Abstract
Background
Little data are available regarding the long-term mortality rate for patients receiving nocturnal home hemodialysis.
Study Design
Post-trial observational study.
Setting & Participants
Frequent Hemodialysis Network (FHN) Nocturnal Trial participants who consented to extended follow-up.
Intervention
The FHN Nocturnal Trial randomized 87 subjects to 6-times-per-week home nocturnal hemodialysis or to 3-times-per-week hemodialysis for one year. Patients were enrolled starting in March 2006 and follow-up was completed by May 2010. After the one year trial concluded, FHN Nocturnal subjects were free to modify their hemodialysis prescription.
Outcomes & Measurements
We obtained dates of death and kidney transplantation through July 2011 using linkage to the US Renal Data System and queries of study centers. We used log-rank tests and Cox regression to relate mortality to the initial randomization assignment.
Results
Median follow-up for the trial and post trial observational period was 3.7 years. In the nocturnal arm, there were 2 deaths during the 12 month trial period and an additional 12 deaths during the extended follow-up. In the conventional arm, the numbers of deaths were 1 and 4, respectively. In the nocturnal dialysis group, the overall mortality HR was 3.88 (95% CI, 1.27-11.79; p = 0.01). Using as-treated analysis with a 12 month running treatment average, the HR for mortality was 3.06 (95% CI, 1.11-8.43; p = 0.03). Six month running treatment data analysis showed an HR of 1.12 (95% CI, 0.44-3.22; p = 0.7).
Limitations
These results should be interpreted cautiously due to a surprisingly low (0.03 per patient-year) mortality rate for subjects randomized to conventional home hemodialysis, low statistical power for the mortality comparison due to the small sample size, and the high rate of hemodialysis prescription changes.
Conclusions
Patients randomized to nocturnal hemodialysis had a higher mortality rate than those randomized to conventional dialysis. The implications of this result require further investigation.
Index words: Nocturnal hemodialysis, frequent hemodialysis, extended hemodialysis, frequent-long dialysis schedule, clinical trial, mortality, hemodialysis prescription, dialysis dose, dialysis adequacy, dialysis regimen, end-stage renal disease (ESRD), renal replacement therapy, Frequent Hemodialysis Network (FHN) Nocturnal Trial
The Frequent Hemodialysis Network (FHN) Nocturnal Trial was designed to evaluate the safety and efficacy of 6-times-weekly nocturnal hemodialysis versus conventional home (3-times-weekly) hemodialysis on intermediate outcomes.1 Details about the trial, including enrollment and randomization challenges,2 baseline characteristics of enrolled participants 3 and the primary results, 4 have been published; as have secondary results concerning mineral metabolism,5 cardiac structure,6 physical performance,7 nutrition and body composition,8 anemia,9 mental health,10 vascular access complications,11 and residual kidney function.12.
Although the FHN Nocturnal Trial was not powered for comparisons of mortality between treatment groups, mortality was specified as a secondary outcome for descriptive purposes.
We now report on the mortality analysis during extended follow-up of the FHN Nocturnal Trial.
Methods
Study Design of Randomized Trial
The FHN Nocturnal Trial (ClinicalTrials.gov study number NCT 00271999) was a multicenter randomized clinical trial in which 87 patients were randomized in a 1:1 ratio to either 6-times-weekly nocturnal hemodialysis (nocturnal hemodialysis) or 3-times-weekly hemodialysis (conventional hemodialysis), with self-care provided in both arms of the study1. Patients in the nocturnal hemodialysis group could follow any dialysis prescription that would deliver a standard Kt/Vurea of at least 4.0, and a treatment time of ≥6 hours/session. Patients in the conventional hemodialysis group remained on their usual 3-times-weekly prescription subject to a prescribed equilibrated Kt/Vurea >1.1, a standard Kt/Vurea of >2.0 and a treatment time between 2.5 and 5 hours per session.
Patients were enrolled starting in March 2006 and follow-up was completed by May 2010. Two intermediate co-primary composite endpoints were defined based on the change in left ventricular mass in conjunction with mortality and the change in the physical health composite score of the SF-36 in conjunction with mortality.1;13 Neither outcome was significantly different between the two randomized arms; however the analysis was underpowered due to small sample size.4
Evaluation of Mortality During Extended Follow-up
Following completion of the randomized trial, the treatment interventions were discontinued and patients were able to modify their dialysis regimens according to their preferences as agreed to by their treating physicians. Institutional review board approval was obtained at each site to gather longer-term information on treatment and mortality status in order to evaluate persisting effects of the original trial intervention. Additional prospective and retrospective data could also be collected from consenting subjects. This extended follow-up data was collected from April 2010 through April 2011 when surviving subjects had reached their 13th to 57th month of follow-up. When analyzing the data through the extended follow-up visit, we noted an imbalance of deaths between the treatment groups. Because of the incomplete nature of these data, this imbalance led us to seek complete follow-up data for mortality and kidney transplantation throughout an even longer extended follow-up period. We also developed a data analysis plan.
For 52 patients who had consented for linkage with the US Renal Data System (USRDS), extended follow-up for mortality and transplantation was obtained through July 31, 2011. We obtained permission to seek mortality and transplantation status for the remaining 31 patients from the institutional review boards of the individual clinical sites, and ascertained mortality and transplantation status for these patients during the summer of 2012. Cause of death was classified by at least two members of the Outcome Committee and by review of clinical and/or USRDS data using a standard classification and methodology that was used in prior hemodialysis studies and with blinding of the treatment status.14 We also requested the number of dialysis treatments and the weekly treatment time for one week during each calendar month following the termination of the clinical trial through the end of each patient's extended follow-up period. For the interaction analysis, we defined a center as “inexperienced” if they had treated five or fewer patients with home nocturnal hemodialysis prior to the initiation of the FHN Nocturnal Trial.
Data Analysis
The total follow-up period was divided into the 12 month period of the randomized trial, the 12 month period following completion of the randomized trial, and the period more than 1 year after the trial which extended until the patient died or reached the administrative censoring data of the extended follow-up period.
The primary analyses of mortality were performed using a log-rank test to compare mortality by the original randomized treatment groups over the full extended follow-up period. In accordance with an intent-to-treat strategy, participants who had undergone kidney transplantation were not censored in the primary analysis. The hazard ratio (HR) representing the treatment effect was calculated using Cox-proportional hazards regression. In a secondary analysis, the log-rank test and Cox regression were repeated after censoring individuals who had undergone kidney transplantation.
