Abstract
Introduction
Lung cancer in never-smokers ranks among the ten most common causes of death due to cancer worldwide and in the U.S. However, it is unknown whether never-smokers at elevated risk for developing lung cancer may benefit from lung cancer screening.
Methods
The MISCAN-Lung microsimulation model was used to assess the effects of lung cancer screening for simulated cohorts of never-smokers at different levels of relative risk (RR) for lung cancer compared to never-smokers at average risk. The benefits and harms of screening were estimated for each cohort and compared to those of a cohort of ever-smokers eligible for lung cancer screening according to the United States Preventive Services Task Force (USPSTF) criteria.
Results
The relative lung cancer mortality reduction in never-smokers was higher than the USPSTF eligible cohort (37% compared to 32%). However, the number of life-years gained per lung cancer death averted was lower (10.4 compared to 11.9) and the proportion of overdiagnosed cancers was higher (9.6% compared to 8.4%) for never-smokers compared to the USPSTF eligible cohort, as never-smokers are diagnosed at a later age. The estimated number of screens per lung cancer death averted ranged from 6,162 for never-smokers at average risk to 151 for never-smokers with a RR of 35, compared to 353 for the USPSTF eligible cohort.
Conclusions
Never-smokers with RRs of 15-35 have similar to better trade-offs between benefits and harms compared to ever-smokers recommended for lung cancer screening by the USPSTF guidelines. For most never-smokers, lung cancer screening is not beneficial.
Keywords: Lung cancer, Screening, Never-smokers
Introduction
While smoking is considered as a main risk factor for developing lung cancer, 10- 25% of all lung cancers occur in never-smokers.1, 2 Lung cancer in never-smokers is a significant public health problem, as it ranks among the ten most common causes of death due to cancer worldwide and in the U.S. 2-4
The results of the National Lung Screening Trial (NLST) have indicated that lung cancer mortality can be reduced by screening ever-smokers with computed tomography (CT). 5 The United States Preventive Services Task Force (USPSTF) recently published the recommendation to implement annual lung cancer screening for ever-smokers aged 55-80 years who have smoked at least 30 pack-years and, if quit smoking, quit less than 15 years ago.6 Other organizations have recommended screening using the NLST eligibility criteria or variations thereof. 7-9 To our knowledge, no organization currently recommends lung cancer screening for never-smokers.
Some lung cancer screening studies have included never-smokers, but these studies used chest radiography or were single arm studies.10-12 A survey on attitudes toward lung cancer screening in the United States showed that a large proportion of never-smokers were willing to consider lung cancer screening, even though few believed that they were at risk for developing lung cancer.13
In addition to tobacco smoking, various risk factors for developing lung cancer have been identified for ever- and never-smokers, such as environmental tobacco smoke (e.g. “second-hand smoking”), exposure to carcinogens (e.g. asbestos, radon gas and ionizing radiation), and genetic susceptibility. 3, 14-16 A number of risk models incorporate these and other risk factors to identify ever- and never-smokers at elevated levels of risk.17-21 Recent studies have identified sub-populations within the NLST who were at a higher level of risk for developing lung cancer compared to the average population of the trial. 20, 22, 23 Screening was more effective for these sub-populations, which indicates that screening recommendations based on an individual's risk could lead to more effective screening programs.20, 22, 23 Therefore, some researchers argue that lung cancer screening may be recommended for never-smokers, provided that they have a high risk for developing lung cancer.24
However, the long-term benefits and harms of implementing a lung cancer screening program for never-smokers are unknown. The USPSTF recommendations were in part based on modeling analyses, which investigated the trade-offs between the long-term benefits and harms of different screening policies for ever-smokers.25 This study aims to investigate the trade-offs between the benefits and harms of lung cancer screening for never-smokers at different levels of risk.
Materials and Methods
MISCAN-Lung
The MIcrosimulation SCreening ANalysis (MISCAN) Lung model is used in this investigation. MISCAN-Lung has been calibrated to the NLST, the Prostate, Lung, Colorectal and Ovarian Cancer Screening trial (PLCO), and data from the Surveillance, Epidemiology and End Results (SEER) Program, from which it derived information on the preclinical duration of lung cancer and CT screening effectiveness.26, 27 Lung cancer incidence and mortality in never-smokers in the PLCO were among the calibration targets of the model.26, 27 MISCAN-Lung aided in informing the USPSTF on their recommendations for lung cancer screening.25, 28
Histological types
There are indications that smoking behavior affects not only a person's risk of developing lung cancer, but also the histological type that develops.29, 30 This suggests that the distribution of histological types of lung cancer in never-smokers may differ from ever-smokers. Subramanian provided an overview of the distribution of histological types of lung cancer in never-smokers across different studies.16 This overview was used to derive the distribution of histological types of lung cancer in never-smokers for this investigation, shown in Table 1.16 To our knowledge, little information is available on differences in the distribution of histological types of lung cancer in never-smokers between genders. Therefore, we assumed the distribution of histological types of lung cancer in never-smokers did not differ by gender.
