Abstract
Mental rotation is a visuo-spatial task associated with pronounced sex differences. Performance is also affected by gonadal hormones such as testosterone and estradiol. To better understand hormonal modulation of the neural substrates of mental rotation, the present study examined the influence of estradiol using functional magnetic resonance imaging. Ten pre-menopausal women were tested on a 3D mental rotation task during the early follicular (EF) and late follicular (LF) phases of the menstrual cycle. Change in estradiol between the two phases was confirmed by hormone assays. Brain activation patterns were similar across the two phases, but the change in estradiol had different associations with the two hemispheres. Better performance in the LF than the EF phase was associated with a pattern of reduced recruitment of the right hemisphere and increased recruitment of the left hemisphere. The increased recruitment of the left hemisphere was directly associated with greater changes in estradiol. Given that the right hemisphere is the dominant hemisphere in visuo-spatial processing, our results suggest that estradiol is associated with reduced functional asymmetry, consistent with recent accounts of hormonal modulation of neurocognitive function.
Keywords: menstrual cycle, estradiol, functional lateralization, fMRI
Introduction
Mental rotation [1] has been a topic of intense investigation, in part due to a fairly consistent finding that men and women perform differently on three-dimensional (3D) mental rotation. Although several functional magnetic resonance imaging (fMRI) studies have examined how functional brain organization in mental rotation relates to sex and hormonal modulation [2–7], results thus far have been inconsistent, or even contradictory. For example, some studies report more widespread activation in women than men [2–3, 8], whereas other studies report otherwise [7, 9]. Some studies found that women in the high estradiol phase have more activation than in the low estradiol phase [8] whereas another study reported the opposite [4]. These inconsistencies could be due to variations in the type of task or stimuli used, lack of direct measurement of circulating estradiol concentration (i.e., estimated by self-report of menstrual phase), and lack of control for the influence of other hormones such as testosterone. The present study will address some of these limitations.
In this study, we probe the effect of estradiol on neural processing during mental rotation using fMRI. Most menstrual cycle studies have compared the follicular to luteal menstrual cycle phases, which are not only associated with different levels of estradiol, but are also associated with differences in the levels of progesterone and gonadotropin [10]. To examine menstrual cycle related changes in estradiol selectively, we compared brain activation in the same individuals during the early (EF) and late follicular (LF) phases of the menstrual cycle. Since other hormones (such as progesterone, testosterone, luteinizing hormone, and follicle-stimulating hormone) remain low from menses to a few days before ovulation, only estradiol levels should fluctuate during these two phases. The goal was to isolate neural components of mental rotation that are selectively associated with variation in estradiol level. Changes in activation were associated with task performance to further confirm that changes in brain activation are related to mental rotation rather than factors unrelated to the task, such as global cerebrovascular/perfusion changes with estradiol [8, 22]. Thus, in the higher estradiol phase, if enhanced brain activation is correlated with improved performance, then estradiol has a potentially facilitating influence; otherwise, estradiol is more disadvantageous.
Methods
Participants
Participants were recruited via advertisement in the local community and compensated for participation. Nineteen women enrolled in the study, but only ten completed both fMRI sessions (EF & LF) and 8 of those women provided blood samples after both sessions. Age of participants who completed the study ranged from 18 to 38 years (25±6.4 years). All participants were right-handed (assessed with the Edinburgh Handedness Inventory [23]), had normal vision, and reported no major medical, neurological or psychiatric conditions. Participants were not using contraceptives and reported menstrual cycles ranged from 18 to 36 days (25.1±6.4 days). Approval to conduct the experiment was obtained from local IRB.
Procedure
Participants completed three sessions. The first session involved informed consent, screening, and practice with the mental rotation task and a verbal working memory task (which has been reported in [11]). During this session, each subject was given the Calendar of Premenstrual Experiences [COPE [12]] to complete on a daily basis in order to monitor physical, emotional and physiological changes across their menstrual cycles and to document the first day of menses. After completing the COPE through one full menstrual cycle, participants were asked to call the lab to schedule a second session that occurred either during EF (Days 3–5 following menses) or LF (Days 10–12 following menses, as confirmed by hormone levels). Half of the subjects were instructed to schedule their first session during the EF phase (5 subjects provided blood samples) and the other half of the subjects were instructed to schedule the first session during the LF phase (3 subjects provided blood samples). The third session occurred during the phase of the menstrual cycle that was not tested in Session 2.