Due to random error, results reaching statistical significance in trials with low power tend to exaggerate the treatment effect and incur an elevated risk of false positive conclusions. 15;16 We therefore performed Bayesian analyses to better quantify the uncertainty of the trial results under two prior distributions in which extreme relative hazards are unlikely. Posterior distributions of the relative hazards were simulated using Monte Carlo Markov chains to describe the implications of the trial results under each prior assumption. 17Posterior probabilities of a relative hazard <0.80 were computed to represent the probabilities of a clinically significant benefit. We considered a “conservative” prior distribution to represent a perspective which assumes equal probabilities of benefit or harm of the intervention, and attributes relatively small (0.05) probabilities both to substantial benefit (a ≥33.3% reduction in hazard for treatment vs. control) and to substantial harm (a ≥33.3% reduction in hazard for control vs. treatment). The small probabilities assigned to substantial treatment effects can be viewed as reflecting a view that even if the intervention might have produced a substantial effect during the one year trial period, this effect would likely have attenuated due to the high proportion of subjects who changed modalities after the trial phase. We also considered a mildly “enthusiastic” prior distribution with a median relative hazard of 0.80 and a 0.74 probability of a relative hazard <1. We also examined the effect of hypothetical re-classifications of mortality status on the log rank test comparing mortality between the randomized groups in order to convey the sensitivity of our results to small numbers of outcomes.18
In further secondary analyses, death rates were calculated by randomized group within each the following pre-specified subgroups, along with p-values for interaction tests under Cox regressions: age group (≤50 and >50 years), sex (male and female), race (black and non-black), nationality of center (United States and Canada), experienced vs. inexperienced clinical center, left ventricular mass (≤132 and >132 g), urine volume (≤500 and >500 ml/day or ≤100 and >100 ml/d), and ESRD vintage (<4 and ≥4 years).
The distribution of the treatment regimens was summarized by first computing separately for each patient the average number of treatments per week and the average weekly treatment time for each patient during the trial, the first year after the trial, and more than one year after the trial, and then summarizing these averaged values for the three periods by randomized group. We also used time-dependent Cox proportional hazards regression to perform as-treated analyses to relate mortality at each follow-up time to indicator variables which defined whether the patients' average treatment regimen over the previous 12 months approximated a frequent nocturnal hemodialysis regimen, pre-defined by a running average treatment frequency >4.5 treatments per week in conjunction with a running average treatment time >27 h/wk. The time-dependent Cox regression was repeated using a running average of 6 months for treatment frequency and weekly treatment time, and with average weekly treatment frequency and weekly treatment time treated as continuous variables. Due to the limited number of deaths and the absence of covariate information after the randomized trial phase, the as-treated analyses are presented without covariate adjustment. In contrast to the intent-to-treat analyses, follow-up time was censored after transplantation in the as-treated analyses.
Results
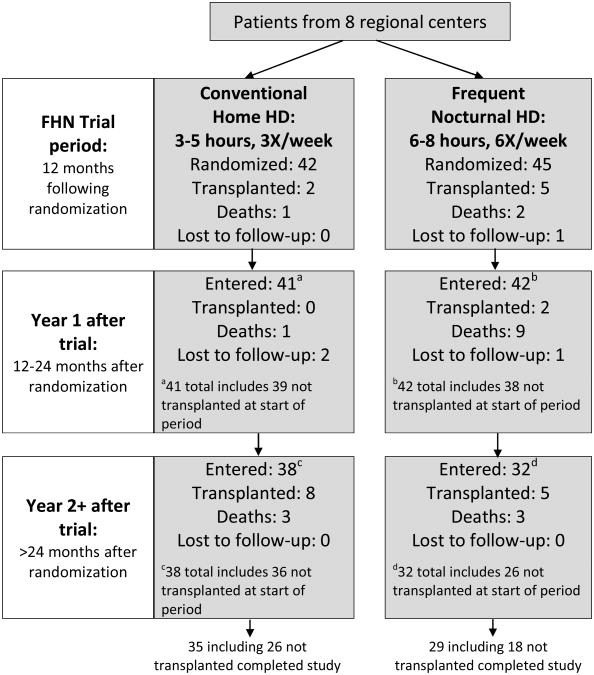
Eighty-seven subjects were recruited from eight regional centers in the United States and Canada, and were randomized in a 1:1 ratio to either 6-times-weekly nocturnal treatments at home (nocturnal hemodialysis group, n=45) or conventional 3-times-weekly treatments, mostly performed at home (conventional hemodialysis group; n=42; Figure 1). Eighty-three of these subjects remained in follow-up at the end of the 12 month randomized trial phase, including 6 who had received a kidney transplant. Characteristics of subjects at baseline and at the conclusion of the randomized trial are shown in Table 1.
Figure 1.
Patient status over the extended follow-up period based upon the original randomized group assignment. The single loss to follow-up during the trial phase in the frequent nocturnal group occurred after transplantation. One of the conventional home hemodialysis patients who was transplanted during the trial subsequently died more than 2 years after the trial was completed.
Table 1. Selected baseline values prior to randomization, and at the end of the trial at 12 months.
