Abstract
Background/Aims
Risks and benefits of simeprevir plus sofosbuvir in patients with advanced cirrhosis are unknown. We assessed the safety and sustained virologic responses (SVR) of simeprevir plus sofosbuvir with and without ribavirin in patients with Child-Pugh (CP)-B/C vs. CP-A cirrhosis and compared to matched untreated controls.
Methods
Multicenter cohort of adults with HCV genotype 1 and cirrhosis treated with simeprevir plus sofosbuvir with/without ribavirin for 12 weeks. Controls were matched on treatment center, age, CP class and model for end-stage liver disease (MELD) score.
Results
Of 160 patients treated with simeprevir plus sofosbuvir with/without ribavirin, 35% had CP-B/C and 64% had CP-A, with median baseline MELD 9 (IQR 8–11). SVR12 was achieved by 73% of CP-B/C vs. 91% of CP-A (p<0.01). CP-B/C vs. CP-A had more early treatment discontinuations (11% vs. 1%), adverse events requiring hospitalization (22% vs. 2%), infections requiring antibiotics (20% vs. 1%) and hepatic decompensating events (20% vs. 3%) (all p<0.01). There were 2 deaths: 1 CP-B/C (liver-related) and 1 CP-A (not liver-related). In multivariate analysis, CP-B/C independently predicted lack of SVR12 (OR 0.27, 95% CI 0.08–0.92). In comparing simeprevir plus sofosbuvir treated patients vs. matched untreated controls, adverse events requiring hospitalization (9% vs. 13%, p=0.55), infections (8% vs. 6%, p=0.47) and events of decompensation (9% vs. 10%, p=0.78) occurred at similar frequency.
Conclusions
Simeprevir plus sofosbuvir with/without ribavirin has lower efficacy and higher rates of adverse events in patients with CP-B/C cirrhosis compared to CP-A. The frequency of adverse safety outcomes were similar to matched untreated controls, suggesting safety events reflect the natural history of cirrhosis and are not related to treatment.
Keywords: Child-Pugh class, Model of end stage liver disease, Sustained virologic response, Adverse events
INTRODUCTION
All-oral hepatitis C virus (HCV) therapy has revolutionized the treatment of patients with cirrhosis, including those with decompensated disease. In December 2013, the Food and Drug Administration (FDA) approved the combination of simeprevir, a second-generation NS3/4A protease inhibitor (PI), and sofosbuvir, the first-in-class nucleotide analogue NS5B polymerase inhibitor, for treatment of genotype 1 patients with cirrhosis. Prior to this time, off-label use of this combination was prompted by the high need for an interferon-free therapy for patients with genotype 1 infection and advanced fibrosis. The phase 2 COSMOS study1 included patients with genotype 1 HCV infection with varying degrees of fibrosis, including compensated cirrhosis, who were treated for 12 or 24 weeks with simeprevir and sofosbuvir with or without ribavirin. Among patients with compensated cirrhosis, sustained virologic response at week 12 (SVR12) was obtained in 93% (38/41).1 Although the FDA has approved sofosbuvir and simeprevir for treatment of genotype 1 HCV, including in those with cirrhosis CP-A and B, safety information for this combination in patients with advanced cirrhosis is very limited and no prior controlled studies have been conducted.
Pharmacokinetic data on simeprevir and sofosbuvir indicate differences in drug levels in patients with decompensated cirrhosis and/or renal dysfunction. Simeprevir is extensively metabolized by the hepatic cytochrome CYP3A system and eliminated via biliary excretion.2 Relative to subjects with normal hepatic function, simeprevir areas-under-the-curves from 0 to 24 hour (AUC0–24) values were 240% and 502% higher in patients with CP class B and class C cirrhosis, respectively.2 In clinical trials, higher exposure to simeprevir was associated with increased frequency of adverse reactions.2 As a result, it is recommended that risks and benefits be carefully considered prior to simeprevir use in patients with CP class B cirrhosis and avoided in patients with CP class C cirrhosis.2 Sofosbuvir is extensively metabolized in the liver to the pharmacologically active metabolite GS-461203 with eventual dephosphorylation to the inactive metabolite GS-331007.3 Relative to subjects with normal hepatic function, the GS-331007 AUCs0-24 are only 18% and 9% higher in patients with CP class B and C cirrhosis respectively and thus no dose adjustments for sofosbuvir are recommended for patients with advanced cirrhosis.3 Renal clearance is the major elimination pathway for sofosbuvir, via GS-331007, and compared to those with normal renal function, sofosbuvir AUC0-∞ was 170% higher and the GS-331007 AUC0-∞ was 450% higher in those with estimated glomerular filtration rate <30 mL/min/1.73m2.3 Consequently, use of sofosbuvir is not recommended for patients with estimated glomerular filtration rate <30 mL/min/1.73m2. While these pharmacokinetic data are helpful, their utility for predicting safety and efficacy may be limited for patients with varying degrees of combined liver and renal dysfunction, as occurs frequently in patients with advanced cirrhosis.
A recent case report suggested simeprevir plus sofosbuvir may be associated with worsening hepatic decompensation in patients with advanced cirrhosis.4 However, establishing a causal relationship between drug exposures and decompensating events in the absence of controlled studies is challenging since decompensating events occur as part of the natural history of advanced liver disease.5
In this multicenter cohort study, we aimed to assess the real-world safety and efficacy of simeprevir and sofosbuvir with and without ribavirin in patients with cirrhosis, focusing specifically on comparing outcomes in patients with CP class B and C cirrhosis versus CP class A cirrhosis. Further, with the goal of assessing the association between drug exposure and safety outcomes with simeprevir plus sofosbuvir, we compared outcomes to matched untreated control patients.
METHODS
Simeprevir and Sofosbuvir Treatment Cohort
This retrospective multicenter cohort study included genotype 1 HCV-infected adult patients (18 years of age or older) with cirrhosis who received simeprevir and sofosbuvir with or without ribavirin from December 2013 through September 2014 in 2 different clinical settings: the University of California at San Francisco (UCSF) and Kaiser Permanente (KP). UCSF is an academic, tertiary care referral center that treats patients referred from northern and central California. KP is an integrated health care delivery organization and the regional sites of KP Northern California, KP Southern California, KP Colorado and KP Mid-Atlantic were included. KP membership is representative of a geographical area’s total insured population except for persons with extremes in income.6,7 The institutional review boards at each of the participating study centers approved this study. This retrospective study was approved by the UCSF institutional review board as a study exempt for need of consent. At part of their clinical care, all patients were informed that they were being treated with an off-label combination of oral antivirals and that treatment in those with advanced cirrhosis had not been previously studied. Patient demographic, virologic and clinical data, including medication dosing were collected by individual health record review and/or programmed capture from health plan databases.