Hormone assays
5ml of blood was drawn into gold top tubes immediately after each session, centrifuged, and transported to the Reproductive Endocrinology Laboratory at the University of Kentucky. Serum was frozen until assayed. Estradiol, progesterone, testosterone, LH and FSH were assayed by a solid-phase, competitive chemiluminescent enzyme immunoassay using an Immulite 1000 (Siemens Healthcare Diagnostics, Los Angeles, CA) according to the manufacturer's recommendations. Serum and alkaline phosphatase labeled hormone were added to antibody coated beads which were then incubated at 37 °C for up to 70 min. Test units were washed after incubation, alkaline phosphatase substrate was added and the samples incubated at 37 °C for 10 min. Counts per second for each sample was converted to analyte concentrations using stored master curves. The assay sensitivities were as follows: estradiol (15 pg/mL), progesterone (0.2 ng/mL), testosterone (15 ng/dL), LH (0.1 mIU/mL) and FSH (0.1 mIU/mL). The intra- and inter-assay coefficient of variation was routinely between 5–8% and 10–13% respectively.
Task and design
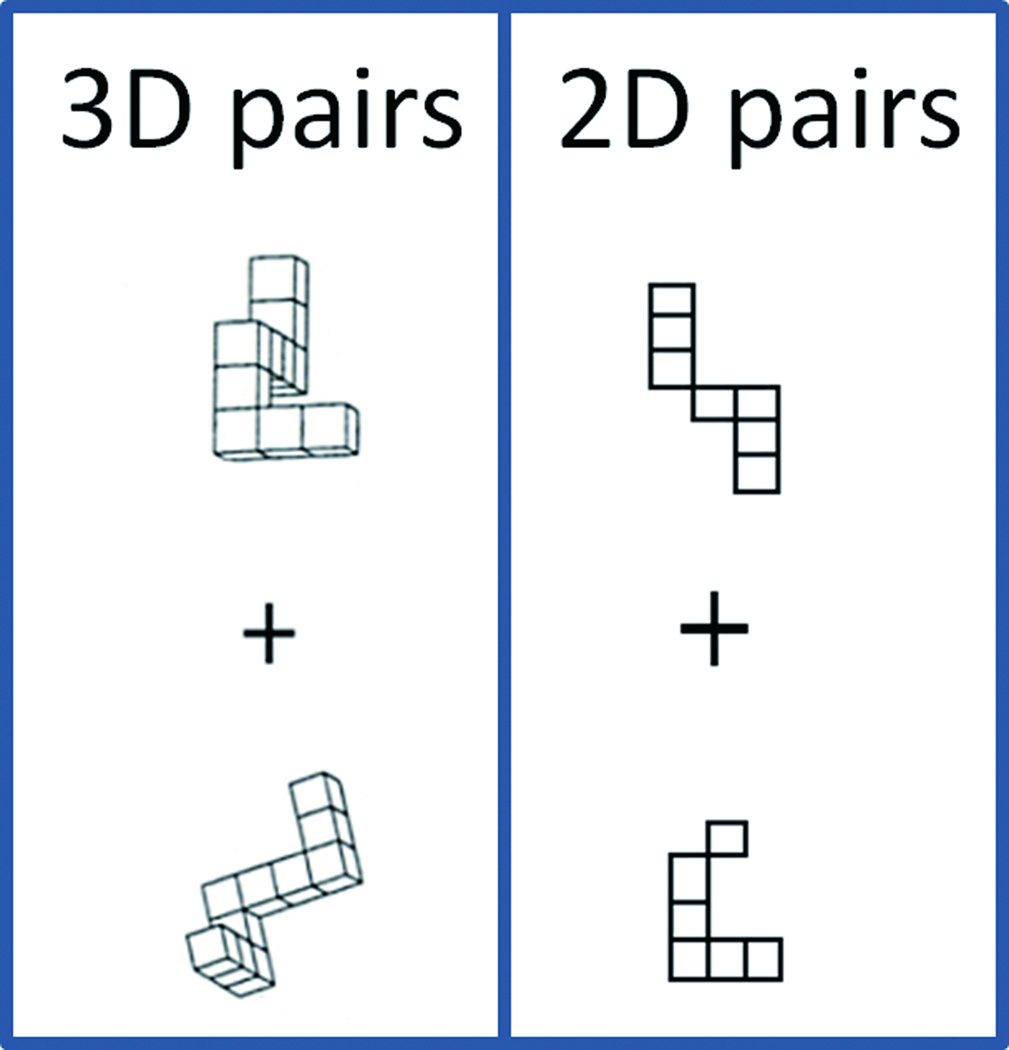
Participants completed a slow event related task with three kinds of stimuli (pairs of 3D or 2D stimuli, or a fixation cross). The 3D mental rotation task was based on the classic paper-and-pencil mental rotation task [13]. Equal numbers of same and different 3D object pairs from the paper-and-pencil test were used. The 2D shapes were drawn based on the 3D shapes so that the number of squares in each shape corresponded to an orthogonal view of the 3D shapes (Fig 1). The 2D-matching task was designed to control for low level visual processing, stimulus discrimination, response selection and execution and rotation within the picture plane. Participants used the index and middle fingers of their right hand to respond (with index finger indicating a match) and were instructed to respond as quickly but as accurately as possible. Each stimulus pair presentation was terminated by the subject’s button press and replaced by a fixation cross for the remainder of the trial (up to a total time of 20.4s). Maximum task presentation time was 17.5s. The reason we chose a slow event-related design is that pilot testing indicated