 48 and 96 h subunit exchange incubation time. Each bar graph is an average of 4 or more individual technical replicates, error bars represent SD. Differences between groups were assessed by t-test (Prism 6). (E) Stability of plasma TTR was assessed as a function of plasma tafamidis concentrations. For the last two points on the graph, excess tafamidis (50 μM or 100 μM) was added ex vivo to the 60 mg dose plasma sample and subunit exchange was initiated with 1.8 μM FT2-WT TTR. (F) TTR tetramer concentrations in the patient plasma samples were assessed by measuring TTR•A2 conjugate fluorescence at 0 h subunit exchange incubation time. Each bar graph is an average of 4 or more individual technical replicates, error bars represent SD. Differences between groups were assessed by t-test (Prism 6).
TTR kinetic stability assessment in patient plasma using subunit exchange analysis
The kinetic stability of endogenous TTR in patient plasma was assessed using the TTR subunit exchange method [25]. We measured the subunit exchange rate of WT TTR in the patient plasma samples before and after each 6-week treatment with the increasing oral doses of tafamidis. Because the half-life of endogenous TTR in plasma is approximately 48 h [27, 28], we chose to quantify the fraction of subunit exchange after 48 and 96 h of incubation. As expected, the fraction of subunit exchange at 48 h (Figure 1B) was lower than the fraction of subunit exchange at 96 h (Figure 1C) without tafamidis treatment, and as a function of oral tafamidis dosing. The rate of subunit exchange, kex, is rate limited by tetramer dissociation [26, 29, 30]. The rate of subunit exchange was calculated from data at the 48 and 96 h time points separately and the averages of the two rates are graphed as a function of the daily dose of tafamidis (Figure 1D), as done previously [25]. The kinetic stability of endogenous TTR in the patient’s plasma (Figure 1D) at the 60 mg dose was significantly higher than at the 40 and 20 mg doses (P<0.0001). Furthermore, the measured kinetic stability of TTR tetramers exhibited a strong correlation with the plasma concentration of tafamidis (Figure 1E). In this patient, maximal TTR kinetic stabilization was achieved with a daily oral dose of 60 mg of tafamidis. This was demonstrated by the fact that ex vivo addition of 50 or 100 μM more tafamidis to the 60 mg tafamidis plasma sample did not significantly further decrease the rate of subunit exchange (increase kinetic stability; Figure 1E). The plasma concentration of normally folded TTR tetramers increased with increasing doses of oral tafamidis. At the 60 mg dose, the WT TTR tetramer concentration increased by 38% relative to the WT TTR tetramer plasma concentration before tafamidis treatment, as calculated from TTR•A2 conjugate fluorescence peak intensity (Figure 1F and S1B). Moreover, consistent with previous findings showing increased plasma TTR levels after tafamidis treatment [5, 7], total TTR levels measured by quantitative Western blotting also increased in the tafamidis-treated samples (Figure S2). This is due to a pharmacologic chaperoning effect–tafamidis enters the endoplasmic reticulum of liver cells, binds to the WT TTR tetramer in the endoplasmic reticulum and shifts the linked folding and assembly process toward tetramer formation, thus enabling more WT TTR tetramer to be formed and secreted [31, 32].
Discussion
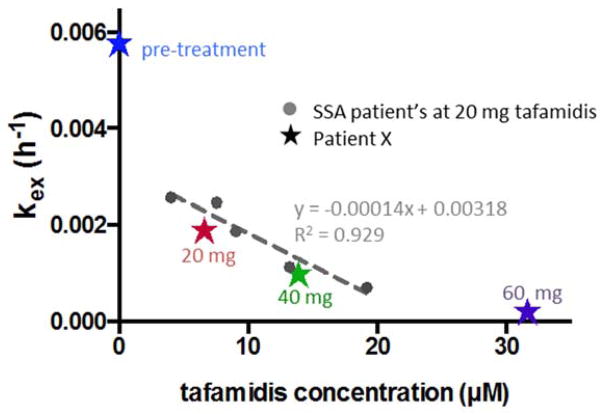
Previously, we have studied the WT TTR subunit exchange rate in plasma as a function of tafamidis plasma concentration at a fixed 20 mg oral dose of tafamidis once daily in the context of ATTRwt cardiomyopathy patients (Figure 2; previous data depicted as filled circles) [25]. Compared to these ATTRwt patients, the patient of focus in this paper exhibits a relatively low plasma tafamidis concentration and a relatively low degree of kinetic stability (Figure 2; new data depicted by stars at the indicated oral doses). The patient’s blood tafamidis concentrations inversely correlate with tetramer dissociation rates, reflected by the TTR subunit exchange rates (kex) (Figure 2). In this patient, we achieve maximal kinetic stabilization of WT TTR at a once daily dose of 60 mg of tafamidis, whereas 20 and 40 mg doses were insufficient (Figure 2). Importantly, at this dose of tafamidis, less than 1% of the patient’s TTR tetramers dissociate over TTR’s half-life of ≈ 2 days (Figure 1B), precluding new TTR synthesis contributing to an additional TTR aggregate load. Since TTR aggregation is a concentration dependent phenomenon, it is possible that incomplete TTR kinetic stabilization could still lead to a good clinical outcome if the concentration of misfolded TTR is below that enabling efficient aggregation. Correlating the extent of kinetic stabilization with clinical outcome will ultimately give us the definitive answer. Other plasma factors besides tafamidis and holo retinol binding protein concentrations may also contribute to TTR kinetic stability, but as yet we do not have enough information to know which factors are important. Based on our current understanding, it seems appropriate to use available methods to maximally kinetically stabilize TTR, especially if the safety profile of tafamidis continues to be acceptable.