The target population was patients with cirrhosis treated with simeprevir and sofosbuvir with and without ribavirin. Cirrhosis was defined by the presence Ludwig-Batts stage 4 fibrosis on liver biopsy or at least 2 of the following 5 criteria: 1) radiographic (ultrasound, computed tomography, magnetic resonance imaging) evidence of liver nodularity, 2) radiographic evidence of portal hypertension, 3) platelet count less than 120 thousand (K)/mm3 (with non-liver causes excluded), 4) endoscopic evidence of varices or portal hypertensive gastropathy, or 5) liver biopsy with Ludwig-Batts stage 3.
Treatment was simeprevir 150 mg once a day and sofosbuvir 400 mg once a day for a target duration of 12 weeks. Use of ribavirin and ribavirin dose adjustments were not protocolized but were at the discretion of the treating physician. If a patient’s estimated glomerular filtration rate (eGFR) dropped below 30 mL/min/1.73m2, the sofosbuvir dose was changed to 200mg once a day. Sofosbuvir 200mg daily dose was achieved by asking patients to cut the 400mg pill in half with a pill cutter. Simeprevir dose reductions were considered in patients with CP-B/C cirrhosis, but not undertaken, in large part, because of simeprevir’s capsule formulation. Growth factor use for management of cytopenias was also at the discretion of the treating physician.
Untreated Matched Control Cohort
The untreated control cohort was followed for 6 months, equivalent to the total time simeprevir and sofosbuvir treated patients were under observation (3 months treatment and 3 months after treatment to ascertain SVR12). Variables measured at “baseline” for the untreated control cohort were measured at the beginning of the 6-month period. HCV-infected controls with cirrhosis were retrospectively matched to cases based on center (UCSF vs. KP), time (6 month period between 1/1/2013 and 10/1/2014), age at treatment start (± 5 years), baseline CP class (A vs. B/C), and baseline model for end-stage liver disease (MELD) score (± 2). The majority of untreated controls were untreated because FDA-approved interferon free regimens were unavailable during the 6-month period they were followed. Many of the untreated controls were later treated when interferon free treatment regimens became available. For establishing CP class among potential controls using programmed data abstraction, specific rules were utilized. All potential controls with encephalopathy received 2 points towards their CP score and no potential controls were given 3 points. All potential controls with ascites were given 2 points if serum sodium was >131 or were given 3 points if serum sodium was ≤ 131.
For UCSF, the charts of all patients seen in hepatology faculty practice between 1/1/2013 and 10/1/2014 were examined. 2,813 potential controls with HCV who were not cases and with complete baseline data were identified. Among the simeprevir and sofosbuvir treated cases who did not have complete baseline CP class data (n=1), matches were identified based on center, baseline age and baseline MELD score only. One UCSF case did not have a single untreated control match. Otherwise, 8 to 143 controls were identified for each case of which 3 were randomly selected with replacement, using a random number generator, to create the UCSF portion of the untreated matched control cohort. Within the UCSF untreated control cohort, only one control was used twice as a result of random selection (Supplementary Figure 1).
For KP, identification of HCV controls with cirrhosis was done using programmed capture from health plan databases. A total of 3,232 potential controls were identified from 6,660 HCV infected patients with cirrhosis with active membership between 1/1/2013 and 10/1/2014 (i.e. no membership gap >180 days). Among those simeprevir and sofosbuvir treated cases who did not have complete baseline CP class data (n=3), matches were identified based on center, baseline age and baseline MELD score only. One KP case did not have a single untreated control match. Otherwise, 1 to 36 controls were identified for each case of which up to 3 were randomly selected with replacement, using a random number generator, to create the KP portion of the untreated matched control cohort. Within the KP untreated control cohort, no control was used twice (Supplementary Figure 2).
Primary Predictor and Study Endpoints
For the simeprevir and sofosbuvir with/without ribavirin treated cohort, patients with CP class B or C (CP score ≥ 7) cirrhosis were compared to those with CP class A (CP score 5–6) cirrhosis. The primary virologic outcome was achievement of SVR12, defined as an undetectable HCV RNA 12 weeks (± 2 weeks) after completion or early discontinuation of HCV therapy. Secondary virologic outcomes included achievement of undetectable HCV RNA at 4 weeks (± 1 week) of HCV therapy and end of treatment response (EOTR), defined as undetectable HCV RNA at the completion or early discontinuation of HCV therapy. Plasma HCV RNA levels were quantified by assays providing lower limits of quantification ranging from 615 to 43 IU/mL. Lower limits of detection of tests used to define “undetectable” levels were 10 IU/mL or lower at all sites. The primary safety outcome was hepatic decompensation (defined as new onset or worsening ascites, hepatic encephalopathy or esophageal variceal bleeding) during therapy. Secondary safety outcomes included MELD and CP score change from baseline to the end of treatment, early treatment discontinuation for any reason, early treatment discontinuation due to adverse event, early treatment discontinuation not due to adverse event, hospitalization due to adverse event, infection requiring antibiotics and death. All patients were included in the analysis of the safety outcomes. The virologic outcomes were analyzed on an intention to treat basis. Safety outcomes for the cohort receiving simeprevir plus sofosbuvir with/without ribavirin were compared to the untreated matched control cohort.
Statistical Analysis
Median with interquartile range (IQR), range and proportions were used for descriptive statistics, as appropriate. For comparisons of those patients with CP-B/C vs. CP-A and for comparisons between case and control cohorts, the Chi-square test was used for dichotomous variables and the Mann-Whitney U test was used for continuous variables. Exact methods were used, as appropriate.