that subjects needed, on average, about 7s to solve the task. Four versions of the task were created (each of which included seven 3D events and seven 2D events), and in each session (EF & LF) subject completed two versions (each in a single functional run), with versions and orders counterbalanced across subjects and sessions to minimize practice and order effects for specific stimuli. Visual angle for individual 3D objects measured approximately 5°.
Figure 1.
Example of 3D and 2D stimulus pairs used in the matching task.
fMRI acquisition and analysis
Data were collected using a Siemens Vision 1.5 T magnet. Subjects completed two 123-volume whole-brain functional runs (3.56×3.56×3mm voxels, TR/TE/flip angle/FOV = 4s/40 ms/90°/228×228 mm, 44 contiguous slices, 3 mm thickness, acquired in an interleaved fashion) per session. T1-weighted anatomical scans (3D MP-RAGE, 150 sagittal slices 1 mm3 voxel) were collected for each participant at the first session. Two other functional scans involving a working memory task (reported in [11]) were also collected during the scanning session.
Using FSL (http://www.fmrib.ox.ac.uk/fsl), functional images were motion corrected, spatially smoothed (FWHM = 8.0 mm), and temporally filtered using a high-pass filter (100 s). All 10 subjects contributed at least one useable run in each phase. We included all 10 subjects that completed fMRI sessions in order to maximize power to detect activation. However, for any analyses involving estradiol, only the 8 subjects that provided data could be analyzed.
Customized square waveforms (on/off) representing each condition (3D & 2D) were convolved with a double-gamma hemodynamic response function, with the event duration determined by the RT associated with that event. Mixed-effects group analysis was conducted by using FSL’s FLAME (FMRIB’s local analysis of mixed effects) stages 1 + 2. The 3D vs. fixation contrast was used to define regions used in the subsequent ROI analysis, using clusters determined by Z > 2.3 and a (whole-brain corrected) cluster significance threshold of p < .05 using Gaussian Random Field theory [24]. The first 6 local maxima of each of the 4 surviving clusters were then identified, and 5-mm spheres were created centered around these local maxima.