Table 1. Histologic types considered in MISCAN-Lung.
| Histologic types considered in MISCAN-Lung | Proportions considered in never-smokers (both genders) | Proportions considered in ever-smokers (men) | Proportions considered in ever-smokers (women) |
|---|---|---|---|
| Adenocarcinoma/large cell carcinoma/bronchioalveolar carcinoma | 66.68% | 41.01% | 50.33% |
| Squamous cell carcinoma | 13.68% | 25.22% | 15.78% |
| Small cell carcinoma | 2.53% | 13.75% | 13.26% |
| Other non-small cell carcinoma | 17.12% | 20.02% | 20.63% |
Table notes: Abbreviations: Microsimulation Screening Analysis model (MISCAN)
Lung cancer survival
It has been suggested that never-smokers may have a better response to certain treatments compared to ever-smokers, such as treatment with epidermal-growth-factor receptor inhibitors, which could lead to differences in survival.31, 32 Some studies suggest that never-smokers have a better survival compared to ever-smokers, while other studies indicate that no significant differences in survival exist.33-36 To our knowledge, no study provides detailed data on lung cancer survival for never-smokers by stage, histology and gender.33-36 Therefore, survival data from SEER was used, which provides detailed information on survival by stage, histology and gender for ever- and never-smokers combined.37
Lung carcinogenesis
MISCAN-Lung utilizes the two-stage clonal expansion model (TSCE) to estimate a person's risk of developing lung cancer as a function of age and smoking history.26, 27, 38, 39 The TSCE has been used to investigate the age-specific incidence of lung cancer in never-smokers previously.14, 39, 40 To assess whether MISCAN-Lung is suitable for investigating the effectiveness of lung cancer screening for never-smokers, the estimated age-group specific mortality rates of lung cancer in never-smokers were compared to those reported by Thun.41
Considered levels of relative risk
If lung cancer screening is to be considered for never-smokers, eligible individuals will need to be identified, for example, through the application of risk models. To our knowledge, the following lung cancer risk models consider never-smokers: the Spitz, PLCOm2011, PLCOm2104 and the Liverpool Lung Project (LLP) models.17-20 The Spitz model incorporates: environmental tobacco smoke exposure (odds ratio (OR) of 1.80, 95% confidence interval (CI) 1.20-2.69)) and a family history of any cancer in two or more first-degree relatives (OR 2.00, 95% CI 1.39-2.90).18 Spitz noted that the ORs of these variables closely approximated the relative risks (RR).18 Thus, the Spitz model considers RRs up to 3.6. Recently, this model was extended to incorporate micronuclei in binucleated cells (BN-MN) (OR 16.72 per unit increase, 95% CI 9.01-31.02) alongside environmental tobacco smoke exposure (OR 1.12, 95% CI 0.47-2.68) and a family history of cancer in two or more first-degree relatives (OR 1.06, 95% CI 0.47-2.43).21 The average difference in BN-MN between cases and controls in the model's development and validation datasets was 1.78-1.79 units.21 Assuming the ORs of the model variables closely approximate the RRs and an increase of 1.80 units of BN-MN compared to a never-smoker at average risk is considered, the model considers RRs up to at least 35.73 for never-smokers.
