Abstract
Background
The impact of catheter ablation of ventricular tachycardia (VT) on all-cause mortality remains unknown.
Objective
To examine the association between VT recurrence after ablation and survival in patients with scar-related VT.
Methods
Analysis of 2,061 patients with structural heart disease referred for catheter ablation of scar-related VT from 12 international centers was performed. Data on clinical and procedural variables, VT recurrence, and mortality were analyzed. Kaplan-Meier analysis was used to estimate freedom from recurrent VT, transplant, and death. Cox proportional hazards frailty models were used to analyze the effect of risk factors on VT recurrence and mortality.
Results
One-year freedom from VT recurrence was 70% (72% in ischemic and 68% in non-ischemic cardiomyopathy). 57 (3%) patients underwent cardiac transplantation and 216 (10%) died during follow-up. At one year, the estimated rate of transplant and/or mortality was 15% (same for ischemic and non-ischemic cardiomyopathy). Transplant-free survival was significantly higher in patients without VT recurrence compared to those with recurrence (90% vs. 71%, p<0.001). In multivariable analysis, recurrence of VT after ablation showed the highest risk for transplant and/or mortality (HR 6.9 (5.3-9.0); p<0.001). In patients with EF<30% and across all NYHA classes, improved transplant-free survival was seen in those without VT recurrence.
Conclusions
Catheter ablation of VT in patients with structural heart disease results in 70% freedom from VT recurrence, with an overall transplant and/or mortality rate of 15% at 1 year. Freedom from VT recurrence is associated with improved transplant-free survival, independent of heart failure severity.
Keywords: ablation, ventricular tachycardia
INTRODUCTION
Catheter ablation has been shown to reduce recurrent ventricular tachycardia (VT) and appropriate implantable cardioverter-defibrillator (ICD) therapies in patients with structural heart disease.1 It has been historically regarded as a palliative option to minimize the morbidity and adverse effects of ICD shocks on quality of life. By reducing ICD shocks and VT recurrence, it is plausible for catheter ablation to have a beneficial impact on mortality. Yet, data on patient outcomes after ablation has been limited to single-center observational cohorts, prospective multi-center registries, and small randomized trials primarily evaluating VT recurrence.2-11 Therefore, the rate of survival in patients undergoing catheter ablation and the impact of VT recurrence on all-cause mortality in patients with structural heart disease is unknown. The aim of this study was to assess the outcomes of patients after catheter ablation and the impact of successful VT ablation on survival in patients with structural heart disease from the largest analysis of multi-center data to date.
METHODS
International VT Ablation Center Collaborative Group (IVTCC)
The IVTCC includes 12 international sites that specialize in VT management (Supplemental Table 1) and have developed a shared database. Retrospective analysis of consecutive VT ablation procedures between 2002-2013 was performed at a coordinating center (UCLA) in patients that met the following inclusion criteria:
1) Structural heart disease with ischemic (ICM) and/or nonischemic (NICM) cardiomyopathy with left ventricular ejection fraction (EF) <55%. Left ventricular EF>55% was included in cases of RV and hypertrophic cardiomyopathy.
2) Catheter ablation for monomorphic VT
3) Myocardial scar identified with electroanatomic mapping
4) Clinical follow-up for VT recurrence, transplant, and mortality.
The diagnosis of ICM was established by history of myocardial infarction with focal wall motion abnormality or fixed perfusion defect correlated with coronary stenosis or prior coronary intervention. Etiologies for NICM included arrhythmogenic right ventricular cardiomyopathy (ARVC), hypertrophic, valvular, sarcoidosis, toxin-induced, congenital, Chagasic, and idiopathic dilated cardiomyopathy. Collection of data was approved by Institutional Review Boards of the participating centers.
Ablation Procedure
The approach to ablation of VT across centers was substrate-based modification of scar guided by electroanatomic mapping. All procedures were performed under conscious sedation or general anesthesia with systemic intravenous anticoagulation administered when mapping the LV endocardium. Epicardial ablation was performed at the discretion of the operator and utilized the percutaneous technique described by Sosa et al.12 In cases of prior cardiac surgery or adhesions that impaired the ability to map the epicardium, surgical access was used to perform epicardial mapping and ablation.13 Hemodynamic support devices (extracorporeal membrane oxygenation, Impella (Abiomed, Danvers, MA), or intra-aortic balloon counterpulsation) were used at the discretion of the operator.