For each sphere ROI, scores that indexed mental rotation activity were computed. Peak hemodynamic change was calculated as the maximum signal after stimulus onset minus the average of the two pre-stimulus time points. Mental rotation signal change was defined as the difference of 3Dpeak minus 2Dpeak (i.e., 3Dsignal=3Dpeak – 2Dpeak), then its change from EF to LF was calculated (i.e., [3DsignalLF – 3DsignalEF]). Using IBM statistics (Chicago, IL) two types of correlations were conducted. One set of correlations examined the association between phase-related changes in brain activity and change in performance, with change in performance defined by change in 3D compare to 2D performance (RT or error rate), from EF to LF (e.g., [3DRTLF – 3DRTEF]). The other set of correlations examined the association between changes of brain activity and hormone levels. Non-parametric correlations (Spearman rank) were used for both kinds of correlations due to the small sample size and the significance of the correlation was evaluated by p<0.05 (two-tailed). An additional bootstrap procedure was used to further construct confidence intervals for the Spearman correlations, using IBM Statistics (v. 21; Chicago IL) with 1,000 bootstrap samples. Correlation reliability was evaluated by whether the 95% confidence interval contained zero or not.
Results
Hormonal analysis
A related samples wilcoxon signed rank test comparing EF and LF sessions for each of five hormones (estradiol, progesterone, luteinizing hormone, follicular stimulating hormone, and testosterone) revealed that only estradiol levels increased from the EF (M = 49.4 ±20.5 pg/mL;, range 25 ~ 94) to the LF phase (M = 110.5± 65.7 pg/mL; range 42 ~ 218), with t=2.521, p = .012. The other hormones showed no significant difference (p > 0.11).
Behavioral performance
Behavioral data ( RT(reaction time), error rate ) were analyzed with IBM Statistics (Chicago, IL) using a 2 (Condition: 2D, 3D) × 2 (Phase: EF, LF) repeated measures ANOVA. RTon each correct trial was log-transformed to satisfy the assumption of normality for the multivariate approach to repeated measures analyses. Based on the 10 participants who completed both fMRI sessions, the 2D shape-matching task led to fewer errors (5.3% vs. 25.2%) and faster responses (2.89 sec vs. 7.17 sec) than 3D-mental rotation task. The main effect of condition was significant for both correct RT (F(1,18) = 73.6, p < 0.001) and error rate (F(1,18) = 144, p < 0.001). However, neither the main effect of menstrual phase nor the Phase × Condition interaction was significant (all p > 0.05).
Imaging results
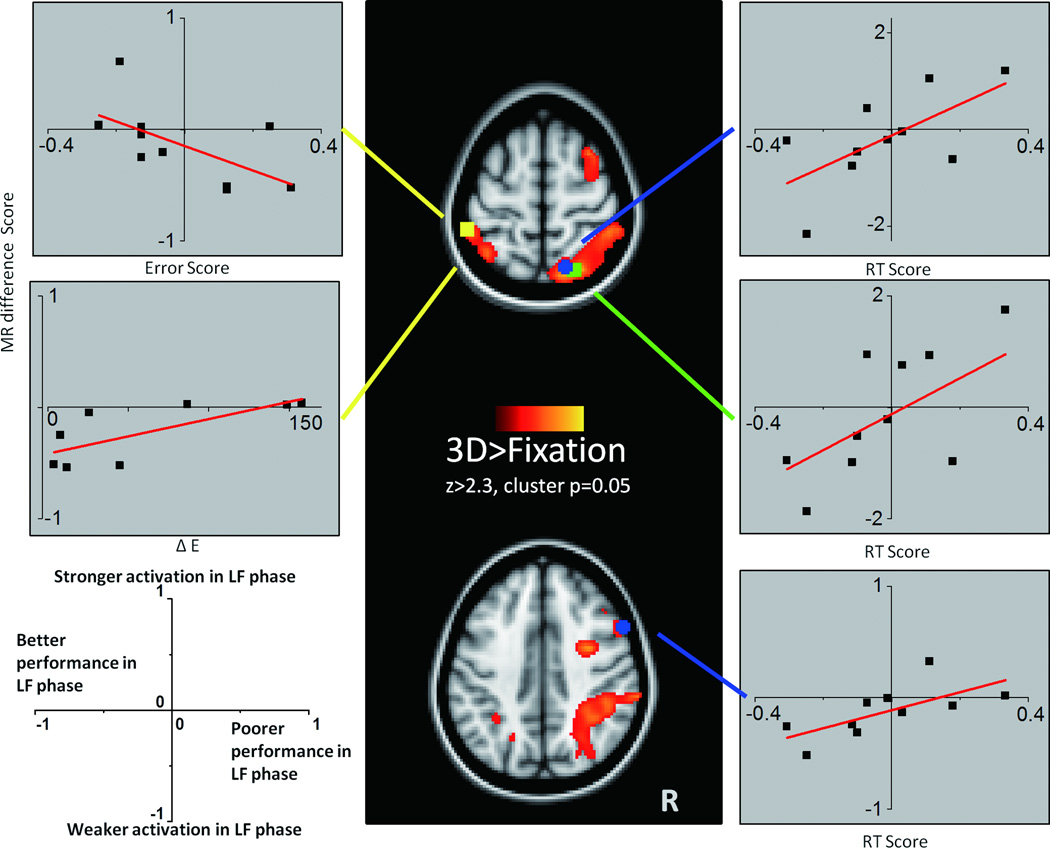