Figure 2.
A comparative plot detailing the effect of tafamidis on TTR tetramer stability of an ATTRwt patient’s plasma with that of multiple SSA patients’ plasma treated with 20 mg, once daily tafamidis [25]. Patient X’s kex values at a daily dose of 0 (blue), 20 (red), 40 (green), and 60 mg tafamidis (purple) are overlaid with previously reported data from five other tafamidis-treated SSA patients [25] (grey filled circles) as a function of the measured blood concentration of tafamidis. The dotted line represents a linear regression fit of the 5 previously reported SSA patients’ data [25].
Conclusions
Personalized medicine, defined as the selection of diagnostic and therapeutic strategies based on prospectively validated patient characteristics, is being explored as a more effective therapeutic strategy compared to the conventional treatment strategy, which applies the same approach to all patients, even those in different disease stages. Herein we report a case study of a male ATTRwt patient in which the dose of tafamidis has been optimize to maximize WT TTR kinetic stabilization. By assessing tafamidis efficacy on TTR kinetic stability as a function of blood tafamidis concentrations, we have determined that this patient needs a three-fold higher dose of tafamidis than that currently being used commercially, and 20 mg less than the highest dose being explored in the cardiomyopathy trial being conducted by Pfizer. We expect that a certain threshold of WT TTR kinetic stabilization will correlate with a maximal clinical response, which may be less than maximal kinetic stabilization sought herein. Until we know what this threshold is, it seems reasonable to maximize TTR kinetic stability as a surrogate biomarker.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant DK046335 (JWK), National Institute on Aging grant AG036778 (MSM) as well as the Skaggs Institute for Chemical Biology and the Lita Annenberg Hazen Foundation. We thank those scientists whose work enabled us to do this clinical study with tafamidis.
Footnotes
Declaration of Interest
Jeffery W. Kelly is a shareholder and a paid consultant for Pfizer who sells tafamidis. Dr. Maurer’s institution, Columbia University Medical Center, received funding for research studies of tafamidis and for consultation for Dr. Maurer’s role as co-Chair of the steering committee for the ATTR-Cardiomyopathy trial.
References
- 1.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. New Engl J Med. 2003;349:583–96. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Kelly JW. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol. 1998;8:101–6. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 4.Givens RC, Russo C, Green P, Maurer MS. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medial center. Aging Health. 2013;9:229–35. doi: 10.2217/ahe.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho T, Maia LF, Martins da Silva A, Cruz MW, Plante-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy A randomized, controlled trial. Neurology. 2012;79:785–92. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarstrom P, Wiseman RL, Powers ET, Kelly JW. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–6. doi: 10.1126/science.1079589. [DOI] [PubMed] [Google Scholar]
- 7.Coelho T, Maia LF, Martins da Silva A, Cruz MW, Plante-Bordeneuve V, Suhr OB, et al. Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol. 2013;260:2802–14. doi: 10.1007/s00415-013-7051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing Diflunisal for Familial Amyloid Polyneuropathy A Randomized Clinical Trial. JAMA. 2013;310:2658–67. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrade CA. Peculiar Form of Peripheral Neuropathy - Familiar Atypical Generalized Amyloidosis with Special Involvement of the Peripheral Nerves. Brain. 1952;75:408–27. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- 10.Coelho T. Familial amyloid polyneuropathy: New developments in genetics and treatment. Curr Opin Neurol. 1996;9:355–9. [PubMed] [Google Scholar]