Programmed stimulation using up to two sites, with two drive drains and triple extrastimuli down to a minimum of 200ms or ventricular refractory period was performed for induction of VT. Electroanatomic maps were created during sinus rhythm using CARTO (Biosense Webster, Diamond Bar, CA) or NAVX (St. Jude Medical, Minneapolis, MN) with standard low voltage settings (<1.5mV).2 Entrainment mapping was performed when VT was hemodynamically tolerated. An isthmus was defined by classical entrainment criteria as a site that demonstrated concealed fusion with a postpacing interval within 30 ms of the VT cycle length, where the stimulus to QRS interval was equal to EGM-QRS.14 Pace-mapping was utilized to help localize ablation in regions where matches with the targeted VT were seen and sites with longer stimulus-QRS latency were ablated.15 Regions of late activation or local conduction delay as evidence by split, fractionated or isolated late potentials were tagged and targeted for ablation.6,16 The elimination of sustained monomorphic VT inducibility served as the common desired procedural endpoint and programmed stimulation was performed after ablation unless hemodynamic instability or procedural duration was prohibitive.1
RF ablation was performed using a standard non-irrigated catheter (Navi-Star, Biosense-Webster, Diamond Bar, CA), open-irrigated catheter (ThermoCool, ThermoCool SF, or Navistar RMT 3.5 mm, Biosense-Webster, Diamond Bar, CA) or closed-loop irrigated catheter (Chili, Boston Scientific, Natick MA) at 30-50W, temperature limit 42-45°C.
Follow-up and Endpoints
Data and follow-up from the most recent ablation was reported in patients who underwent multiple procedures. Patients were seen in follow-up with office visits and device interrogations to monitor for VT recurrence. Recurrent VT/VF was defined as documented sustained VT/VF or any appropriate ICD therapy, including anti-tachycardia pacing. The date of VT recurrence, cardiac transplant, or death was noted in addition to the last follow-up date. Antiarrhythmic therapy after ablation was at the discretion of the treating physician. Transplant-free survival in patients with follow-up to 12 months was assessed in those with and without recurrence of VT.
Statistical Analysis
Categorical variables are summarized as frequencies and percentages, and compared using chi-square or Fisher’s exact tests. Continuous data are reported as mean±standard deviation or medians with 25%-75% percentiles. A two-sample Student’s t-test or Wilcoxon rank-sum test was used to determine differences between groups.
Kaplan-Meier survival curves were used to estimate freedom from recurrent VT, transplant, and death. Separate Kaplan-Meier curves are displayed for patients with and without VT recurrence at one year to visualize differences in survival times. Log-rank tests were not performed because VT recurrence is time-dependent, which is explicitly modeled in the multivariable analyses. Subgroup analysis was performed in patients with ICM and NICM, EF <30%, and NYHA class I-IV. Univariate analysis was used to evaluate the association of clinical and procedural variables on VT recurrence and mortality.
Multivariable Cox proportional hazards frailty model with VT recurrence as a time-dependent covariate was used to analyze the association between VT recurrence and mortality adjusted for age, sex, EF, NYHA class, diabetes mellitus, chronic kidney disease, ischemic cardiomyopathy, atrial fibrillation, cardiac resynchronization (CRT), ICD shocks, VT storm (>3 episodes/24 hours), antiarrhythmics, prior ablation, beta-blockers, history of ICD, number of VTs induced, epicardial ablation, procedural time, procedural complications, hemodynamic support device, post-ablation noninducibility with centers as random effects. Additionally, interactions between EF and ICM with VT recurrence and NYHA class with VT recurrence were evaluated.
Incomplete variables were handled using a multiple imputation approach with 10 imputations for multivariable analysis; imputations were based on an iterative Markov chain Monte Carlo method and initial values were generated by an expectation–maximization algorithm (missing at random assumption). The number of observations was indicated in the univariate analysis. In addition to performing multiple imputation analysis for missing data, a Cox hazard regression analysis with frailty model of the subgroup of patients who had complete data was performed (n=751). The patient characteristics of patients with complete cases compared to those with missing variables (incomplete cases) are shown in (Supplemental Table 2). A p value of <0.05 was considered statistically significant. All analyses were performed using SAS 9.3 (SAS institute, Cary, NC).