The initial voxel-wise analysis that defined the ROIs was based on the 10 participants who completed both fMRI sessions, to maximize statistical power (Fig. 2). The general patterns of 3D > fix activation are consistent with previous fMRI studies, including strong bilateral parietal activation, which is considered a core region for mental rotation processes (see Zacks[14]), as well as precentral and occipito-temporal regions which are also implicated in mental rotation [15–17]. The 3D > fixation map yielded 24 ROIs. For each ROI, correlations between change in 3D mental rotation activation (3DsignalLF – 3DsignalEF) and change in hormone level (LF – EF estradiol) or performance (RT: (3DRTLF – 3DRTEF) or error rate) were conducted and result summarized in Fig.2 and Table 1. An increase in mental rotation RT score was correlated with increased mental rotation activation in the right superior parietal cortex (ROI-A: rho=0.511, p=0.040, n=10, CI =0~0.902; ROI-B: rho=0.511, p=0.040, n=10, CI =-0.077~1;) and right superior frontal cortex (rho=0.600, p=0.016, n=10, CI=0.20 to 0.95); whereas a decrease in mental rotation error score from EF to LF was correlated with greater recruitment of left superior parietal cortex(rho=-0.535, p=0.036, n=10, CI=-0.026~-0.950), and increased estradiol level from EF to LF (rho=0.571, p=0.048, n=8, CI=0~1). Of note, this region did not show correlations with testosterone changes across the two phases (rho=-0.265, p=0.373, CI=-0.959~0.652) suggesting that the correlation between estradiol and fMRI signal magnitude change in this region is specific to estradiol and not related to some other variable that might be associated with individual differences in activation level. Although we used non-parametric statistics for these tests (given the small sample size), we acknowledge that these correlations would not survive correction for multiple tests across 24 different ROIs.
Figure 2.
Changes in mental rotation fMRI difference scores plotted as a function of performance or hormonal change. For all plots, the y-axis is the mental rotation activation score (3DsignalLF – 3DsignalEF) and the x-axis (labeled for each plot) is performance score: (3D-2D)LF - (3D-2D)EF, or change in estradiol: ∆E=ELF-EEF. As shown in the legend on the bottom left, a higher value on the y-axis represents stronger 3D mental rotation activation in the LF phase and a lower value represents weaker 3D mental rotation activation in the LF phase. A higher value on the x-axis represents poorer performance (higher errors or RT) in the LF phase whereas a lower value on the x-axis represents better performance in the LF phase.
Table 1.
Regions that show a significant correlation between mental rotation activation score and performance or hormonal change score.
| Cluster | MNI coordinates |
Notes | Correlation coefficient (rho=) |
||||
|---|---|---|---|---|---|---|---|
| x | y | z | ∆ Estradiol | RT Score |
Error Score |
||
| Right superior parietal Cx | 22 | −68 | 62 | local maxima A | ns | 0.511 | ns |
| 16 | −66 | 64 | local maxima B | ns | 0.511 † | ns | |
| Right superior frontal Cx | 56 | 14 | 36 | ns | 0.6 | ns | |
| cerebellum | nothing in this cluster | ns | ns | ns | |||
| left superior parietal Cx | −52 | −40 | 58 | 0.571 | ns | −0.535 | |
ns: not significant, all other correlations significant at p<0.05; Cx: cortex.