Logistic regression was used to examine predictors of the primary virologic and safety outcomes, with p<0.05 defining statistical significance. The primary predictor of interest was baseline CP class (CP-B/C vs. CP-A). Baseline characteristics including age, sex, race/ethnic group (White, non-Hispanic vs. Hispanic vs. Black vs. Asian vs. Other), HCV genotype 1a (vs. 1b or other), HCV treatment experienced (vs. no treatment), baseline laboratory indices [bilirubin, creatinine, international normalized ratio (INR), albumin and platelet count], baseline MELD, baseline log-transformed HCV viral load (VL), and presence/absence of varices were examined in regression models. Those covariates with p<0.20 in univariate analysis were evaluated in multivariate models. Models were built using backward elimination of covariates, using a p<0.05 as the criterion for inclusion in the final model. The primary predictor, baseline CP score (CP ≥ 6 vs. CP = 5), was forced into all models. For virologic and safety outcomes, logistic regression models with the individual components of CP class were also examined. Multicollinearity within the models was examined using the variance inflation factor (VIF). If the VIF was >10, then multicollinearity was established and the offending covariate was removed from the model. Optimization of baseline bilirubin, INR, albumin and platelet count as a predictor of SVR12 was examined post-hoc using receiver operator curves (Supplementary Figure 3). The optimal point that maximized sensitivity and specificity within the simeprevir and sofosbuvir cohort for each covariate (bilirubin of 1.3 mg/dL, INR of 1.3, albumin of 3.5 g/dL, and platelet count of 100 K/mm3) was used in univariate and multivariate analyses of SVR12 and of decompensation. All final models were adjusted for sex, race/ethnicity and clinical center (UCSF and KP).
Statistical analyses were performed using STATA version 13 software (Stata Corporation, College Station, Texas).
RESULTS
A total of 160 patients with cirrhosis were treated with simeprevir and sofosbuvir, with 56 (35%) receiving ribavirin and 104 (65%) not. Ribavirin treated patients were similar to those not treated with ribavirin except that ribavirin-treated patients were more frequently Hispanic and less frequently Black, were more frequently previous null/partial responders and were more frequently treated at center #1 (Supplemental Table 1). The median age of the cohort was 62 years (IQR: 58 – 65), with 39% female, 11% Hispanic, 16% Black, and 26% with diabetes with a median hemoglobin A1c of 6.7% (IQR: 6.1% – 7.9%). A total of 56% were HCV treatment-experienced including 15% (n=13) with prior telaprevir- or boceprevir-triple therapy, and 62% with genotype 1a with 11 of 25 tested (44%) positive for the Q80K polymorphism (Table 1). In terms of severity of cirrhosis, the median baseline albumin count was 3.6 g/dL (IQR: 3.1 – 4.0), median baseline platelet count was 98 K/mm3 (IQR: 73 – 140), 24% had ascites, 23% had hepatic encephalopathy, and 15% had documented varices, Information to establish baseline CP class was missing in only 4 patients, yielding 35% with CP-B/C and 65% with CP-A. Only 4% (n=6) patients with CP-C cirrhosis were treated with simeprevir and sofosbuvir. All 6 CP-C patients were listed for liver transplant prior to treatment initiation, had median MELD score of 15 (range: 14–17). The decision to treat these 6 patients reflects their high need for treatment and was undertaken only after thorough discussion of potential risks and benefits and with close monitoring for adverse effects. Compared to patients with CP-A cirrhosis, those with CP-B/C cirrhosis were more frequently female and Hispanic, less frequently had hypertension requiring medication, had higher baseline bilirubin and INR and lower baseline albumin and platelet count. Hepatic encephalopathy, ascites, varices and listing for liver transplantation prior to starting treatment were all more frequent in the CP-B/C group. The median baseline MELD scores in patients with CP-B/C and CP-A cirrhosis were 12 (IQR: 10 – 14) and 8 (IQR: 7 – 9), respectively (Table 1). Ribavirin was used in 35% of CP-BC patients and 37% of CP-A patients (p=0.80).
Table 1.
Characteristics of Patients Treated with Simeprevir Plus Sofosbuvir by Baseline CP Class (B/C vs. A)
| Characteristic at start of treatment | CP Class B/C (n = 55) | CP Class A (n = 101) | p-value |
|---|---|---|---|
|
| |||
| Age, yrs, median (IQR) | 61 (58 – 64) | 62 (58–67) | 0.14 |
|
| |||
| Male, no. (%) | 27 (49) | 68 (67) | 0.03 |
|
| |||
| Race/ethnicity, no. (%) | 0.01 | ||
| White, Non-Hispanic | 31 (56) | 68 (67) | |
| Hispanic | 12 (22) | 5 (5) | |
| Black | 9 (16) | 17 (17) | |
| Asian | 0 (0) | 6 (6) | |
| Other | 3 (5) | 5 (5) | |
|
| |||
| Diabetes, no. (%) | 15 (35) | 40 (72) | 0.84 |
| Hemoglobin A1c, %, median (IQR) | 6.9 (6.4 – 8.6) | 6.5 (6.1–7.5) | 0.13 |
|
| |||
| Clinical diagnosis of hypertension, no. (%) | 18 (33) | 51 (51) | 0.03 |
|
| |||
| Dyslipidemia requiring medication, no. (%) | 2 (4) | 13 (13) | 0.07 |
|
| |||
| Genotype 1a (vs. 1b), no. (%) | 32 (58) | 66 (65) | 0.41 |
|
| |||
| Q80K polymorphism, no. (%) [N=24] | 5 (45) | 6 (46) | 0.97 |
|
| |||