| Baseline | End of trial at 12 mo | |||||||
|---|---|---|---|---|---|---|---|---|
| Nocturnal HD | Conventional HD | Nocturnal HD | Conventional HD | |||||
| No. Analyzed | Mean±SD or No. (%) | No. Analyzed | Mean±SD or No. (%) | No. Analyzed | Mean±SD or No. (%) | No. Analyzed | Mean±SD or No. (%) | |
| Left ventricular mass (g/m2) | 45 | 138.5 ± 47.9 | 42 | 134.9 ± 41.8 | 35 | 133.3 ± 56.5 | 39 | 132.8 ± 41.7 |
| Physical health composite score | 45 | 37.5 ± 9.6 | 41 | 38.2 ± 8.3 | 37 | 40.4 ± 12.4 | 39 | 40.1 ± 9.7 |
| Age (y) | 45 | 51.7 ± 14.4 | 42 | 54.0 ± 12.9 | 38 | 52.0 ± 14.0 | 39 | 54.9 ± 12.2 |
| Diabetes | 45 | 19 (42) | 42 | 18 (43) | 38 | 18 (47) | 39 | 16 (41) |
| Female sex | 45 | 16 (36) | 42 | 14 (33) | 38 | 13 (34) | 39 | 14 (36) |
| Black race | 45 | 12 (27) | 42 | 11 (26) | 38 | 10 (26) | 39 | 11 (28) |
| ESRD vintage (y)* | 45 | 1.3 [0.1-12.5] | 42 | 0.5 [0.1- 6.0] | 38 | 1.0 [0.1-, 12.6] | 39 | 0.5 [0.1- 6.1] |
| Cause of ESRD | 43 | 41 | 36 | 38 | ||||
| Diabetic nephropathy | 15 (35) | 15 (37) | 14 (39) | 13 (34) | ||||
| Glomerulonephritis | 14 (33) | 17 (42) | 9 (25) | 16 (42) | ||||
| Hypertensive nephrosclerosis | 4 (9) | 3 (7) | 3 (8) | 3 (8) | ||||
| Polycystic kidney disease | 3 (7) | 0 (0) | 3 (8) | 0 (0) | ||||
| Comorbid medical conditions | 45 | 42 | 38 | 39 | ||||
| Hypertension | 41 (91) | 39 (93) | 35 (92) | 36 (92) | ||||
| Myocardial infarction | 5 (11) | 4 (10) | 4 (11) | 4 (10) | ||||
| Heart failure | 5 (11) | 7 (17) | 3 (8) | 6 (15) | ||||
| Atrial fibrillation | 6 (13) | 0 (0) | 5 (13) | 0 (0) | ||||
| Peripheral vascular disease | 8 (178) | 7 (17) | 7 (18) | 6 (15) | ||||
| Abdominal aortic aneurysm repair or bypass grafting | 2 (4) | 5 (12) | 1 (3) | 4 (10) | ||||
| Stroke/CVA | 1 (2) | 1 (2) | 1 (3) | 0 (0) | ||||
| Dementia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Tumor without metastases | 1 (2) | 0 (0) | 1 (3) | 0 (0) | ||||
| Diabetes and diabetic complications | 19 (42) | 18 (43) | 18 (47) | 16 (41) | ||||
| Hemiplegia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Chronic pulmonary disease | 2 (4) | 2 (5) | 2 (5.) | 2 (5) | ||||
| Weekly predialysis DBP (mm Hg) | 45 | 79.6 ± 10.6 | 42 | 83.1 ± 13.5 | 37 | 75.9 ± 14.2 | 38 | 82.9 ± 12.9 |
| Weekly postdialysis weight (kg) | 45 | 87.6 ± 27.0 | 42 | 83.3 ± 23.8 | 37 | 90.0 ± 28.4 | 38 | 84.4 ± 25.9 |
| Predialysis serum albumin (g/dl) | 45 | 3.90 ± 0.48 | 42 | 3.92 ± 0.51 | 36 | 4.06 ± 0.52 | 38 | 4.13 ± 0.39 |
| Predialysis phosphorus (mg/dl) | 45 | 5.82 ± 1.59 | 42 | 5.77 ± 1.65 | 36 | 4.73 ± 1.33 | 38 | 5.96 ± 1.97 |
| Predialysis hemoglobin (mg/dl) | 45 | 11.63 ± 1.12 | 42 | 11.93 ±1.09 | 37 | 11.73 ± 1.17 | 39 | 11.94 ± 1.10 |
| Urine volume | ||||||||
| < 50 ml/d | 45 | 13 (29) | 42 | 11 (26) | 34 | 26 (76) | 33 | 19 (58) |
| 50-500 ml/d | 14 (31) | 8 (19) | 4 (12) | 6 (18) | ||||
| > 500 ml/d | 18 (40) | 23 (55) | 4 (12) | 8 (24) | ||||
| Access type | 45 | 42 | 38 | 39 | ||||
| Fistula | 22 (49) | 17 (41) | 21 (55) | 20 (51) | ||||
| Graft | 3 (7) | 4 (10) | 3 (8) | 4 (10) | ||||
| Catheter | 20 (44) | 21 (50) | 14 (37) | 15 (39) | ||||
| Household income | 45 | 42 | ||||||
| <$20,000 | 11 (24) | 7 (17) | 38 | 10 (26) | 39 | 7 (18) | ||
| $20,000-$29,999 | 9 (20) | 8 (19) | 7 (18) | 8 (21) | ||||
| $30,000-$39,999 | 7 (16) | 6 (14) | 7 (18) | 6 (15) | ||||
| $40,000-$49,999 | 5 (11) | 6 (14) | 4 (11) | 4 (10) | ||||
| >$50,000 | 8 (18) | 11 (26) | 6 (16) | 10 (26) | ||||
| Unknown | 5 (11) | 4 (10) | 4 (11) | 4 (10) | ||||
| Education | 45 | 42 | 38 | 39 | ||||
| No diploma | 8 (18) | 5 (12) | 7 (18) | 5 (13) | ||||
| High school graduate | 12 (27) | 9 (21) | 10 (26) | 8 (21) | ||||
| Vocational/technical/business/some college, no degree | 13 (29) | 16 (38) | 11 (29) | 15 (39) | ||||
| Associate degree or beyond | 11 (24) | 12 (29) | 9 (24) | 11 (28) | ||||
| Unknown | 1 (2) | 0 (0) | 1 (3) | 0 (0) | ||||
| US citizens health insurance | 29 | 25 | 24 | 23 | ||||
| None | 1 (3) | 0 (0) | 1 (4) | 0 (0) | ||||
| Health insurance | 28 (97) | 24 (96) | 23 (96) | 22 (96) | ||||
| Unknown | 0 (0) | 1 (4) | 0 (0) | 1 (4) | ||||
| US citizens: Medicare | 29 | 25 (86) | 25 | 22 (88) | 24 | 22 (92) | 23 | 20 (87) |
| US citizens: Medicaid or State Medical Assistance | 29 | 9 (31) | 25 | 4 (16) | 24 | 9 (38) | 23 | 4 (17) |
| Canadian health care benefits | 16 | 16 (100) | 17 | 17 (100) | 14 | 14 (100) | 16 | 16 (100) |
Note: End of trial measurements represent 77 patients who completed the trial and who did not undergo transplantation. There are additional missing data for individual parameters, including one participant in whom race was listed as “unknown”.
CVA, cerebrovascular accident; DBP, diastolic blood pressure; ESRD, end-stage renal disease; HD, hemodialysis; SD, standard deviation
Values are given as median [interquartile range].