this correlation coefficient’s confidence interval contains 0 according to bootstrap
Notes mental rotation activation score: 3DsignalLF – 3DsignalEF; performance score(RT and Error): (3D–2D)LF– (3D–2D)EF
Discussion
In this study, we systematically investigated the association between change in mental rotation-related fMRI activation and change in estradiol level. The major observation is that the influence of estradiol, measured by correlations between fMRI signal change and behavioral or hormonal changes from EF to LF, was different in the two hemispheres. Compared to the EF phase, in the LF phase the frontal and parietal cortex in the right hemisphere (which is dominant for mental rotation) showed increased activation in the subjects with poorer task performance (longer RT), while the non-dominant (left) hemisphere showed increased activation in the subjects with better task performance (fewer errors; Fig. 2). This means that non-dominant hemisphere recruitment in the LF (high estradiol) phase was associated with performance improvements. In addition, the left superior parietal cortex showed increased activation in proportion to increased estradiol from the EF to LF phase. In contrast, the right hemisphere did not show significant correlations with estradiol changes. This suggests that the greater the estradiol change in the LF phase, the more the activation in the non-dominant hemisphere. In turn, we suggest that greater recruitment of the non-dominant hemisphere with a greater increase in estradiol is one way to characterize reduced functional asymmetry.
The finding of reduced functional cerebral asymmetry due to increased estradiol levels during LF phase is consistent with Hausmann’s theory of reduced functional asymmetries by estradiol and progesterone in performance of different cognitive tasks [6, 18–20]. Hausmann [15, 18] suggests that gonadal steroids could reduce cortical-cortical transmission via glutamatergic and GABA-ergic action. Since the dominant hemisphere typically inhibits the activity in the corresponding non-dominant hemisphere regions via glutamatergically induced excitatory post-synaptic potentials, a higher gonadal steroid concentration would lead to reduced inhibition of the non-dominant hemisphere, resulting in temporary reduced asymmetry. Importantly, in the present study, the correlation appeared to be specific to estradiol changes across the menstrual cycle and not related to testosterone changes.
Results from a verbal working memory task which was completed by the same set of subjects [11] also showed a similar reduction in asymmetry across the two menstrual cycle phases. Specifically, better performance in the LF than the EF phase was associated with a pattern of reduced recruitment of the left-hemisphere (dominant hemisphere) and increased recruitment of the right-hemisphere (non-dominant hemisphere), which also reflects reduced functional asymmetry for working memory. For both studies, increased activation from the EF to LF phase in the non-dominant hemisphere was positively correlated with improved performance, but increased activation in the dominant hemisphere was negatively correlated with performance. The different asymmetry patterns between these two tasks (for mental rotation, the right hemisphere is dominant, and for working memory the left hemisphere is dominant) suggest that these findings are not likely to be anatomically driven (such as estradiol-related perfusion differences across the brain [8, 21]); instead, they are related to the functionality attributed to each hemisphere.
Conclusion
The present study demonstrated that increased estradiol in the LF phase was associated with reduced functional asymmetry involving a number of critical cortical nodes for mental rotation. These findings, together with the prior companion study on verbal working memory in the same sample [11] are consistent with the proposal by Hausmann [15, 18] that estradiol is associated with reduced functional cerebral asymmetry. Collectively, the present findings contribute to a better understanding of the influence of estradiol on functional brain activation, which may ultimately have an impact on treatment of medical conditions related to reductions in estradiol, as in aging and menopause. Nevertheless, given the small sample size and the finding that the statistical tests were significant at an uncorrected alpha level, the present results should be considered preliminary for guiding future studies with larger samples.
Acknowledgements
This work has been supported by NIH grant (P20-RR015592; K12-DA14040).