| Previous treatment, no. (%) | 34 (62) | 52 (51) | 0.22 |
| Telaprevir- or boceprevir-triple therapy, no. (%) | 7 (21) | 6 (12) | 0.26 |
| Null/partial responders, no. (%) | 26 (76) | 37 (71) | 0.81 |
|
| |||
| Total bilirubin, mg/dL, median (IQR) | 1.7 (1.2 – 2.4) | 0.9 (0.7–1.2) | <0.01 |
|
| |||
| INR, median (IQR) | 1.3 (1.2–1.4) | 1.1 (1.0–1.2) | <0.01 |
|
| |||
| Albumin, g/dL, median (IQR) | 3 (2.7–3.3) | 3.9 (3.5–4.2) | <0.01 |
|
| |||
| Platelet count, 1K/mm3, median (IQR) | 78 (62 – 107) | 111 (81–160) | <0.01 |
|
| |||
| Creatinine, mg/dL, median (IQR) | 0.85 (0.7–1.05) | 0.83 (0.7–0.97) | 0.40 |
|
| |||
| Creatinine clearance*, mL/min/1.73m2, median (IQR) | 77 (53–91) | 81 (67–97) | 0.09 |
|
| |||
| Baseline HCV VL, log IU/mL, median (IQR) | 5.9 (5.4–6.4) | 6.4 (6.1–6.7) | <0.01 |
|
| |||
| Any hepatic encephalopathy§, no. (%) | 27 (49) | 8 (8) | <0.01 |
|
| |||
| Any ascites¶, no. (%) | 35 (64) | 3 (3) | <0.01 |
|
| |||
| Non-bleeding or bleeding varices, no. (%) | 19 (35) | 5 (5) | <0.01 |
|
| |||
| HCC, no. (%) | 4 (7) | 12 (12) | 0.37 |
|
| |||
| MELD, median (IQR) | 12 (10–14) | 8 (7–9) | <0.01 |
|
| |||
| Listed for liver transplantation, no. (%) | 24 (44) | 5 (5) | <0.01 |
|
| |||
| Center, no. (%) | 0.91 | ||
| #1 | 30 (55) | 56 (55) | |
| #2 | 25 (45) | 45 (45) | |
Calculated using Crockcroft-Gault equation
Either medically controlled hepatic encephalopathy or medically uncontrolled hepatic encephalopathy
Either medically controlled ascites or medically uncontrolled ascites
CP = Child-Pugh, HCC = hepatocellular carcinoma, INR = international normalized ratio, IQR = interquartile range, MELD = model for end-stage liver disease, VL = viral load
Virologic Outcomes in Patients Treated with Simeprevir and Sofosbuvir
The overall SVR12 frequency was 85%; 73% (40/55) in CP-B/C patients and 91% (92/101) in CP-A patients (p<0.01). Comparing CP-B (n=49) to CP-C (n=6) patients, SVR12 frequencies were similar (75% vs. 50%, p=0.33). The overall SVR12 frequency among those who received ribavirin was 89% vs. 82% among those who did not (p=0.23); 79% (15/19) vs. 69% (25/36) among CP-B/C patients (p=0.45), and 95% (35/37) vs. 89% (57/64) among CP-A patients (p=0.35). Among 13 patients previously treated with either telapravir- or boceprevir-based triple therapy, 10 (77%) achieved SVR12 (6 CP-B/C and 4 CP-A patients). Frequencies of undetectable HCV RNA at 4 weeks and end of treatment were similar between groups (Figure 1). Among those who achieved EOTR, the overall relapse frequency was 11%; 23% in CP-B/C patients and 4% in CP-A patients (p<0.01). In a per-protocol analysis excluding patients who discontinued therapy early, the overall SVR12 frequency was 86%; 75% (37/49) in CP-B/C patients and 91% (91/100) in CP-A patients (p<0.01).
Figure 1.
In univariate analysis, SVR12 was associated with CP-A (vs. CP-B/C), lower baseline bilirubin and MELD, higher baseline albumin and platelets, and absence of any hepatic encephalopathy or ascites (Table 2). Use of ribavirin with simeprevir and sofosbuvir was not associated with SVR12 in univariate analysis (OR: 1.74, 95% CI: 0.65 – 4.68, p=0.27) nor was previous telaprevir- or boceprevir-based triple therapy experience (OR: 0.45, 95% CI: 0.10 – 1.94, p=0.28). In multivariate models, bilirubin ≥1.3 mg/dL, INR ≥1.3, albumin ≥3.5 g/dL, any hepatic encephalopathy, any ascites and MELD were found to be collinear with CP-B/C group (VIF > 10) likely because all either are components or share components of the CP scoring system. Therefore, multivariate model 1 excluded baseline bilirubin, INR, albumin, any hepatic encephalopathy, any ascites and MELD (Table 2). CP-B/C cirrhosis and platelets ≥ 100 K/mm3 were factors significantly associated with SVR12 in this model. In multivariate model 2 where CP-B/C was replaced by its individual components and in this model, albumin ≥ 3.5 g/dL and platelets ≥100 K/mm3 were significantly associated with SVR12 (Table 2).
Table 2.
Univariate and Multivariate Predictors of SVR12 in Patients Treated with Simeprevir Plus Sofosbuvir
| Baseline Covariate | All Patients (n=160) | |||||
|---|---|---|---|---|---|---|
| Univariate* | Multivariate 1§ ¶ | Multivariate 2§ $ | ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| CP-B/C cirrhosis | 0.26 (0.11–0.65) | <0.01 | 0.27 (0.09–0.80) | 0.02 | -- | -- |
| Total bilirubin ≥1.3 mg/dL | 0.45 (0.19–1.068 | 0.07 | -- | -- | -- | -- |
| Albumin ≥ 3.5 g/dL | 5.25 (1.95–14.1) | <0.01 | -- | -- | 4.17 (1.44–11.8) | 0.01 |
| Platelets ≥ 100 K/mm3 | 2.73 (1.06–7.01) | 0.04 | 2.19 (1.17–6.45) | 0.02 | 2.42 (1.09–7.12) | 0.04 |
| Any hepatic encephalopathy | 0.41 (0.16–1.04) | 0.06 | -- | -- | -- | -- |
| Any ascites | 0.47 (0.19–1.18) | 0.11 | -- | -- | -- | -- |
| MELD, per 1 unit | 0.90 (0.77–1.04) | 0.15 | -- | -- | -- | -- |
Univariate results of variables with p<0.20. Baseline covariates examined included age, sex, race/ethnic group (White, non-Hispanic vs. Hispanic vs. Black vs. Asian vs. Other), HCV genotype 1a (vs. 1b or other), HCV treatment experienced (vs. no treatment), laboratory indices [bilirubin, creatinine, international normalized ratio (INR), albumin and platelet count], CP class (CP-B/C vs. CP-A), MELD, HCV viral load, log-transformed (VL) and presence/absence of varices.