The PLCOm2011 model was the first model based on data from PLCO to provide risk estimates for never-smokers.17 Recently, an updated version of this model (PLCOm2014) was published that incorporates five risk factors (excluding age and race) for never-smokers: education (OR 0.92 per 1 of 6 levels change, 95% CI 0.87-096), body mass index (BMI) (OR 0.97 per 1 unit change, 95% CI 0.95-0.99), chronic obstructive pulmonary disease (OR 1.41, 95% CI 1.15-1.73), a personal history of cancer (OR 1.623, 95% CI 1.22-2.16) and a family history of lung cancer (OR 1.80, 95% CI 1.48-2.18).20 The calculator provided by the authors was used to verify that the ORs closely approximate the RRs (available at http://www.brocku.ca/lung-cancer-risk-calculator). A BMI of 18 is the lowest for which the model is valid and assuming the base BMI and education levels are similar to those in the PLCOm2012 model (a BMI of 27 and “some college education”), implies that the PLCOm2014 model considers RRs up to 6.98 (disregarding age and race).20, 23
The LLP model incorporates four risk factors for never-smokers: a history of pneumonia (OR 1.83, 95% CI 1.26-2.64), asbestos exposure (OR 1.89, 95% CI 1.35-2.62), a history of cancer (OR 1.96, 95% CI 1.22-3.14), and a family history of lung cancer (OR 2.02 for age of onset <60, 95% CI 1.18-3.45 and 1.18 for age of onset ≥60, 95% CI 0.79-1.76).19 The model was replicated in R software (version 3.0.1) and analyses indicate that the ORs of the risk factors closely approximate the RRs. Thus, the LLP model considers RRs up to 13.69.
Therefore cohorts of never-smokers with the following levels of RR will be simulated: 1, 2, 5, 10, 15, 20 and 35. For cohorts with RRs higher than 1, the hazard function of the TSCE at each age was multiplied by the considered level of RR.25 In addition, a “USPSTF eligible” cohort is simulated, composed of individuals who would be eligible for at least one screening at some point in their life according to the USPSTF recommendations. The Smoking History Generator developed by the National Cancer Institute was used to generate the probability of death from causes other than lung cancer by smoking behavior (including never smoking).42-44
Considered screening programs, benefits and harms
The investigated cohorts are assumed to be born in 1950 and followed from ages 45-90, similar to de Koning.25 To allow comparison to the screening policy recommended by the USPSTF, never-smokers are assumed to be screened annually from ages 55-80 with a perfect adherence to screening. The investigated benefits and harms of lung cancer screening include the relative reduction in lung cancer mortality, the number of life-years gained and overdiagnosis.
Results
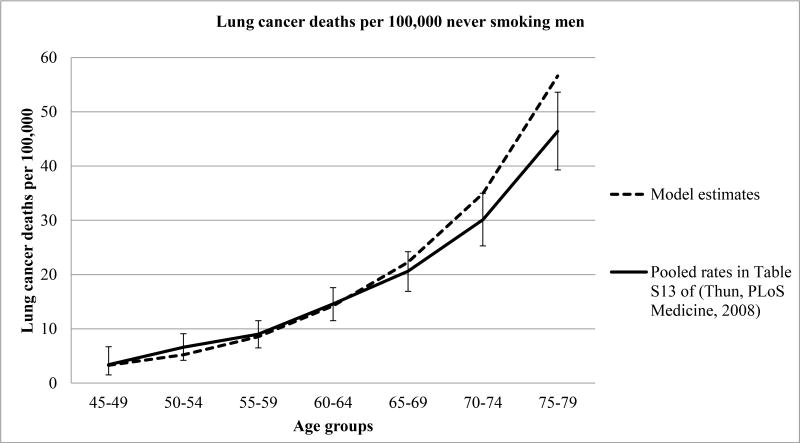
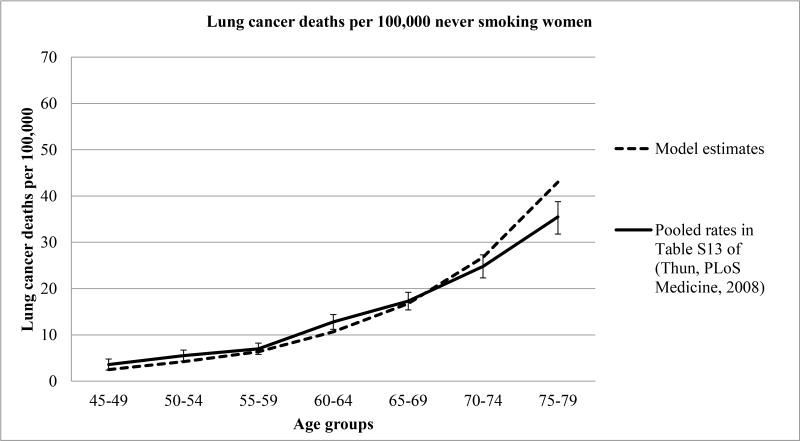
The age-group specific lung cancer mortality rates for never-smoking men and women estimated by MISCAN-Lung were compared to those reported by Thun (Table S13 in their report), in Figures 1A and 1B respectively.41 Overall, the model reproduced the reported age-group specific lung cancer mortality rates well for both genders, but somewhat overestimated the lung cancer mortality rate for ages 75-79.