After the initial randomized trial period of 12 months, patients were able to modify their dialysis regimens according to their preferences as agreed to by their treating physicians. After the randomized trial phase, mortality and kidney transplantation status were obtained retrospectively, with a median total follow-up time over both the trial and post-trial phases of 3.7 (10th-90th percentile, 1.3-5.1) years. At the administrative close of the extended study, a total of 19 subjects had died, with 14 deaths in the nocturnal group (0.099 deaths per patient-year) and 5 deaths in the conventional group (0.032 deaths per patient-year, Table 2). Nine of the frequent nocturnal deaths occurred during the first year after the randomized trial ended. Twenty-one additional subjects had undergone transplantation, 3 subjects without transplants were lost to follow-up, and 44 subjects were confirmed alive without transplants.
Table 2. Mortality frequencies and rates by hemodialysis group.
| Nocturnal | Conventional | ||
|---|---|---|---|
| Full Extended Follow-up* | No. of patients | 45 | 42 |
| No. of deaths | 14 | 5 | |
| Total Patient-Years | 140.9 | 154.5 | |
| Death Rate/Patient-Year | 0.099 | 0.032 | |
| Trial Only | No. of patients | 45 | 42 |
| No. of deaths | 2 | 1 | |
| Total Patient-Years | 46.7 | 44.2 | |
| Death Rate/Patient-Year | 0.043 | 0.023 | |
| First Year After Trial | No. of patients | 42 | 41 |
| No. of deaths | 9 | 1 | |
| Total Patient-Years | 36.5 | 39.1 | |
| Death Rate/Patient-Year | 0.247 | 0.026 | |
| >1 Year After Trial | No. of patients | 32 | 38 |
| No. of deaths | 3 | 3 | |
| Total Patient-Years | 57.6 | 71.2 | |
| Death Rate/Patient-Year | 0.052 | 0.042 |
Including the trial and posttrial periods
In the primary intent-to-treat analysis that included the full follow-up period, the relative HR for mortality for patients randomized to the nocturnal group was 3.88 (95% confidence interval [CI], 1.27-11.79, p=0.01; Kaplan Meier survival plots in Figure 2). None of the 14 deaths in the nocturnal group and 1 of 5 deaths in the conventional group occurred after transplantation. Hence, censoring for transplantation increased the relative HR for mortality for those assigned to the nocturnal group to 5.98 (95% CI, 1.71-20.92; p=0.002). The mortality rate differential between randomized arms was unaffected by the pre-specified subgroups of age, sex, race, US vs Canadian center, experienced vs. inexperienced center, ESRD vintage, baseline left ventricular mass, or baseline urine volume levels (Table S1, provided as online supplementary material). We did note that the higher mortality in the nocturnal group persisted among patients with ≤500 ml/d of urine volume at baseline (11 deaths in 27 subjects vs. 1 death in 19 subjects). The cause of death in the two treatment groups included 9 deaths related to cardiac causes, 2 to infection, and 1 to the dialysis procedure itself (Table 3). Three deaths, all in the conventional group, were due to cancer.
Figure 2.
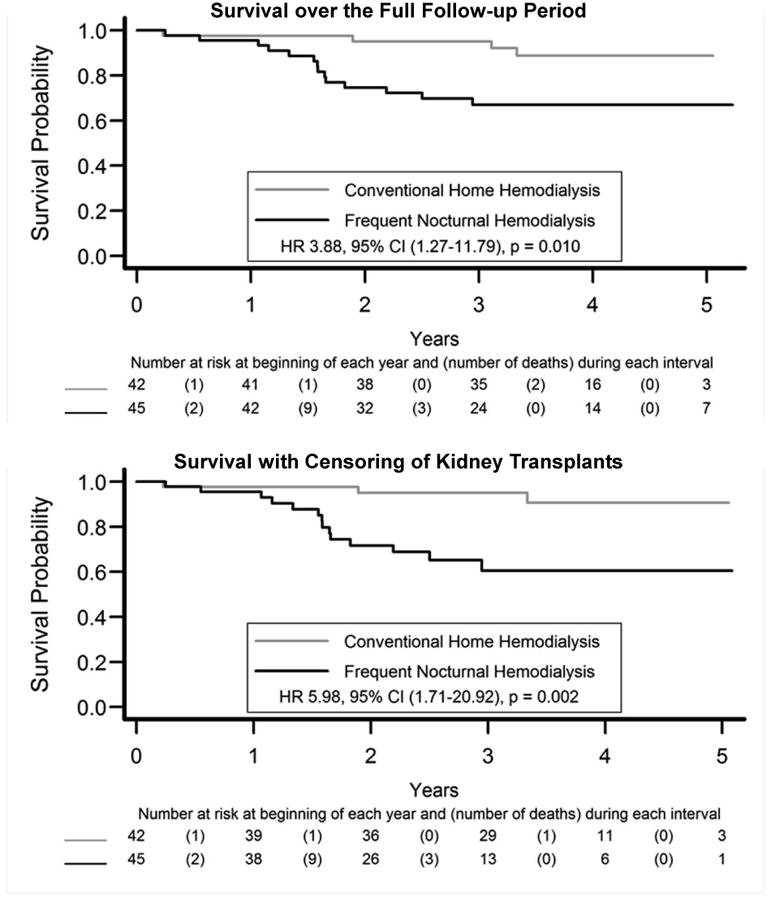
Survival during the randomized trial and the post-trial period. Note that one subject in the conventional arm who died 5.8 years after randomization is not shown.
Table 3. Cause of death by hemodialysis group.
| Category | Total | Nocturnal | Conventional |
|---|---|---|---|
| Atherosclerosis / Ischemic | 2 | 1 | 1 |
| CHF / cardiomyopathy | 1 | 1 | 0 |
| Arrhythmia | 3 | 2 | 1 |
| Other cardiac | 3 | 3 | 0 |
| Non-access infection | 2 | 2 | 0 |
| Other dialysis | 1 | 1 | 0 |
| GI bleed | 1 | 1 | 0 |
| Cancer | 3 | 0 | 3 |
| Other | 2 | 2 | 0 |
| Unknown | 1 | 1 | 0 |
| TOTAL | 19 | 14 | 5 |
| All cardiac deaths | 9 | 7 | 2 |
| All infectious deaths | 2 | 2 | 0 |
| All deaths excluding cancer/accidental deaths | 16 | 14 | 2 |
Note: Other cardiac deaths include one death from CHF with volume overload, one sudden death due to an arrhythmia and one sudden death thought to be secondary to a cardiac arrest. Infection deaths include one death from endocarditis and one death from a perirenal abscess. Other deaths include one death for dementia and failure to thrive and one death due to subdural hematoma.