RESULTS
Patient and Procedural Characteristics
Between 2002 and 2013, 2,061 patients (87% male, median age 65 years (55-72 years)) underwent catheter ablation for scar-mediated VT at 12 centers with median follow-up of 527 days (208-1048 days). Complete follow-up through one year was available for 79% of the cohort with censoring of 444 patients before 1 year. The median EF was 31% (24-42%) and 29% of patients had NYHA class I, 37% NYHA class II, 28% NYHA class III, and 6% NYHA class IV functional status. Etiologies of NICM included 72% idiopathic, 9.2% ARVC, 5.0% valvular, 4.3% myocarditis, 3.6% hypertrophic, 1.8% congenital, 0.8% toxin-induced, and 0.1% Chagasic cardiomyopathy.
An ICD was present in 87% of the population, and 26% had CRT. The presenting indication for ablation was ICD shocks in 65% and electrical storm (>3 VT episodes in 24 hours) in 35%. Medical therapy included beta blockers in 79% and amiodarone in 55%. Overall, 18% of the population was refractory to ≥2 antiarrhythmic drugs and 39% had a history of prior ablation (1, n=536 and ≥2, n=276) . The baseline patient characteristics are summarized in TABLE 1.
TABLE 1.
Patient characteristics by VT recurrence and mortality
| VT Recurrence within 12 Months | Transplant/Death within 12 Months | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| N (%) or median (Q1-Q3) | Yes (N=536) |
No (N=1525) |
p value | Yes (N=273) |
No (N=1788) |
p value |
|
| ||||||
| Age (y) | 65.0 (55.0-72.0) | 64.0 (54.0-72.0) | 0.341 | 66.0 (59.0-74.0) | 64.0 (54.0-72.0) | <0.001 |
| Female | 81 (15.1) | 185 (12.1) | 0.083 | 35 (12.8) | 230 (12.9) | 1 |
| ICM | 257 (47.9) | 838 (55.0) | 0.006 | 147 (53.8) | 948 (53.0) | 0.850 |
| EF Pre-Ablation | 28.0 (20.0-40.0) | 35.0 (25.0-45.0) | <0.001 | 24.0 (20.0-30.0) | 35.0 (25.0-45.0) | <0.001 |
| NYHA | <0.001 | <0.001 | ||||
| I | 92 (18.8) | 466 (32.5) | 23 (8.9) | 535 (32.1) | ||
| II | 193 (39.5) | 525 (36.6) | 71 (27.6) | 647 (38.8) | ||
| III | 164 (33.5) | 372 (25.9) | 113 (44.0) | 423 (25.4) | ||
| IV | 40 (8.2) | 71 (5.0) | 50 (19.5) | 61 (3.7) | ||
| ICD | 487 (92.8) | 1251 (84.2) | <0.001 | 250 (94.7) | 1488 (85.2) | <0.001 |
| CRT | 165 (31.4) | 348 (23.4) | <0.001 | 107 (40.5) | 406 (23.3) | <0.001 |
| Electrical Storm | 220 (43.8) | 464 (32.3) | <0.001 | 147 (57.4) | 537 (31.9) | <0.001 |
| ICD Shocks | 344 (72.7) | 869 (62.7) | <0.001 | 176 (76.2) | 1037 (63.7) | <0.001 |
| Hypertension | 285 (58.8) | 753 (56.4) | 0.407 | 140 (58.6) | 898 (56.8) | 0.662 |
| Atrial Fibrillation | 163 (33.5) | 394 (28.7) | 0.052 | 97 (39.3) | 460 (28.5) | 0.004 |
| Diabetes Mellitus | 128 (24.4) | 305 (20.6) | 0.073 | 92 (35.4) | 341 (19.5) | <0.001 |
| Chronic Kidney Disease | 169 (31.7) | 436 (28.7) | 0.213 | 126 (46.5) | 479 (26.9) | <0.001 |
| Baseline Creatinine | 1.1 (0.9-1.5) | 1.1 (0.9-1.4) | 0.199 | 1.3 (1.0-1.7) | 1.1 (0.9-1.3) | <0.001 |
| Beta-Blocker | 433 (81.5) | 1173 (78.3) | 0.123 | 226 (83.7) | 1380 (78.4) | 0.056 |
| Amiodarone | 283 (59.1) | 737 (54.0) | 0.061 | 172 (71.1) | 848 (52.9) | <0.001 |
| ≥2 AAD | 110 (23.0) | 228 (16.7) | 0.003 | 66 (27.3) | 272 (17.0) | <0.001 |