Figure 1A. Observed and estimated lung cancer death rates in never smoking men Figure notes.
Adapted from.41 Error bars denote 95% confidence intervals for the incidence rate difference.
Figure 1B. Observed and estimated lung cancer death rates in never smoking women Figure notes.
Adapted from.41 Error bars denote 95% confidence intervals for the incidence rate difference.
Table 2 shows the benefits of lung cancer screening for the investigated cohorts. The proportion of lung cancers detected at an early stage was higher for never-smokers compared to the USPSTF eligible cohort (65.8-65.9% of all cases compared to 59.4%). This may be a result of the higher proportion of adenocarcinomas in never-smokers (see Table 1), which have a longer preclinical sojourn time and are more likely to be detected at an early stage by CT screening compared to other histologies.26 As a result of the larger proportion of lung cancers detected at an early stage, the relative reduction in lung cancer mortality was higher for never-smokers compared to the USPSTF eligible cohort: 37.0-37.3% compared to 32.7%. However, the number of lung cancer deaths averted (per 100,000) was lower for most cohorts of never-smokers, ranging from 354 deaths averted for never-smokers at average risk to 12,509 for never-smokers with a RR of 35, compared to 4,305 for the USPSTF eligible cohort. The same holds for the number of life-years gained (per 100,000), which ranged from 3,669 for never-smokers at average risk to 129,509 for never-smokers with a RR of 35, compared to 51,035 for the USPSTF eligible cohort.
Table 2. Benefits of screening.
| Scenario | Lung cancers detected at an early stage (stage I-II) % | Lung cancer mortality reduction % | Absolute number of lung cancer deaths averted per 100,000 | Life-years gained per 100,000 | Life-years gained per lung cancer death averted | Screens per life-year gained | Screens per lung cancer death averted |
|---|---|---|---|---|---|---|---|
| USPSTF (ever eligible only) | 59.4% | 32.7% | 4,305 | 51,035 | 11.9 | 30 | 353 |
| Never-smokers at average risk | 65.8% | 37.1% | 354 | 3,669 | 10.4 | 594 | 6,162 |
| Never-smokers at 2 times average risk | 65.9% | 37.0% | 706 | 7,332 | 10.4 | 296 | 3,075 |
| Never-smokers at 5 times average risk | 65.8% | 37.0% | 1,764 | 18,359 | 10.4 | 117 | 1,216 |
| Never-smokers at 10 times average risk | 65.8% | 37.1% | 3,541 | 36,809 | 10.4 | 57 | 593 |
| Never-smokers at 15 times average risk | 65.8% | 37.1% | 5,322 | 55,247 | 10.4 | 37 | 387 |
| Never-smokers at 20 times average risk | 65.8% | 37.1% | 7,118 | 73,892 | 10.4 | 27 | 283 |
| Never-smokers at 35 times average risk | 65.9% | 37.3% | 12,509 | 129,786 | 10.4 | 15 | 151 |
Table notes: Abbreviations: United States Preventive Services Task Force (USPSTF)
The number of lung cancer deaths averted and life-years gained for never-smokers with a RR of 15 were higher compared to the USPSTF cohort. However, due to the high number of screens, the number of screens per life-year gained and the number of screens per lung cancer death averted were still higher compared to the USPSTF cohort. However, screening never-smokers with a RR of 20 leads to a slightly lower number of screens per life-year gained and a much lower number of screens per lung cancer death averted compared to the USPSTF cohort.
Table 3 shows the harms of lung cancer screening for the investigated cohorts. The number of screens (per 100,000) was much greater for the USPSTF eligible and never-smoker cohorts compared to the 1950 cohort examined in de Koning et al, as in the latter cohort only 19.3% of the cohort received at least one screen.25 The number of screens was higher for the never-smoker cohorts compared to the USPSTF eligible cohort: approximately 1.8-2.1 million screens compared to 1.5 million screens. This is due to two reasons: first, never-smokers live longer compared to ever-smokers and will be able to attend more screenings during their life-time.43, 45 Second, the USPSTF criteria indicate that ever-smokers may not be eligible for screening at the earliest starting age, as some current, continuing smokers may not reach the minimum number of pack-years at age 55 but at a later age. In addition, eligible former smokers may not complete the full screening program due to becoming ineligible by reaching the maximum years since cessation. As a result, the average number of screening examinations per person screened was higher for the cohorts of never-smokers compared to the USPSTF eligible cohort (20-22 compared to 16). As the never-smoker cohorts receive a higher number of screening examinations and are diagnosed at a later age (when no screening occurs), the proportion of overdiagnosis was higher compared to the USPSTF eligible cohort (9.5-9.6% of all screen-detected cases compared to 8.4%).