Adjusted for sex, race and center effect
Multivariate model 1 with CP-B/C cirrhosis included in the model
Multivariate model 2 without CP-B/C cirrhosis included in the model
CP = Child-Pugh, HCV = hepatitis C virus, MELD = model for end-stage liver disease, RNA = ribonucleic acid, SVR12 = sustained virologic response at 12 weeks
Safety Outcomes in Patients Treated with Simeprevir and Sofosbuvir
Compared to patients with CP-A cirrhosis, CP-B/C patients more frequently stopped treatment early and did so more frequently due to adverse events (Table 3). CP-B/C patients also more frequently were hospitalized due to an adverse event, more frequently developed infections requiring antibiotics and more frequently developed de novo or worsening hepatic decompensation (Table 3). CP and MELD score change from baseline to the end of treatment were similar between the two groups (Table 3). Frequency of all safety outcomes were similar between those who received ribavirin compared to those who did not. For wait-listed patients, any changes in bilirubin, creatinine and INR during therapy were used for MELD score updating. However, the increase in bilirubin seen with simeprevir and ribavirin did not influence MELD score enough to significantly change prioritization for liver transplant. Reasons for early treatment discontinuation due to an adverse event, hospitalization due to an adverse event, infection requiring antibiotics, hepatic decompensation and death are provided in Table 4. Median time on simeprevir and sofosbuvir was 31 days (IQR: 13 – 56) among those who discontinued therapy early and was 44 days (IQR: 12 – 56) in those who discontinued therapy early due to adverse event. Among the seven patients who discontinued therapy early, 2 (29%) were receiving ribavirin. Four patients who stopped therapy early went on to achieve SVR12 after being maintained on therapy for a median 69 days (IQR: 44 – 82). Compared to patients with CP-B cirrhosis (n=49), CP-C patients (n=6) had numerically higher frequency of early treatment discontinuation (8% vs. 33%, p=0.12), hospitalizations due to an adverse event (20% vs. 33%, p=0.60), infections requiring antibiotics (18% vs. 33%, p=0.39) and worsening hepatic decompensation (18% vs. 33%, p=0.39).
Table 3.
Safety Outcomes in Patients Treated with Simeprevir Plus Sofosbuvir
| Safety Outcome | CP Class B/C (n = 55) | CP Class A (n = 101) | p-value |
|---|---|---|---|
| CP score change from baseline to end of treatment, median (range) | 0 (−2–5) | 0 (−1–2) | 0.46 |
| MELD score change from baseline to end of treatment, median (range) | 0 (−4–7) | 0 (−3–8) | 0.36 |
| Early treatment discontinuation, no (%) | 6 (11) | 1 (1) | <0.01 |
| Early treatment discontinuation due to adverse event, no (%) | 5 (9) | 1 (1) | 0.01 |
| Hospitalization due to adverse event, no (%) | 12 (22) | 2 (2) | <0.01 |
| Infection requiring antibiotics, no (%) | 11 (20) | 1 (1) | <0.01 |
| Hepatic decompensation, no (%) | 11 (20) | 3 (3) | <0.01 |
| Death, no (%) | 1 (2)* | 1 (1)§ | 0.66 |
liver-related death
not liver-related death
CP = Child-Pugh; Model for End-Stage Liver Disease = MELD
Table 4.
Safety Outcomes in Patients Treated with Simeprevir and Sofosbuvir
| Reason | CP Class B/C (n = 55) | CP Class A (n = 101) |
|---|---|---|
|
| ||
| Early Treatment Discontinuation due to Adverse Event (n=6)* | ||
| Infection and hepatic decompensation, no (%) | 3 (50) | 0 (0) |
| Skin/soft tissue infection, no | 1 | 0 |
| Cholangitis, no | 1 | 0 |
| Infection, no (%) | 1 (17) | 0 (0) |
| Bacteremia (unclear source), no | 1 | 0 |
| Hepatic decompensation, no (%) | 1 (17) | 1 (17) |
| De novo B-cell lymphoma, no (%) | 1 (17) | 0 (0) |
|
| ||
| Hospitalization due to Adverse Event (n=14) | ||
| Infection and hepatic decompensation, no (%) | 4 (29) | 0 (0) |
| Skin/soft tissue infection, no | 1 | 0 |
| Bursitis, no | 1 | 0 |
| Cholangitis, no | 1 | 0 |
| Urinary tract infection, Pneumonia, Dental abscess, no | 1 | 0 |
| Infection, no (%) | 3 (21) | 0 (0) |
| Skin/soft tissue infection, no | 2 | 0 |
| Bacteremia (unclear source), no | 1 | 0 |
| Hepatic decompensation, no (%) | 3 (21) | 0 (0) |
| Other, no (%) | 2 (14) | 2 (14) |
| Rash, no | 1 | 0 |
| Headache, no | 0 | 1 |
| Gout, no | 0 | 1 |
| Hypoglycemia in diabetic, no | 1 | 0 |
|
| ||
| Infection Requiring Antibiotics (n=12) | ||
| Skin/soft tissue infection, no (%) | 5 (42) | 2 (17) |
| Bursitis, no (%) | 1 (8) | 0 (0) |
| Cholangitis, no (%) | 1 (8) | 0 (0) |
| Bacteremia (unclear source), no (%) | 1 (8) | 0 (0) |
| Urinary tract infection, Pneumonia, Dental abscess, no (%) | 1 (8) | 0 (0) |
| Urinary tract infection, no (%) | 2 (17) | 0 (0) |
|
| ||
| Hepatic Decompensation (n=14) | ||
| Hepatic encephalopathy, no (%) | 7 (50) | 2 (14) |
| Ascites, no (%) | 3 (21) | 1 (7) |
| Variceal bleed, no (%) | 1 (7) | 0 (0) |
|
| ||
| Death (n=2) | ||
| Hepatic decompensation with seizures, no (%) | 1 (50) | 0 (0) |
| Non-Hodgkins lymphoma, present pre-treatment, no (%) | 0 (0) | 1 (50) |
One patient discontinued treatment early not due to an adverse event. Due to “forgot to take medications” in a patient with CP-B/C cirrhosis and baseline hepatic encephalopathy
CP = Child-Pugh
Among patients treated with ribavirin, 16% (3/19) of patients with CP-B/C cirrhosis vs. 5% (2/37) of CP-A cirrhosis had ribavirin added-on mid-therapy. Median ribavirin starting dose per day did not vary by CP-B/C (1000mg, IQR: 800–1200) vs. CP-A (1200mg, IQR: 900–1200) (p=0.31). Comparing patients with CP-B/C vs. CP-A cirrhosis, frequency of ribavirin dose reductions [32% (6/19) vs. 30% (11/37), p=1.00] and ribavirin dose discontinuations [11% (2/19) vs. 3% (1/37), p=0.26] were not significantly different. In CP-B/C patients, SVR12 was achieved in 57% (4/7) of CP-B/C patients who underwent ribavirin dose reductions and/or discontinuations compared to 92% (11/12) who did not (p=0.08). Two CP-B/C patients had baseline eGFR < 30 ml/min/1.73m2; neither received ribavirin and both received sofosbuvir 200mg daily (increased to 400mg daily if eGFR ≥ 30 on-treatment) and both achieved SVR12. Two additional CP-B/C patients, one with baseline eGFR of 35 ml/min/1.73m2 and the other with eGFR of 51 ml/min/1.73m2, developed eGFR < 30 ml/min/1.73m2 on-treatment resulting in sofosbuvir dose change to 200mg daily. The patient with baseline eGFR of 51 ml/min/1.73m2 was treated with ribavirin, required ribavirin dose reduction and achieved SVR12 while the patient with baseline eGFR of 35 ml/min/1.73m2 was treated without ribavirin and achieved SVR12.