CHF, congestive heart failure; GI, gastrointestinal
In the nocturnal group, 5 post-trial deaths were recorded in 22 subjects without vascular access events during the trial phase and 7 were recorded in 21 subjects with access events. In the conventional group, 4 post-trial deaths occurred among 26 subjects with no access events compared to no deaths among 15 subjects with access events during the randomized trial phase. None of the deaths were clearly related to a vascular access event.
For the purposes of the as-treated analyses, a frequent and long schedule was defined as the combination of >4.5 hemodialysis sessions per week and a weekly dialysis time of >27 hours. The proportions of subjects whose average treatment frequency and weekly treatment time met both of these criteria for frequent long hemodialysis were 61% vs 0% in the nocturnal and conventional groups, respectively, during the trial period, and were 51% vs. 27% during the first year after the trial, and 52% vs. 33% subsequently (Table S2 and Figure S1). The cumulative exposure of patients to the ″frequent, long-weekly time (frequent-long) schedule″ based on running averages of the weekly treatment frequencies and treatment times over the previous 12 or 6 months of follow-up is depicted in Figure 3. Patients randomized to the nocturnal and conventional groups, respectively, were exposed to frequent nocturnal dialysis for 62% vs. 13% of all recorded patient-months of follow-up based on 12-month running averages, and for 60% vs. 18% of all patient-months based on 6-month running averages.
Figure 3.
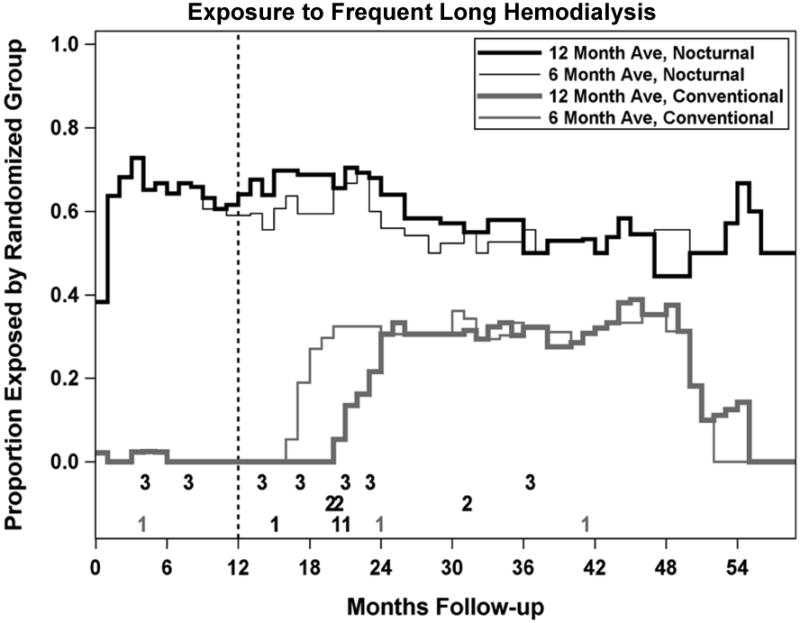
The graph displays the percent of treatments greater than 4.5 sessions/week and more than 27 hours/week over the preceding 6 or 12 months in subjects randomized to either conventional or more frequent-long hemodialysis.
Deaths prior to transplant are indicated by the numbers at the bottom of the plot; 1 indicates deaths classified as not occurring on frequent-long by both 6 and 12 month moving averages; 2 indicates deaths classified as occurring on frequent-long by the 12-month moving average only; 3 indicates deaths classified as occurring on frequent-long by both 6 and 12-month moving averages.
Numbers in black refer to deaths in subjects randomized to the nocturnal arm and numbers in gray refer to deaths in subjects randomized to the conventional arm. No deaths were classified as occurring on frequent-long by the 6-month but not the 12-month moving average.
Of the 18 deaths which occurred prior to kidney transplantation, two are not shown: one death that occurred during month 70 for a subject not following a frequent-long schedule and an additional death for a patient with no follow-up visits. Both these patients had been randomized to the conventional hemodialysis group.
The results of as-treated analyses relating mortality to a patient's exposure to frequent-long hemodialysis over the previous 12 or 6 months is shown in Table 4. In the nocturnal group, 10 of 13 deaths occurred in subjects who had been following a frequent-long schedule. Using the 12-month running average, all four deaths in the conventional group occurred in subjects not following a frequent-long prescription. No deaths occurred in conventional group patients who decided, after the trial ended, to move to a frequent-long schedule. The as-treated mortality HR associated with a frequent-long schedule was 3.06 (95% CI, 1.11-8.43; p=0.03). In contrast, when the 6-month running average was used, the as-treated HR associated with a frequent-long schedule was only 1.19 (95% CI, 0.44-3.21; p=0.7). The attenuation of the as-treated HR reflected the reclassification of 3 deaths from the frequent long category using the 12-month running average to the non-frequent long category using the six-month moving average (Figure 3). Mortality was not significantly associated with number of treatments per week or weekly treatment time when averaged over either the prior 6 months or the prior 12 months when these measures were analyzed as continuous variables (Table S3).
Table 4. As-treated analysis relating mortality to averaged prior exposures to frequent long dialysis, with and without stratification by randomized treatment group.