| Prior VT Ablations | 0.0 (0.0-1.0) | 0.0 (0.0-1.0) | 0.019 | 0.0 (0.0-1.0) | 0.00 (0.0-1.0) | 0.001 |
| Min-Max | 0.0-7.0 | 0.0-10.0 | 0.0-6.0 | 0.0-10.0 | ||
Procedural characteristics are shown in TABLE 2. A median of 2 VTs was induced per patient, with 12% having no inducible VT throughout the procedure. In 56% of patients, induced VT was unmappable due to hemodynamic instability. Epicardial ablation was performed in 25% of cases. After ablation, 67% of patients were non-inducible for sustained monomorphic VT, although 5% were not tested.
TABLE 2.
Procedural characteristics by VT recurrence and mortality
| VT Recurrence within 12 Months | Transplant/Death within 12 Months | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| N (%) or median (Q1-Q3) | Yes (N=536) |
No (N=1525) |
p value | Yes (N=273) |
No (N=1788) |
p value |
|
| ||||||
| Hemodynamic Support Device | 32 (7.9) | 63 (5.3) | 0.068 | 39 (19.9) | 56 (4.0) | <0.001 |
| Epicardial Access | 178 (37.5) | 404 (29.3) | 0.001 | 83 (33.7) | 499 (31.1) | 0.440 |
| Ablation | 0.040 | 0.131 | ||||
| Endo | 350 (72.2) | 1049 (75.6) | 186 (74.1) | 1213 (74.8) | ||
| Epi | 24 (4.9) | 94 (6.8) | 9 (3.6) | 109 (6.7) | ||
| Endo+Epi | 106 (21.9) | 236 (17.0) | 54 (21.5) | 288 (17.8) | ||
| Number of VTs Induced | <0.001 | <0.001 | ||||
| 0 | 40 (8.4) | 176 (12.7) | 15 (6.0) | 201 (12.5) | ||
| 1 | 116 (24.4) | 503 (36.2) | 52 (20.8) | 567 (35.2) | ||
| 2 | 131 (27.6) | 318 (22.9) | 72 (28.8) | 377 (23.4) | ||
| ≥3 | 188 (39.6) | 391 (28.2) | 111 (44.4) | 468 (29.0) | ||
| VT Mappability | 0.193 | <0.001 | ||||
| All Unmappable | 149 (40.4) | 394 (38.8) | 93 (46.3) | 450 (38.0) | ||
| All Mappable | 150 (40.7) | 462 (45.5) | 63 (31.3) | 549 (46.4) | ||
| Both Unmappable and | ||||||
| Mappable | 70 (19.0) | 160 (15.7) | 45 (22.4) | 185 (15.6) | ||
| Fastest TCL | 340 (290-394) | 330 (280-392) | 0.563 | 362 (310-428) | 330 (280-390) | <0.001 |
| Slowest TCL | 410 (344-500) | 390 (323-470) | 0.012 | 450 (380-540) | 384 (320-460) | <0.001 |
| Acute Outcome | <0.001 | <0.001 | ||||
| Noninducible | 286 (57.5) | 991 (70.5) | 105 (42.5) | 1172 (70.8) | ||
| Partial/Failure | 176 (35.4) | 365 (26.0) | 116 (47.0) | 425 (25.7) | ||
| Not Tested | 35 (7.0) | 50 (3.6) | 26 (10.5) | 59 (3.6) | ||
| Total Lesion Time (m) | 35.5 (20.2-54.0) | 32.1 (17.0-53.8) | 0.392 | 38.1 (24.1-60.5) | 32.0 (17.0-53.2) | 0.012 |
| Procedure Time (m) | 294.0 (222.0-390.0) | 247.4 (187.1-339.9) | <0.001 | 300 (230-418) | 251 (193-344) | <0.001 |
| Procedural Complications | 30 (6.3) | 97 (7.0) | 0.671 | 30 (12.3) | 97 (6.0) | <0.001 |
Safety and Efficacy
Procedure-related complications occurred in 127 (6%) patients. Of these, 2 patients (0.1%) died during the procedure and 6 (0.3%) required cardiopulmonary resuscitation. 35 (1.7%) cases were complicated by hemopericardium (16 related to epicardial approach), with 8 patients (0.4%) requiring surgical intervention. Complications related to vascular access occurred in 32 (1.6%) patients. Stroke or transient ischemic attack was observed in 10 patients (0.5%), heart block in 19 (0.9%), venous thrombo-embolism in 7 (0.3%), and coronary artery injury in 4 (0.2%).