References
- 1.Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(3972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo B, et al. Cortical activation during mental rotation in male-to-female and female-to-male transsexuals under hormonal treatment. Psychoneuroendocrinology. 2010;35(8):1213–1222. doi: 10.1016/j.psyneuen.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Jordan K, et al. Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia. 2002;40(13):2397–2408. doi: 10.1016/s0028-3932(02)00076-3. [DOI] [PubMed] [Google Scholar]
- 4.Mendrek A, Lakis N, Jimenez J. Associations of sex steroid hormones with cerebral activations during mental rotation in men and women with schizophrenia. Psychoneuroendocrinology. 2011;36(9):1422–1426. doi: 10.1016/j.psyneuen.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Schoning S, et al. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007;45(14):3203–3214. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Weis S, et al. Dynamic changes in functional cerebral connectivity of spatial cognition during the menstrual cycle. Hum Brain Mapp. 2011;32(10):1544–1556. doi: 10.1002/hbm.21126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss E, et al. Sex differences in brain activation pattern during a visuospatial cognitive task: a functional magnetic resonance imaging study in healthy volunteers. Neurosci Lett. 2003;344(3):169–172. doi: 10.1016/s0304-3940(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich T, et al. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. Neuroimage. 2001;13(3):425–432. doi: 10.1006/nimg.2001.0703. [DOI] [PubMed] [Google Scholar]
- 9.Levin SL, Mohamed FB, Platek SM. Common ground for spatial cognition? A behavioral and fMRI study of sex differences in mental rotation and spatial working memory. Evolutionary Psychology. 2005;3:227–254. [Google Scholar]
- 10.Levy MN, Kopeppen BM, stanton BA, editors. Berne & Levy Principles of Physiology. 4E ed. Elsevier Health Sciences; 2007. pp. 724–729. [Google Scholar]
- 11.Joseph JE, et al. Influence of estradiol on functional brain organization for working memory. Neuroimage. 2012;59(3):2923–2931. doi: 10.1016/j.neuroimage.2011.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortola J, et al. Diagnosis of Premenstrual Syndrome by a Simple, Prospective, and Reliable Instrument: The Calendar of Premenstrual Experiences. Obstetrics & Gynecology. 1990;76(2):302–307. [PubMed] [Google Scholar]
- 13.Vandenberg SG, Kuse AR. Mental Rotations, A Group Test of Three-Dimensional Spatial Visualization. Perceptual and Motor Skills. 1978;47:599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- 14.Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. J Cogn Neurosci. 2008;20(1):1–19. doi: 10.1162/jocn.2008.20013. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann M, et al. Sex hormones affect spatial abilities during the menstrual cycle. Behav Neurosci. 2000;114(6):1245–1250. doi: 10.1037//0735-7044.114.6.1245. [DOI] [PubMed] [Google Scholar]
- 16.Kosslyn SM, et al. Imagining rotation by endogenous versus exogenous forces: distinct neural mechanisms. Neuroreport. 2001;12(11):2519–2525. doi: 10.1097/00001756-200108080-00046. [DOI] [PubMed] [Google Scholar]
- 17.Barnes J, et al. Cortical activity during rotational and linear transformations. Neuropsychologia. 2000;38(8):1148–1156. doi: 10.1016/s0028-3932(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 18.Belger A, Banich MT. Costs and benefits of integrating information between the cerebral hemispheres: a computational perspective. Neuropsychology. 1998;12(3):380–398. doi: 10.1037//0894-4105.12.3.380. [DOI] [PubMed] [Google Scholar]
- 19.Hausmann M. Hemispheric asymmetry in spatial attention across the menstrual cycle. Neuropsychologia. 2005;43(11):1559–1567. doi: 10.1016/j.neuropsychologia.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Hausmann M, et al. Functional cerebral asymmetries during the menstrual cycle: a cross-sectional and longitudinal analysis. Neuropsychologia. 2002;40(7):808–816. doi: 10.1016/s0028-3932(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 21.Weis S, et al. Estradiol modulates functional brain organization during the menstrual cycle: an analysis of interhemispheric inhibition. J Neurosci. 2008;28(50):13401–13410. doi: 10.1523/JNEUROSCI.4392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens MC, Clark VP, Prestwood KM. Low-dose estradiol alters brain activity. Psychiatry Res. 2005;139(3):199–217. doi: 10.1016/j.pscychresns.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 24.Worsley Worsley K. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Ch 14, in Functional MRI: An Introduction to Methods. OUP; 2001. [Google Scholar]