| Period | Patients Exposed To Frequent Long Dialysis? | Randomized Treatment Groups | Using 6-mo Averages for Treatment Exposure | Using 12-mo Averages for Treatment Exposure | ||||
|---|---|---|---|---|---|---|---|---|
| Patient-Y at Risk | No. of Deaths | Death Rate/Patient-Y | Patient-Y at Risk | No. of Deaths | Death Rate/Patient-Y | |||
| Trial | No | All Patients | 57.52 | 2 | 0.035 | 57.44 | 2 | 0.035 |
| N | 16.26 | 1 | 0.062 | 16.17 | 1 | 0.062 | ||
| C | 41.26 | 1 | 0.024 | 41.26 | 1 | 0.024 | ||
| Yes | All Patients | 25.57 | 1 | 0.039 | 25.66 | 1 | 0.039 | |
| N | 25.49 | 1 | 0.039 | 25.57 | 1 | 0.039 | ||
| C | 0.08 | 0 | 0.000 | 0.08 | 0 | 0.000 | ||
| First Year After Trial | No | All Patients | 37.82 | 6 | 0.159 | 40.53 | 4 | 0.099 |
| N | 10.93 | 5 | 0.457 | 9.07 | 3 | 0.331 | ||
| C | 26.89 | 1 | 0.037 | 31.47 | 1 | 0.032 | ||
| Yes | All Patients | 24.83 | 4 | 0.161 | 22.11 | 6 | 0.271 | |
| N | 18.58 | 4 | 0.215 | 20.45 | 6 | 0.293 | ||
| C | 6.25 | 0 | 0.000 | 1.67 | 0 | 0.000 | ||
| >1 Year After Trial | No | All Patients | 75.99 | 3 | 0.039 | 74.25 | 2 | 0.027 |
| N | 30.32 | 1 | 0.033 | 19.14 | 0 | 0.000 | ||
| C | 55.68 | 2 | 0.036 | 55.11 | 2 | 0.036 | ||
| Yes | All Patients | 40.16 | 1 | 0.025 | 41.91 | 2 | 0.048 | |
| N | 21.72 | 1 | 0.046 | 22.90 | 2 | 0.087 | ||
| C | 18.44 | 0 | 0.000 | 19.00 | 0 | 0.000 | ||
Note: Exposure to frequent long dialysis defined as at least 4.5 treatments per week in conjunction with a weekly treatment time of at least 27 hours with both the number of treatments and weekly treatment times averaged over the preceding 6 months, and over the preceding 12 months. (Both averages excluded pre-randomization data.) For the 6-month exposure averages, the hazard ratio relating mortality to the time-dependent indicator for frequent long nocturnal dialysis was 1.19 (95% confidence interval, 0.44-3.22; p = 0.7). For the 12-month exposure averages, the same hazard ratio was 3.06 (95% confidence interval, 1.11-8.43; p = 0.03). Note that these data exclude 2 of the 19 deaths, including 1 death in conventional group that occurred after kidney transplantation, and 1 death in nocturnal group for a subject with no follow-up visits.
C, conventional; N, nocturnal
Given the small sample size, we examined the numbers of patients for whom hypothetical changes in their vital status would have increased the p-value for the log-rank test comparing mortality between the randomized groups to greater than 0.05. Switching the vital status of the first 4 of the 14 deaths in the nocturnal group to “survivor” with follow-up to July 31, 2011, would increase the p-value of the mortality comparison from 0.01 to 0.06. Similarly, changing the vital status of the 2 surviving patients in the conventional group with the longest follow-up to death on the first day of follow-up would also have increased the p-value from 0.01 to 0.06.
A conceptual background to the Bayesian analyses may be found in Item S1. The posterior probabilities of a clinically significant benefit with a relative hazard below 0.80 were 0.01 and 0.04 under the “conservative” and “enthusiastic” prior perspectives, respectively, and the 95% Bayesian CIs were 0.84-1.97 and 0.76-2.32, respectively (Figures 4A and 4B).
Figure 4.
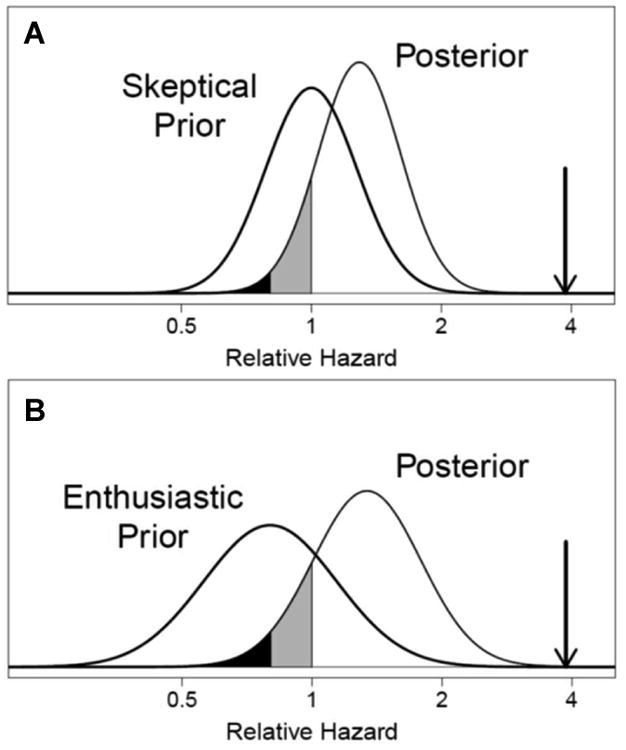
Posterior distributions of the relative hazard comparing mortality during extended follow-up between the randomized frequent and conventional hemodialysis groups under prior distributions representing “conservative” (Panel A) and “enthusiastic” (Panel B) perspectives on the probability of a treatment benefit. The posterior distributions characterize the implications of the observed relative hazard of 3.88 (downward pointing arrow) for individuals with the perspectives indicated by the conservative and enthusiastic priors before seeing the results. The posterior probabilities of harm (unshaded), a small benefit with relative hazard between 0.8 and 1 (grey shading), and a substantial benefit with relative hazard ≤ 0.8 (black shading) are 0.88, 0.11, and 0.01, respectively, under the “conservative” prior, and are 0.85, 0.11, and 0.04, respectively, under the “enthusiastic” prior.
Discussion
The finding that patients randomized to the frequent nocturnal hemodialysis group had higher mortality compared to the conventional hemodialysis group was unexpected and occurred during extended follow-up in a trial that was not powered to determine mortality differences; therefore, it is important that this result be interpreted cautiously. This result was observed consistently for all-cause mortality with and without censoring for transplantation, deaths due to cardiac or infectious causes, and all deaths excluding cancer.
Four considerations, however, point to a risk of a false positive finding. First, the FHN Nocturnal Trial was not powered to detect differences in mortality. More than 1000 patients would be required to detect a 25% reduction in mortality with 4 years of follow-up, assuming a 12.5% annual mortality rate in the control group. It has been well documented that positive findings from underpowered comparisons have an inflated false positive rate. Second, only 0.032 deaths per patient-year were observed in the conventional hemodialysis group. This unexpectedly low death rate in the conventional group may have led to an exaggerated relative hazard for the nocturnal hemodialysis group. Third, the possibility that frequent nocturnal dialysis increases mortality has not been suggested by observational studies 19-23. Fourth, the high rate of modality switches after the completion of the trial limits the plausibility of a large effect of the original randomized assignment on mortality, either positive or negative, over the extended follow-up period.