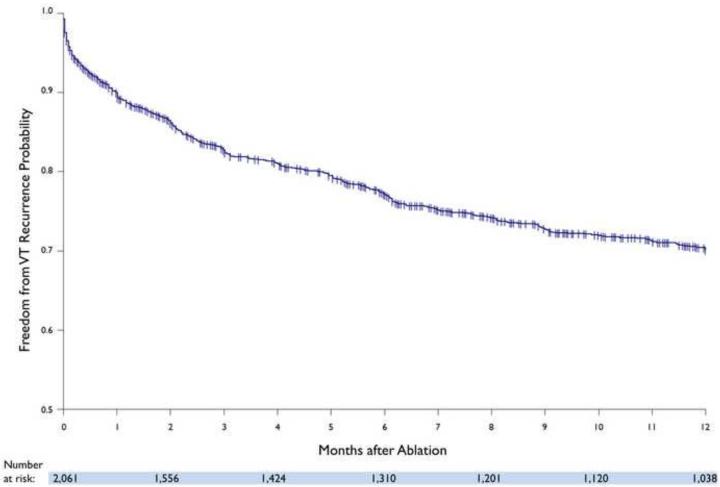
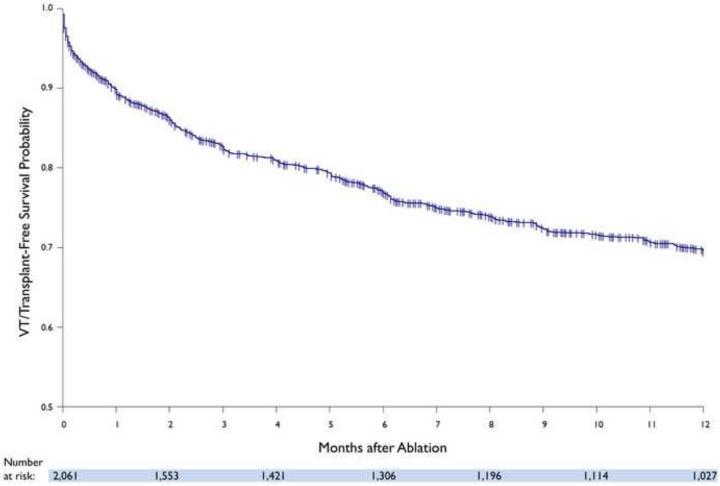
Freedom from VT recurrence in the overall cohort was 70% (72% in patients with ICM and 68% in NICM), with 28% patients taking amiodarone and 10% on other anti-arrhythmic drugs. (FIGURE 1) During follow-up, 57 (3%) patients underwent cardiac transplantation (12% for refractory VT, 68% for advanced heart failure, and 20% for both) and 216 (10%) died. The estimated rate of transplant and/or death was 15% (ICM: 15%, NICM: 15%) at one year. Kaplan-Meier estimate for the combined freedom from VT recurrence, transplant, and mortality was 70% (72% in patients with ICM and 67% in NICM) at one year. (FIGURE 2) Amongst patients without prior ablation, outcomes were similar to the overall cohort, with 73% freedom from VT recurrence, 12% rate of transplant and/or death, and 72% combined freedom from VT recurrence, transplant, and mortality.