Table 3. Harms of screening.
| Scenario | Screens per 100,000 | CT examinations per 100,000 (includes screenings) | Average screening examinations per person screened | % of cases overdiagnosed | % of screened cases overdiagnosed |
|---|---|---|---|---|---|
| USPSTF (ever eligible only) | 1,520,632 | 1,776,046 | 16.2 | 4.6% | 8.4% |
| Never-smokers at average risk | 2,179,173 | 2,544,605 | 22.5 | 5.2% | 9.5% |
| Never-smokers at 2 times average risk | 2,170,544 | 2,534,532 | 22.5 | 5.2% | 9.6% |
| Never-smokers at 5 times average risk | 2,144,601 | 2,504,249 | 22.2 | 5.3% | 9.6% |
| Never-smokers at 10 times average risk | 2,101,248 | 2,453,645 | 21.9 | 5.3% | 9.6% |
| Never-smokers at 15 times average risk | 2,057,745 | 2,402,865 | 21.5 | 5.3% | 9.6% |
| Never-smokers at 20 times average risk | 2,014,118 | 2,351,940 | 21.1 | 5.3% | 9.6% |
| Never-smokers at 35 times average risk | 1,882,721 | 2,198,562 | 19.9 | 5.3% | 9.6% |
Table notes: Abbreviations: United States Preventive Services Task Force (USPSTF)
Discussion
Suggestions to recommend lung cancer screening based on an individual's risk and the growing awareness of lung cancer in never-smokers have raised the question of whether never-smokers at high risk for lung cancer should be screened.2-4, 12, 14, 20, 23, 30, 33, 35, 41, 46 Our study is the first to provide indications whether never-smokers may benefit from lung cancer screening through quantifying the benefits and harms of screening never-smokers at different levels of risk.
Screening never-smokers at average risk or an RR of 2 compared to average risk has unfavorable trade-offs between benefits and harms, requiring 3,000-6,000 screens to prevent one death. However, the trade-off for never-smokers with a RR of 5 is more favorable than breast cancer screening: 1,216 screens per death averted compared to 1,558 (Model E in Mandelblatt et al), however, the number of screens per life-year gained is less favorable: 117 compared to 91.47 Never-smokers with a RR of 10 have more favorable trade-offs in deaths prevented and life-years gained per screen compared to breast cancer screening, but less favorable compared to ever-smokers for whom the USPSTF recommends screening.25, 47 However, never-smokers with a RR of 15-35 have similar to more favorable trade-offs between the benefits and harms compared to smokers for whom the USPSTF recommends screening.
Lung cancer screening for never-smokers may lead to a higher relative reduction in lung cancer mortality compared to screening ever-smokers. However, while the cohorts of never-smokers have a higher relative reduction in lung cancer mortality compared to the USPSTF eligible cohort, the increase in number of life-years gained is less than one would anticipate. For example, the number of lung cancer deaths averted for never-smokers with a RR of 15 was 23.62% higher compared to the USPSTF eligible cohort, while the number of life-years gained was only 8.25% higher (Table 2). This can be explained by the lower number of life-years gained per lung cancer death averted, which was 11.9 years for the USPSTF eligible cohort compared to 10.4 for the never-smoker cohorts. This may seem counterintuitive, as ever-smokers have a higher all-cause mortality compared to never-smokers, but can be explained through the differences in the average age of lung cancer diagnosis and average age of death between these groups.43, 45 Table S1 (in Supplemental Digital Content 1) shows the average age of lung cancer diagnosis (given that the cancer is diagnosed after age 45) and the average age of death (given that the person is alive at age 45) for the investigated cohorts. Table S1 (in Supplemental Digital Content 1) indicates that persons in the USPSTF eligible cohort die younger compared to never-smokers, due to the detrimental effects of smoking.43, 45 However, due to the carcinogenic effects of smoking, patients in the USPSTF eligible cohort developed lung cancer at a younger age compared to never-smokers. In addition, the high proportion of adenocarcinoma in never-smokers, which have a longer preclinical sojourn time compared to other histologies, may further contribute to the later age of diagnosis 26.