We attempted to quantify the implications of these uncertainties by simulating the relative hazards of mortality under two prior perspectives in which extremely large treatment effects are unlikely: a “conservative” prior perspective in which the treatment is presumed equally likely to increase and decrease mortality, and an “enthusiastic” prior perspective in which the median relative hazard was 0.80. The posterior estimates of the relative HR for mortality were substantially attenuated towards the null hypothesis of no treatment effect and the associated 95% Bayesian CIs include a relative hazard of 1 (Figure 4A and 4B). The probability of a clinically significant treatment benefit with a relative hazard <0.80, however, is less than 0.04 under both prior perspectives, suggesting that there was no survival advantage to nocturnal dialysis.
Previously reported secondary analyses of the FHN Nocturnal Trial did identify two areas of increased risk for the frequent-long schedule, vascular access events 11 and loss of residual kidney function12. However, apart from one nocturnal group patient who died due to unrecognized disconnection of a central venous catheter, we found no evidence that the higher mortality among subjects randomized to the nocturnal group was associated with either vascular access events or loss of residual kidney function.
As-treated analyses relating mortality to the treatment modality (frequent-long vs. not-frequent-or-long) defined by 6- or 12-month running averages were equivocal; mortality risk was directly associated with average exposure to frequent-long treatments over the prior 12 months, but not over the prior 6-month period that reflected the participant's more recent treatment regimen. Furthermore, mortality was not significantly related to continuous measures of treatment frequency or weekly treatment time, irrespective of whether these measures were averaged over 6 or 12 months. It is important to note, however, that as-treated analyses are subject to bias since the patient's treatment selection may reflect their mortality risk.
In sum, our findings should not be interpreted as demonstrating an adverse effect of frequent nocturnal dialysis on mortality, but they do appear to indicate that it is unlikely that frequent nocturnal dialysis substantially reduces mortality for the patient population that was represented in the trial. The low rate of 0.03 deaths per patient-year in the conventional hemodialysis group raises the possibility that the participants entering the trial represented an unusually healthy group of dialysis patients. If so, our results allow for the possibility that nocturnal dialysis could improve survival among dialysis patients with multiple medical comorbidities. It might also be conjectured that the beneficial effects of long nocturnal therapy were attenuated in the FHN Nocturnal Trial by inclusion of subjects with elevated residual kidney function and/or residual urine output. Our subgroup analyses do not support this conjecture, as similar or greater relative mortality hazards were observed for the nocturnal group compared to the conventional group for patients with residual urine volumes ≤100 ml/d or ≤500 ml/d as in the full study cohort. Nonetheless, the small sample size precludes a definitive answer to this question.
How do the survival results from the FHN Trial compare with reported randomized and observational data? In the Alberta randomized clinical trial of nocturnal hemodialysis, in which 52 patients were enrolled, there were 5 deaths in the nocturnal arm and 4 deaths in the conventional arm during the extended follow-up period.24 In the London Nocturnal controlled clinical trial, there were 3 deaths among 12 patients in the nocturnal hemodialysis arm and 3 deaths among 13 patients in the control arm.25 In ACTIVE (A Clinical Trial of Intensive Dialysis), participants were randomized to ≥24 hours versus 12-15 hours of hemodialysis and followed up for 12 months. There were 5 deaths in the extended arm and 2 in the standard arm. 26 Observational studies include one conducted by Nesrallah et al., 23 who found an almost 50% reduction in mortality rate in 338 patients treated with mostly home nocturnal hemodialysis (average session duration, 7.4 hours; average frequency, 4.8 times per week) compared to 1388 treated with conventional 3-times-weekly in-center dialysis (average session duration, 3.9 hours). Lacson and colleagues found a 25% improved survival in 746 patients treated with 3-times-weekly in-center nocturnal hemodialysis (average session duration, 7.85 hours) compared to matched conventional 3-times-weekly HD patients (average session duration, 3.75 hours).22 In a Turkish study 21, the relative mortality ratio was 0.28 among 269 patients treated with thrice weekly in-center nocturnal HD (average session duration, 455 minutes) compared to 970 control patients receiving thrice-weekly in-center hemodialysis (average session duration, 236 minutes). Pauly et al., who noted that nocturnal hemodialysis patients are likely to be a highly selective group, compared survival in such a group vs. patients receiving a kidney transplant from a deceased donor, and found similar survival rates 20. None of these observational studies suggested an increased mortality risk associated with nocturnal hemodialysis.
What are some of the important differences between the FHN Nocturnal Trial and the other trials cited above? The Alberta trial included mostly prevalent patients with a median dialysis vintage of about five years, suggesting that most patients in this trial had little to no residual kidney function at baseline. In comparison, the FHN Nocturnal Trial subjects had a median dialysis vintage of 0.9 years and 46% had a baseline urine output >500 mL/d. The Nesrallah study compared patients receiving conventional in-center dialysis with those treated with home nocturnal hemodialysis; so, the results are potentially confounded by the location where dialysis was provided 23. In the studies by Lacson 22 and Ok 21 and their colleagues, both treatment arms were in-center; however the total time receiving dialysis per week was <24 hours and dialysis was performed in-center. The latter observation raises the possibility that dialysis time in excess of 24 hours per week may result in intravascular volume depletion, resulting in organ hypoperfusion that could negate the other benefits of a nocturnal long hemodialysis prescription, the removal of one or more beneficial substances or exposure to a harmful substance present in the dialyzer circuit (plasticizer, sterilant, etc.), hypokalemia, hypophosphatemia or exposure to the extracorporeal circuit itself (platelet activation,27 exposure to microbubbles28).
In summary, our observation of higher mortality among subjects randomized to the nocturnal hemodialysis group compared to the conventional hemodialysis group raises concerns regarding the long-term effects of frequent nocturnal dialysis, but should be interpreted cautiously due to a surprisingly low (0.03 per patient-year) mortality for subjects randomized to the conventional group, low statistical power for the mortality comparison, and the high rate of hemodialysis prescription changes.
Supplementary Material
Figure S1. Relation of treatment frequency by weekly time by period of trial.
Table S1. Subgroup Results: Event Rates by Treatment Arm and Selected Subgroups, with Treatment by Subgroup Interactions
Table S2. Treatment frequency per week and weekly treatment time by time period.
Table S3. Time-Dependent Cox Regressions Relating Mortality to Alternative Measures of the Dialysis Treatment Regimen Over the Preceding 6 or 12 Months
Item S1. Risk of Reporting Bias and Bayesian Analyses for Frequent Hemodialysis Network Nocturnal Study
Acknowledgments
The authors are indebted to the patients who participated in the study.