In univariate analysis, hepatic decompensation during treatment was associated with CP-B/C (vs. CP-A), higher baseline bilirubin, INR and MELD, lower baseline albumin and platelets, absence of any hepatic encephalopathy and any ascites (Table 4). Use of ribavirin was not associated with decompensation (OR: 1.71, 95% CI: 0.59 – 5.00, p=0.32). In multivariate models, bilirubin ≥1.3 mg/dL, INR ≥1.3, albumin ≥ 3.5 g/dL, any hepatic encephalopathy, any ascites and MELD were found to be collinear with CP-B/C group (VIF > 10) likely because all either are components or share components of the CP scoring system. Therefore, multivariate model 1 excluded baseline bilirubin, INR, albumin, any hepatic encephalopathy, any ascites and MELD (Table 5). CP-B/C cirrhosis was the only factor significantly associated with hepatic decompensation. In multivariate model 2 where CP-B/C was replaced by its individual components, total bilirubin ≥1.3 mg/dL and any hepatic encephalopathy were significantly associated with hepatic decompensation (Table 5).
Table 5.
Univariate and Multivariate Predictors of Hepatic Decompensation in Patients Treated with Simeprevir Plus Sofosbuvir
| Baseline Covariate | Univariate* | Multivariate 1§ ¶ | Multivariate 2§ $ | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| CP-B/C cirrhosis | 8.12 (2.17–30.7) | <0.01 | 10.5 (2.03–54.1) | <0.01 | -- | -- |
| Total bilirubin ≥ 1.3 mg/dL | 9.81 (2.63–36.5) | <0.01 | -- | -- | 7.29 (1.72–31.0) | <0.01 |
| INR ≥ 1.3 | 1.88 (0.74–3.01) | <0.01 | -- | -- | ||
| Albumin ≥ 3.5 g/dL | 0.17 (0.05–0.65) | <0.01 | -- | -- | -- | -- |
| Platelets ≥ 100 K/mm3 | 0.48 (0.16–1.47) | 0.19 | -- | -- | -- | -- |
| Any hepatic encephalopathy | 6.56 (2.15–19.8) | <0.01 | -- | -- | 4.82 (1.27–18.4) | 0.02 |
| Any ascites | 4.20 (1.41–12.5) | 0.01 | -- | -- | -- | -- |
| MELD, per 1 unit | 1.34 (1.13–1.59) | <0.01 | -- | -- | -- | -- |
Univariate results of variables with p<0.20. Baseline covariates examined included age, sex, race/ethnic group (White, non-Hispanic vs. Hispanic vs. Black vs. Asian vs. Other), HCV genotype 1a (vs. 1b or other), HCV treatment experienced (vs. no treatment), laboratory indices [bilirubin, creatinine, international normalized ratio (INR), albumin and platelet count], CP class (CP-B/C vs. CP-A), MELD, HCV viral load, log-transformed (VL) and presence/absence of varices.
Adjusted for sex, race and center effect
Multivariate model 1 with CP-B/C cirrhosis included in the model
Multivariate model 2 without CP-B/C cirrhosis included in the model
CP = Child-Pugh, INR = international normalized ratio, MELD = model for end-stage liver disease
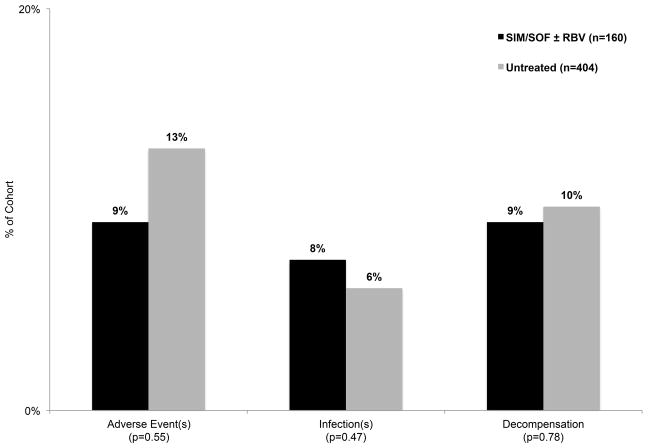
Outcomes in Patients Treated with Simeprevir plus Sofosbuvir vs. Untreated Matched Controls
Simeprevir and sofosbuvir treated patients (with or without ribavirin) were similar to untreated matched controls except that simeprevir-sofosbuvir treated patients were more frequently female and of Hispanic or Black ethnicity/race and HCV treatment-experienced (Supplement Table 1). Cases had a similar rate of adverse events, infections requiring antibiotics and hepatic decompensation as controls (Figure 2). Simeprevir-sofosbuvir treated patients versus controls had similar rates of liver transplantation (1% vs. 2%, p=0.68) and death (1% vs. 1%, p=0.87). When comparing ribavirin treated cases (n=56) to their matched untreated controls (n=167), similar rates of adverse events (11% vs. 10%, p=0.97), infections requiring antibiotics (10% vs. 6%, p=0.35) and hepatic decompensation (13% vs. 8%, p=0.36) were observed. In multivariate analysis adjusting for baseline CP class, viral load, HCC status, varices, sex, race and treating center, simeprevir and sofosbuvir with/without ribavirin treatment was not associated with hepatic decompensation (OR 1.02, 95% CI 0.42–2.49, p=0.97).
Figure 2.