Our investigation has some limitations. We assume that the preclinical duration of lung cancer in never-smokers is similar to that of ever-smokers, while there are indications that lung cancer biology may differ in never-smokers.3 However, while the carcinogenesis process may differ in never-smokers, to our knowledge, there are no indications that differences in the preclinical duration of lung cancer exist between never- and ever- smokers.3
Another limitation is that the investigated levels of RR are assumed to be constant over a person's life. While the elevation in risk may be constant over a person's life for some risk factors, such as genetic susceptibility, this may not be the case for risk factors such as asbestos or radon exposure.3, 14, 16 However, the benefits and harms of screening never-smokers at specific levels of RR are more easily interpreted by assuming the RR is constant over a person's lifetime.
Finally, though our research indicates at what level of risk for developing lung cancer never-smokers could benefit from lung cancer screening, our findings are based on model-based extrapolations. Further research is needed to accurately identify never-smokers at high risk for developing lung cancer, which would allow us to further validate our findings. However, although a number of risk factors for developing lung cancer in never-smokers have been identified, the etiology of lung cancer in never-smokers is not well understood.3, 14-16, 46 While lung cancer risk models for never-smokers exist, the performance of the majority of these models is limited.17-19, 48
This is further demonstrated by comparing the risk of developing lung cancer for an average 67-year old (the age between the USPSTF recommended screening ages of 55-80) never-smoker (by gender) for different timeframes in MISCAN-Lung (Table S2 in Supplemental Digital Content 1) to those of the investigated risk models (Table S3 in Supplemental Digital Content 1). Table S3 (in Supplemental Digital Content 1) indicates that the investigated lung cancer risk models for never-smokers generally predict higher lung cancer risks compared to MISCAN-Lung. However, the age-group specific lung cancer mortality rates for never-smoking men and women estimated by MISCAN-Lung are comparable to those reported by Thun (Table S13 in their report). 41 This indicates that the majority of lung cancer risk models may overestimate the risk of lung cancer for never-smokers.
Finally, the proportion of never-smokers with RRs of 15 compared to never-smokers at average risk is uncertain as information on many risk factors (and the joint distribution thereof) is scarcely available at the population level. The application of the PLCOm2014 model to the PLCO dataset and the application of the LLP model for recruiting participants for the UK Lung Cancer Screening Trial (UKLS) may currently provide the best information on the expected levels of risk for never-smokers.20, 49
The PLCOm2014 model suggests that the maximum observed risk in 65,711 never-smokers in the PLCO was 1.47% over a 6-year period.20 A white never-smoker attaining the highest level of RR (6.98, disregarding age and race) would not reach this level of risk until age 73 (verified using the calculator provided by the authors, available at http://www.brocku.ca/lung-cancer-risk-calculator). The theoretical maximum possible 6-year risk of lung cancer for never smokers in the PLCOm2014 model is 3.5%, however, the necessary combination of risk factors to achieve this level of risk is expected to be rare.20
The LLP model was used to recruit participants for the UKLS trial, including never-smokers.49 Only 4 (0.04% of 10,697) never-smokers had a high LLP risk (a risk of 5% or higher over a 5-year period) and all were aged at least 73 years.49 Analyses using the model (replicated in R software) suggest that both men and women can only achieve this absolute level of risk at this age at the highest level of RR (13.69).
In conclusion, this study is the first to investigate the long term benefits and harms of lung cancer screening for never-smokers. Screening never-smokers at high levels of elevated risk for developing lung cancer (RRs of 15 or higher compared to average risk) is indicated to have similar or better trade-offs between benefits and harms as the population for which the USPSTF recommends screening. However, most lung cancer risk models for never-smokers consider RRs of lower than 15 for never-smokers at elevated risk compared to never-smokers at average risk. In addition, the majority of lung cancer risk models for never-smokers may overestimate the average risk of never-smokers. Applications of lung cancer risk models to populations of never-smokers suggest few never-smokers attain high levels of risk.20, 49 Thus, few never-smokers are expected to attain RR's of 15 compared to never-smokers at average risk. Therefore, for most never-smokers, lung cancer screening is not beneficial.
Supplementary Material
Acknowledgments
We would like to thank M.C. Tammemägi for providing useful comments with regards to the PLCOm2011 and PLCOm2014 models. We thank our colleagues from the CISNET Lung working group (in particular J. Jeon) and F. van Hees (dept. of Public Health, Erasmus MC) for providing useful comments.
Conflicts of Interest and Source of Funding: This publication was supported by Grant 5U01CA152956-04 from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
H.J. de Koning is the principal investigator of the Dutch–Belgian Lung Cancer Screening Trial (Nederlands-Leuvens Longkanker Screenings onderzoek; the NELSON trial). K. ten Haaf is a researcher affiliated with the NELSON trial.