Support: Supported by the National Institute of Diabetes and Digestive and Kidney Diseases, the Centers for Medicare & Medicaid Services and the National Institutes of Health (NIH) Research Foundation. The investigators and sponsors are grateful for the support of contributors to the NIH Research Foundation—Amgen Inc., Baxter, and Dialysis Clinics Inc—and support from Fresenius Medical Care. The funders of this study did not have any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Aspects of this work were presented in abstract form at the 51st European Renal Association–European Dialysis and Transplant Association Congress, May 31-June 3, 2014, Amsterdam, The Netherlands.
Financial Disclosure: Dr Chertow: Member of Scientific Advisory Board of DaVita Clinical Research and Board of Directors of Satellite Healthcare. Dr Greene: Consultant for Eli Lilly, Amgen, Cormedix, Keryx Biopharmaceuticals, and Nephrogenex. Dr Kliger: Investigator originated research, Amgen. Dr Rocco: Consultant for DaVita. The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: MVR, JTD, TG, RSL, CC, AP, RL, GMC, GJB, PWE, ASK; data acquisition: TG, BL, GJB; data analysis and interpretation: TG, BL, GJB; statistical analysis: TG, BL, GJB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. MVR takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71(4):349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 2.Sergeyeva O, Gorodetskaya I, Ramos R, et al. Challenges to enrollment and randomization of the Frequent Hemodialysis Network (FHN) Daily Trial. J Nephrol. 2012;25:302–309. doi: 10.5301/jn.5000160. [DOI] [PubMed] [Google Scholar]
- 3.Rocco MV, Larive B, Eggers PW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis. 2011;57:90–100. doi: 10.1053/j.ajkd.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocco MV, Lockridge RS, Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daugirdas JT, Chertow GM, Larive B, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012;23:727–738. doi: 10.1681/ASN.2011070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CT, Greene T, Chertow GM, et al. Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging. 2012;5:251–261. doi: 10.1161/CIRCIMAGING.111.969923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall YN, Larive B, Painter P, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning: Frequent Hemodialysis Network (FHN) randomized trials. Clin J Am Soc Nephrol. 2012;7:782–794. doi: 10.2215/CJN.10601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaysen GA, Greene T, Larive B, et al. The effect of frequent hemodialysis on nutrition and body composition: frequent Hemodialysis Network Trial. Kidney Int. 2012;82:90–99. doi: 10.1038/ki.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ornt DB, Larive B, Rastogi A, et al. Impact of frequent hemodialysis on anemia management: results from the Frequent Hemodialysis Network (FHN) Trials. Nephrol Dial Transplant. 2013;28:1888–1898. doi: 10.1093/ndt/gfs593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unruh ML, Larive B, Chertow GM, et al. Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. Am J Kidney Dis. 2013;61:748–758. doi: 10.1053/j.ajkd.2012.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suri RS, Larive B, Sherer S, et al. Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol. 2013;24:498–505. doi: 10.1681/ASN.2012060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daugirdas JT, Greene T, Rocco MV, et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The FHN Trial Group. In-Center Hemodialysis Six Times per Week versus Three Times per Week. New England Journal of Medicine. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocco MV, Yan G, Gassman J, et al. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration. Am J Kidney Dis. 2002;39(1):146–153. doi: 10.1053/ajkd.2002.29905. [DOI] [PubMed] [Google Scholar]
- 15.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19:640–648. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- 17.Gelman A, Carlin JB, Stern HS, Rubin DB. FDA Protocol Registration Receipt NCT00302835. First. London: Chapman and Hall; 1995. [Google Scholar]
- 18.Walsh M, Srinathan SK, McAuley DF, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol. 2014;67:622–628. doi: 10.1016/j.jclinepi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Woods JD, Port FK, Stannard D, Blagg CR, Held PJ. Comparison of mortality with home hemodialysis and center hemodialysis: a national study. Kidney Int. 1996;49(5):1464–1470. doi: 10.1038/ki.1996.206. [DOI] [PubMed] [Google Scholar]
- 20.Pauly RP, Gill JS, Rose CL, et al. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transplant. 2009;24:2915–2919. doi: 10.1093/ndt/gfp295. [DOI] [PubMed] [Google Scholar]
- 21.Ok E, Duman S, Asci G, et al. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: a prospective, case-controlled study. Nephrol Dial Transplant. 2011;26:1287–1296. doi: 10.1093/ndt/gfq724. [DOI] [PubMed] [Google Scholar]
- 22.Lacson E, Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23:687–695. doi: 10.1681/ASN.2011070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23:696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manns BJ, Walsh MW, Culleton BF, et al. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney Int. 2009;75:542–549. doi: 10.1038/ki.2008.639. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay RM, Leitch R, Heidenheim AP, Kortas C London Daily/Nocturnal Hemodialysis Study. The London Daily/Nocturnal Hemodialysis Study- study design, morbidity, and mortality results. Am J Kidney Dis. 2003;42(Suppl 1):5–12. doi: 10.1016/s0272-6386(03)00531-6. [DOI] [PubMed] [Google Scholar]
- 26.Jardine MJ, Gray NA, deZoysa J, Chan C, Gallagher MP, C A, Perkovic V. Impact of Extended Weekly Hemodialysis Hours on Quality of Life and Clinical OUtcomes: The ACTIVE Dialysis Multinational Trial. J Am Soc Nephrol. 2014;25(Abstract Suppl):B2. abstract. [Google Scholar]
- 27.Daugirdas JT, Bernardo AA. Hemodialysis effect on platelet count and function and hemodialysis-associated thrombocytopenia. Kidney Int. 2012;82:147–157. doi: 10.1038/ki.2012.130. [DOI] [PubMed] [Google Scholar]
- 28.Barak M, Nakhoul F, Katz Y. Pathophysiology and clinical implications of microbubbles during hemodialysis. Semin Dial. 2008;21:232–238. doi: 10.1111/j.1525-139X.2008.00424.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relation of treatment frequency by weekly time by period of trial.
Table S1. Subgroup Results: Event Rates by Treatment Arm and Selected Subgroups, with Treatment by Subgroup Interactions
Table S2. Treatment frequency per week and weekly treatment time by time period.
Table S3. Time-Dependent Cox Regressions Relating Mortality to Alternative Measures of the Dialysis Treatment Regimen Over the Preceding 6 or 12 Months
Item S1. Risk of Reporting Bias and Bayesian Analyses for Frequent Hemodialysis Network Nocturnal Study