DISCUSSION
Capturing real-life treatment outcomes from diverse treatment settings, we provide critical information on the safety and effectiveness of simeprevir plus sofosbuvir in patients with decompensated cirrhosis. We found the efficacy of simeprevir plus sofosbuvir is significantly reduced in those with decompensation, with 73% of patients with CP-B/C cirrhosis achieving SVR12 compared to 91% of those with compensated cirrhosis. This highlights the need for more efficacious therapies for those with decompensated cirrhosis, a group that remains “difficult to cure” even in the current era of all oral antiviral therapy. Moreover, patients with decompensated cirrhosis (CP-B/C) more frequently required treatment discontinuation and experienced more adverse events including hepatic decompensation compared to patients with compensated cirrhosis. The recognition of the heightened rates of adverse events during treatment points to the need for treating providers to have expertise in management of liver complications and to consider whether evaluation for liver transplantation is needed prior to the start of treatment.
Simeprevir plus sofosbuvir is not recommended in patients with CP-C cirrhosis. Our study cohort included only 6 patients with CP-C cirrhosis and their treatment reflects the limited peginterferon-free therapies available at the time of this study and the high unmet need in these advanced disease patients. Efficacy and safety outcomes were numerically less favorable among patients with CP-C cirrhosis compared to CP-B cirrhosis supporting the recommendation that simeprevir plus sofosbuvir not be used in CP-C cirrhosis. The HCV-TARGET cohort included 227 patients with history of decompensation who underwent simeprevir and sofosbuvir therapy and preliminary data on SVR4 showed a lower rate in patients with cirrhosis, but data on virologic and safety outcomes by baseline CP class were not included8. Along with patients with CP-C cirrhosis, our multivariate logistic analysis suggests that patients with baseline bilirubin > 1.3 or any presence of hepatic encephalopathy may be a group at particularly high risk of decompensating events and for whom simeprevir plus sofosbuvir should be contraindicated.
The most recent AASLD-IDSA recommendations for patients with genotype 1 disease and decompensated cirrhosis was daily fixed-dose combination ledipasvir/sofosbuvir and ribavirin for 12 weeks or ledipasvir/sofosbuvir alone for 24 weeks in those who are deemed ribavirin intolerant.9 These recommendations are based on the preliminary results of the SOLAR-1 study,10 which was a multicenter randomized controlled trial of 108 patients with HCV genotypes 1 and 4 with predominantly CP class B cirrhosis (only 9 CP-C patients). The AASLD-IDSA treatment guideline recommends against the use of protease inhibitors, including simeprevir, in patients with decompensated cirrhosis. Simeprevir plus sofosbuvir is approved for 24 weeks in patients with cirrhosis, including CP-B patients, and in countries where this combination may be the only all-oral therapy available, simeprevir plus sofosbuvir can be considered. However, based upon our safety analysis, we would advise against use of simeprevir and sofosbuvir in patients with an elevated bilirubin or hepatic encephalopathy at baseline, and that patients be monitored closely during treatment. Across a variety of HCV treatments, patients with decompensated cirrhosis have a lower response than patients without.1,11–15 Indeed, our study highlights the importance of CP score as a predictor of both efficacy and safety outcomes, and supports the importance of garnering real-life data for each approved drug combination to guide clinician decisions and to better inform patients about the risks and benefits of a specific treatment regimen.
We found that CP and MELD scores remained on average unchanged from baseline to the end of treatment among patients treated with simeprevir plus sofosbuvir with or without ribavirin, regardless of baseline severity of liver disease. These results are in contrast to the preliminary results of the SOLAR-1 study in which mean CP and MELD scores improved in the majority of patients treated with ledipasvir/sofosbuvir with or without ribavirin for 12 or 24 weeks.10 We speculate that improvements of CP and MELD scores with simeprevir-sofosubvir may be offset by the elevation in unconjugated bilirubin caused by simeprevir. Median albumin change from baseline to end of treatment increased by 0.15 g/dL (IQR: 0 – 0.4) suggesting that liver function improved with simeprevir plus sofosbuvir therapy. Additionally, our finding of similar frequencies of safety outcomes between matched untreated controls and simeprevir plus sofosbuvir treated patients suggests the natural history of HCV was not altered by treatment and may be related to the short duration of follow-up. Studies of longer-term outcomes including survival will be important to guide future decisions about antiviral therapy among patients with advanced cirrhosis, especially those on the waiting list.
The reasons for this differential rate of response in patients with decompensated cirrhosis are unknown. Previous studies have shown that increased portal hypertension, measured directly using the hepatic vein pressure gradient, independently predicted non-SVR possibly due to altered pharmacokinetics of drug uptake, distribution and metabolism.16 Another study showed that increased intrahepatic resistance with increased shear in the hepatic sinusoids that dampens the chemoattraction of T lymphocytes and the interaction of T lymphocytes and HCV-infected hepatocytes, an interaction that appears crucial for viral clearance.17 These and other factors may explain the lower treatment response among patients with cirrhosis, factors that may be more exaggerated in patients with cirrhosis and decompensation.
In our study, we found that presence of clinically overt hepatic encephalopathy independently predicted hepatic decompensation. In a recent case report of two patients experiencing worsening decompensation during simeprevir plus sofosbuvir, both had undergone prior transjugular intrahepatic portosystemic shunt procedures. Portal-systemic shunting, the underlying mechanism of hepatic encephalopathy that is exacerbated by transjugular intrahepatic portosystemic shunts, may substantially decrease the pre-systemic elimination (i.e., first-pass effect) of high extraction drugs, thus leading to alterations in drug delivery and metabolism.18 For sofosbuvir, shunting would decrease both the hepatic conversion to the pharmacologically active metabolite as well as the eventual hepatic dephosphorylation that leads to drug elimination,3 potentially contributing to decreased efficacy and possibly higher risk of toxicity. Since simeprevir is extensively metabolized by the hepatic cytochrome CYP3A system, shunting would certainly be expected to increase drugs levels and lead to possible drug toxicity.2
There are some limitations of our study. First, due to its retrospective nature and reliance on medical records from routine clinical practice, factors of potential importance in predicting response to therapy, such as Q80K status, are lacking in all patients. Second, the control group was not matched on baseline eGFR, which may have resulted in a closer matching of disease severity and likelihood of safety outcomes. However, we did match on baseline MELD score, which contains creatinine, and therefore patients were at least matched on an indirect measure of renal function. Third, we did not collect laboratory and clinical data beyond the end of treatment, data that would have provided insights into the impact of viral clearance on stabilizing or reversing complications of liver disease. Fourth, given the inclusion of multiple centers with multiple treating physicians, there was likely variability in criteria for hospitalization of patients with adverse events as well as thresholds for discontinuing treatment. However, we believe capturing the real-life clinical use of simeprevir and sofosbuvir in different clinical settings is a strength that increases the generalizability of our findings.