- 11.Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci USA. 2012;109:9629–34. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The Transthyretin Amyloidoses: From Delineating the Molecular Mechanism of Aggregation Linked to Pathology to a Regulatory-Agency-Approved Drug. J Mol Biol. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westermark P, Sletten K, Johansson B, Cornwell GG. Fibril in Senile Systemic Amyloidosis Is Derived from Normal Transthyretin. Proc Natl Acad Sci USA. 1990;87:2843–5. doi: 10.1073/pnas.87.7.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammed SF, Mirzoyez SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, et al. Left Ventricular Amyloid Deposition in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2014;2:113–22. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson MD. Familial Amyloidotic Polyneuropathy. Trends Neurosci. 1989;12:88–92. doi: 10.1016/0166-2236(89)90162-8. [DOI] [PubMed] [Google Scholar]
- 16.Falk RH. Cardiac Amyloidosis A Treatable Disease, Often Overlooked. Circulation. 2011;124:1079–85. doi: 10.1161/CIRCULATIONAHA.110.010447. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. New Engl J Med. 1997;336:466–73. doi: 10.1056/NEJM199702133360703. [DOI] [PubMed] [Google Scholar]
- 18.Monaco HL, Rizzi M, Coda A. Structure of a Complex of 2 Plasma-Proteins - Transthyretin and Retinol-Binding Protein. Science. 1995;268:1039–41. doi: 10.1126/science.7754382. [DOI] [PubMed] [Google Scholar]
- 19.Schreiber G, Richardson SJ. The evolution of gene expression, structure and function of transthyretin. Comp Biochem Phys B. 1997;116:137–60. doi: 10.1016/s0305-0491(96)00212-x. [DOI] [PubMed] [Google Scholar]
- 20.Blake CCF, Geisow MJ, Oatley SJ, Rerat B, Rerat C. Structure of Pre-Albumin - Secondary, Tertiary and Quaternary Interactions Determined by Fourier Refinement at 1. 8-A. J Mol Biol. 1978;121:339–56. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 21.Colon W, Kelly JW. Partial Denaturation of Transthyretin Is Sufficient for Amyloid Fibril Formation Invitro. Biochemistry. 1992;31:8654–60. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- 22.Hurshman AR, White JT, Powers ET, Kelly JW. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry. 2004;43:7365–81. doi: 10.1021/bi049621l. [DOI] [PubMed] [Google Scholar]
- 23.Babbes ARH, Powers ET, Kelly JW. Quantification of the thermodynamically linked quaternary and tertiary structural stabilities of transthyretin and its disease-associated variants: The relationship between stability and amyloidosis. Biochemistry. 2008;47:6969–84. doi: 10.1021/bi800636q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiseman RL, Johnson SM, Kelker MS, Foss T, Wilson IA, Kelly JW. Kinetic stabilization of an oligomeric protein by a single ligand binding event. J Am Chem Soc. 2005;127:5540–51. doi: 10.1021/ja042929f. [DOI] [PubMed] [Google Scholar]
- 25.Rappley I, Monteiro C, Novais M, Baranczak A, Solis G, Wiseman RL, et al. Quantification of Transthyretin Kinetic Stability in Human Plasma Using Subunit Exchange. Biochemistry. 2014;53:1993–2006. doi: 10.1021/bi500171j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiseman RL, Green NS, Kelly JW. Kinetic stabilization of an oligomeric protein under physiological conditions demonstrated by a lack of subunit exchange: Implications for transthyretin amyloidosis. Biochemistry. 2005;44:9265–74. doi: 10.1021/bi050352o. [DOI] [PubMed] [Google Scholar]
- 27.Oppenhei Jh, Surks MI, Bernstein G, Smith JC. Metabolism of Iodine-131-Labeled Thyroxine-Binding Prealbumin in Man. Science. 1965;149:748–50. doi: 10.1126/science.149.3685.748. [DOI] [PubMed] [Google Scholar]
- 28.Socolow EL, Woeber KA, Purdy RH, Holloway MT, Ingbar SH. Preparation of I131-Labeled Human Serum Prealbumin and Its Metabolism in Normal and Sick Patients. J Clin Invest. 1965;44:1600–9. doi: 10.1172/JCI105266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider F, Hammarstrom P, Kelly JW. Transthyretin slowly exchanges subunits under physiological conditions: A convenient chromatographic method to study subunit exchange in oligomeric proteins. Protein Sci. 2001;10:1606–13. doi: 10.1110/ps.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keetch CA, Bromley EHC, McCammon MG, Wang N, Christodoulou J, Robinson CV. L55P transthyretin accelerates subunit exchange and leads to rapid formation of hybrid tetramers. J Biol Chem. 2005;280:41667–74. doi: 10.1074/jbc.M508753200. [DOI] [PubMed] [Google Scholar]
- 31.Genereux JC, Qu S, Zhou M, Ryno LM, Wang S, Shoulders MD, et al. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. The EMBO J. 2015;34:4–19. doi: 10.15252/embj.201488896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoulders MD, Ryno LM, Genereux JC, Moresco JJ, Tu PG, Wu C, et al. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013;3:1279–92. doi: 10.1016/j.celrep.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.