References
- 1.Koo LC, Ho JHC. Worldwide Epidemiological Patterns of Lung Cancer in Nonsmokers. Int J Epidemiol. 1990;19:S14–S23. doi: 10.1093/ije/19.supplement_1.s14. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers - a different disease. Nature Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 4.Thun MJ, Henley SJ, Burns D, et al. Lung Cancer Death Rates in Lifelong Nonsmokers. J Natl Cancer Inst. 2006;98:691–699. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 5.Aberle DR, Adams AM, Berg CD, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyer VA. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013 doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 7.Wood DE, Eapen GA, Ettinger DS, et al. Lung Cancer Screening. Journal of the National Comprehensive Cancer Network. 2012;10:240–265. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 9.Detterbeck FC, Mazzone PJ, Naidich DP, et al. Screening for lung cancer: Diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. CHEST Journal. 2013;143:e78S–e92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996–1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer. 2007;58:329–341. doi: 10.1016/j.lungcan.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Sone S, Takashima S, Li F, et al. Mass screening for lung cancer with mobile spiral computed tomography scanner. The Lancet. 1998;351:1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 12.Oken M, Hocking WG, Kvale PA, et al. Screening by chest radiograph and lung cancer mortality: The prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA: The Journal of the American Medical Association. 2011;306:1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 13.Silvestri GA, Nietert PJ, Zoller J, et al. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62:126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy WJ, Meza R, Jeon J, et al. Chapter 6: Lung Cancer in Never Smokers: Epidemiology and Risk Prediction Models. Risk Anal. 2012;32:S69–S84. doi: 10.1111/j.1539-6924.2012.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couraud S, Zalcman G, Milleron B, et al. Lung cancer in never smokers – A review. Eur J Cancer. 2012;48:1299–1311. doi: 10.1016/j.ejca.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian J, Govindan R. Lung Cancer in Never Smokers: A Review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 17.Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung Cancer Risk Prediction: Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Models and Validation. J Natl Cancer Inst. 2011;103:1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitz MR, Hong WK, Amos CI, et al. A Risk Model for Prediction of Lung Cancer. J Natl Cancer Inst. 2007;99:715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 19.Raji OY, Duffy SW, Agbaje OF, et al. Predictive Accuracy of the Liverpool Lung Project Risk Model for Stratifying Patients for Computed Tomography Screening for Lung CancerA Case–Control and Cohort Validation Study. Ann Intern Med. 2012;157:242–250. doi: 10.7326/0003-4819-157-4-201208210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tammemägi MC, Church TR, Hocking WG, et al. Evaluation of the Lung Cancer Risks at Which to Screen Ever- and Never-Smokers: Screening Rules Applied to the PLCO and NLST Cohorts. PLoS Med. 2014;11:e1001764. doi: 10.1371/journal.pmed.1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Zein RA, Lopez MS, D'Amelio AM, et al. The Cytokinesis Blocked Micronucleus Assay as a Strong Predictor of Lung Cancer: Extension of a Lung cancer risk prediction model. Cancer Epidemiology Biomarkers & Prevention. 2014 doi: 10.1158/1055-9965.EPI-14-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of Low-Dose CT Screening According to the Risk of Lung-Cancer Death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tammemägi MC, Katki HA, Hocking WG, et al. Selection Criteria for Lung-Cancer Screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldwin DR, Duffy SW, Wald NJ, et al. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66:308–313. doi: 10.1136/thx.2010.152066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and Harms of Computed Tomography Lung Cancer Screening Strategies: A Comparative Modeling Study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiology Biomarkers & Prevention. 2014 doi: 10.1158/1055-9965.EPI-14-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meza R, ten Haaf K, Kong CY, et al. Comparative analysis of 5 lung cancer natural history and screening models that reproduce outcomes of the NLST and PLCO trials. Cancer. 2014;120:1713–1724. doi: 10.1002/cncr.28623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMahon PM, Meza R, Plevritis SK, et al. Comparing Benefits from Many Possible Computed Tomography Lung Cancer Screening Programs: Extrapolating from the National Lung Screening Trial Using Comparative Modeling. PLoS ONE. 2014;9:e99978. doi: 10.1371/journal.pone.0099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenfield SA, Wei EK, Stampfer MJ, et al. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17:198–204. doi: 10.1136/tc.2007.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and Racial Differences in the Smoking-Related Risk of Lung Cancer. N Engl J Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 32.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) The Lancet. 366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi T, Matsumura A, Fukai S, et al. Japanese Ethnicity Compared with Caucasian Ethnicity and Never-Smoking Status Are Independent Favorable Prognostic Factors for Overall Survival in Non-small Cell Lung Cancer: A Collaborative Epidemiologic Study of the National Hospital Organization Study Group for Lung Cancer (NHSGLC) in Japan and a Southern California Regional Cancer Registry Databases. J Thorac Oncol. 2010;5:1001–1010. doi: 10.1097/JTO.0b013e3181e2f607. 1010.1097/JTO.1000b1013e3181e1002f1607. [DOI] [PubMed] [Google Scholar]
- 34.Meguid RA, Hooker CM, Harris J, et al. Long-term survival outcomes by smoking status in surgical and nonsurgical patients with non-small cell lung cancer: Comparing never smokers and current smokers. CHEST Journal. 2010;138:500–509. doi: 10.1378/chest.08-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian J, Velcheti V, Gao F, et al. Presentation and Stage-Specific Outcomes of Lifelong Never-smokers with Non-small Cell Lung Cancer (NSCLC) J Thorac Oncol. 2007;2:827–830. doi: 10.1097/JTO.0b013e318145af79. 810.1097/JTO.1090b1013e318145af318179. [DOI] [PubMed] [Google Scholar]
- 36.Toh CK, Gao F, Lim WT, et al. Never-Smokers With Lung Cancer: Epidemiologic Evidence of a Distinct Disease Entity. J Clin Oncol. 2006;24:2245–2251. doi: 10.1200/JCO.2005.04.8033. [DOI] [PubMed] [Google Scholar]
- 37.Surveillance Epidemiology End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2012 Sub (1973-2010 varying) - Linked To County Attributes - Total U.S., 1969-2011 Counties, National Cancer Institute DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013 based on the November 2012 submission Accessed March, 29 2014
- 38.Heidenreich WF, Luebeck EG, Moolgavkar SH. Some Properties of the Hazard Function of the Two-Mutation Clonal Expansion Model. Risk Anal. 1997;17:391–399. doi: 10.1111/j.1539-6924.1997.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 39.Meza R, Hazelton W, Colditz G, et al. Analysis of lung cancer incidence in the nurses' health and the health professionals' follow-up studies using a multistage carcinogenesis model. Cancer Causes Control. 2008;19:317–328. doi: 10.1007/s10552-007-9094-5. [DOI] [PubMed] [Google Scholar]
- 40.Hazelton WD, Clements MS, Moolgavkar SH. Multistage Carcinogenesis and Lung Cancer Mortality in Three Cohorts. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1171–1181. doi: 10.1158/1055-9965.EPI-04-0756. [DOI] [PubMed] [Google Scholar]
- 41.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung Cancer Occurrence in Never-Smokers: An Analysis of 13 Cohorts and 22 Cancer Registry Studies. PLoS Med. 2008;5:e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeon J, Meza R, Krapcho M, et al. Chapter 5: Actual and Counterfactual Smoking Prevalence Rates in the U.S. Population via Microsimulation. Risk Anal. 2012;32:S51–S68. doi: 10.1111/j.1539-6924.2011.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenberg MA, Feuer EJ, Yu B, et al. Chapter 3: Cohort Life Tables by Smoking Status, Removing Lung Cancer as a Cause of Death. Risk Anal. 2012;32:S25–S38. doi: 10.1111/j.1539-6924.2011.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holford TR, Levy DT, McKay LA, et al. Patterns of Birth Cohort–Specific Smoking Histories, 1965–2009. Am J Prev Med. 2014;46:e31–e37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thun MJ, Carter BD, Feskanich D, et al. 50-Year Trends in Smoking-Related Mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakelee HA, Chang ET, Gomez SL, et al. Lung Cancer Incidence in Never Smokers. J Clin Oncol. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of Mammography Screening Under Different Screening Schedules: Model Estimates of Potential Benefits and Harms. Ann Intern Med. 2009;151:738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenner D, Hung R, Tsao MS, et al. Lung cancer risk in never-smokers: a population-based case-control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McRonald FE, Yadegarfar G, Baldwin DR, et al. The UK Lung Screen (UKLS): Demographic Profile of First 88,897 Approaches Provides Recommendations for Population Screening. Cancer Prevention Research. 2014;7:362–371. doi: 10.1158/1940-6207.CAPR-13-0206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.