In summary, we have shown that simeprevir and sofosbuvir with and without ribavirin is highly effective and safe in patients with CP-A cirrhosis but associated with lower SVR rates and higher rates of safety events in patients CP-B/C cirrhosis. The frequency of safety outcomes, however, were not different than matched untreated controls of similar disease severity, suggesting that these safety events are not related to the drugs per se but rather the natural history of decompensated cirrhosis. We present very limited data on CP-C cirrhosis and those treated had a perceived critical need for treatment, given the absence of other interferon-free regimens at the time. Understanding the reasons for the reduced efficacy of current all-oral regimens in those with advanced cirrhosis, especially CP-C, remains a top priority for the HCV treatment community.
Supplementary Material
Acknowledgments
Grant Support:
The author VS was supported in part by an NIH-funded postdoctoral research training program grant (T32 DK060414). At Kaiser Permanente, this work was supported by The Permanente Medical Group and Southern California Permanente Medical Group, Department of Research and Evaluation. At the University of California San Francisco, this work was supported by the Liver Center Grant (NIH P30 DK 026743).
The authors gratefully acknowledge the contributions of Dr. Jean-Luc Szpakowski, Dr. Jose Pio, Robert Rathbun PharmD, Susan Caparosa MA, Xia Li MS, Su-Jau Yang PhD, Renee Cunanan, Karen Forte RN, Jonie Ishikawa RN, Jeanette Sanchez PharmD, Kali Sommer PharmD, Gayle Witt RN, Lisa Catalli NP-C and Stephanie Straley PA-C to this work.
Abbreviations
- AASLD-IDSA
American Association for the Study of Liver Disease – Infectious Disease Society of America
- AUC0–24
Areas-under-the-curves from 0 to 24 hours
- CP
Child-Pugh
- FDA
Food and Drug Administration
- HCV
Hepatitis C virus
- MELD
Model for end-stage liver disease
- PI
Protease inhibitor
- RBV
Ribavirin
- SIM+SOF
Simeprevir plus sofosbuvir
- SVR
Sustained virologic response
- SVR12
Sustained virologic response at week 12
Footnotes
Author Contributions:
Authors VS, LN and NT were instrumental in conception and design of this study, generation, collection, assembly, analysis and interpretation of data, drafting and revising the manuscript and approval of the final version of the manuscript. All authors participated in the interpretation of data and approved the final version of the manuscript.
Disclosures:
NT discloses research support from Gilead, Genetech/Roche, Vertex, Novartis, Eisai and AbbVie and has served as consultant for Bristol-Myers Squibb. None of these companies had any role in this study.
LN discloses research support from Gilead, AbbVie, Merck, Vertex and Roche/Genentech. All grant support is paid to the institution only. None of these companies had any role in this study.
AN discloses research support from Gilead, AbbVie, Merck, Vertex, Alvine/Hologic and Roche/Genentech. All grant support is paid to the institution only. None of these companies had any role in this study.
MP discloses research support from Merck and Gilead. All grant support is paid to the institution only. None of these companies had any role in this study.
VS, AD, BP, BW, JRed and JRea have nothing to disclose.
References
- 1.Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014;384:1756–65. doi: 10.1016/S0140-6736(14)61036-9. [DOI] [PubMed] [Google Scholar]
- 2.Simeprevir [package insert] Titusville, NJ: Janssen Therapeutics; 2013. [Google Scholar]
- 3.Sofosbuvir [package insert] Foster City, CA: Gilead Sciences; 2013. [Google Scholar]
- 4.Stine JG, Intagliata N, Shah NL, et al. Hepatic Decompensation Likely Attributable to Simeprevir in Patients with Advanced Cirrhosis. Digestive diseases and sciences. 2014 doi: 10.1007/s10620-014-3422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena V, Terrault NA. Hepatitis C Direct Antiviral Drugs and Hepatic Decompensation in Patients with Advanced Cirrhosis: Culprit or Innocent Bystander? Digestive diseases and sciences. 2015 doi: 10.1007/s10620-015-3535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon N. Similarity of the adult Kaiser Permanente membership in northern California to the insured and general population in northern California: statistics from the 2007 California Health Interview Survey. Internal Report: Kaiser Permanente Division of Research. 2012 [Google Scholar]
- 7.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen DM, O’Leary JG, Pockros PJ, et al. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: real-world experience in a diverse, longitudinal observational cohort [abstract]. Program and abstracts of the 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, Massachusetts. p. Abstract 45. [Google Scholar]
- 9.AASLD/IDSA/IAS-USA. [Accessed on January 26, 2015];Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org.
- 10.Flamm SL, Everson GT, Charlton M, et al. Ledipasvir/sofosbuvir with ribavirin for the treatment of HCV in patients with decompensated cirrhosis: preliminary results of a prospective, multicenter study. 65th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); November 1–5, 2014; Boston, MA. [Google Scholar]
- 11.Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–23. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 12.Curry MP, Forns X, Chung RT, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015;148:100–7. e1. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 13.Charlton M, Gane E, Manns MP, et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology. 2015;148:108–17. doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. The New England journal of medicine. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 15.Iacobellis A, Siciliano M, Annicchiarico BE, et al. Sustained virological responses following standard anti-viral therapy in decompensated HCV-infected cirrhotic patients. Aliment Pharmacol Ther. 2009;30:146–53. doi: 10.1111/j.1365-2036.2009.04025.x. [DOI] [PubMed] [Google Scholar]
- 16.Reiberger T, Rutter K, Ferlitsch A, et al. Portal pressure predicts outcome and safety of antiviral therapy in cirrhotic patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2011;9:602–8. e1. doi: 10.1016/j.cgh.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Zhu H, Tu Z, Xu YL, Nelson DR. CD8+ T-cell interaction with HCV replicon cells: evidence for both cytokine- and cell-mediated antiviral activity. Hepatology. 2003;37:1335–42. doi: 10.1053/jhep.2003.50207. [DOI] [PubMed] [Google Scholar]
- 18.Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. European journal of clinical pharmacology. 2008;64:1147–61. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.