FIGURE 1.
Kaplan-Meier estimate of freedom from VT in the overall cohort.
FIGURE 2.
Kaplan-Meier estimate of VT and transplant-free survival in the overall cohort.
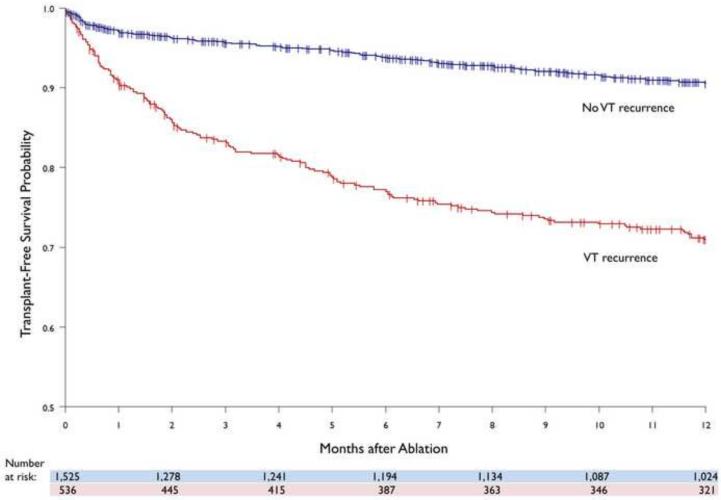
Amongst patients who died, a higher rate of VT recurrence was observed compared to those who survived (55% vs. 22%, p<0.001). The one-year overall probability of transplant-free survival was higher in patients without VT recurrence compared to those who recurred (90% vs. 71%). (FIGURE 3). The observed difference was independent of cardiomyopathy type (ICM: 89% vs. 72%, p<0.001; NICM: 92% vs. 72%, p<0.001).
FIGURE 3.
Kaplan-Meier display of transplant-free survival between patients with and without VT recurrence.
Predictors Of VT Recurrence
Patients with recurrent VT were more likely to have NICM, advanced NYHA status, ICD, CRT, lower EF, electrical storm, shocks, ≥2 antiarrhythmic drugs. (TABLE 1) During the procedure, epicardial ablation, a greater number of induced VTs, longer procedure time, and sustained monomorphic VT post-ablation was observed more frequently in patients with VT recurrence. (TABLE 2) Univariate Cox analysis is shown in Supplemental Table 3.
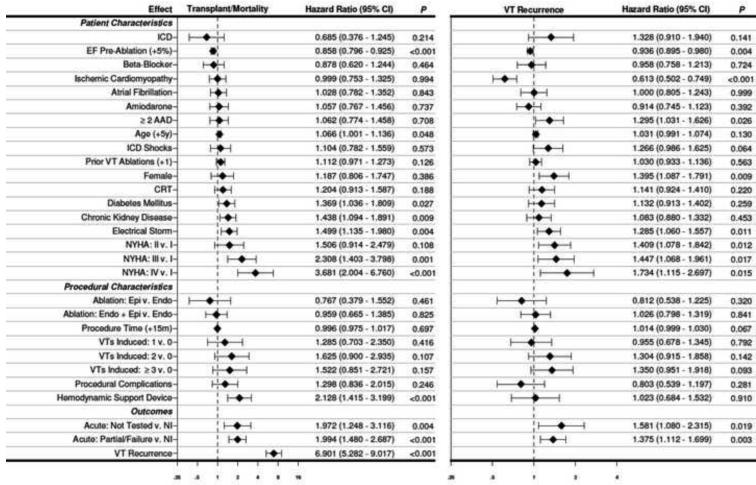
In the Cox multiple regression frailty analysis, the highest probability of VT recurrence was associated with increasing NYHA class, female gender, ≥2 antiarrhythmic drugs, electrical storm, deferred post-ablation testing, and any sustained monomorphic VT inducible after ablation. Ischemic cardiomyopathy and higher EF were associated with lower probability of VT recurrence. (FIGURE 4)
FIGURE 4.
Hazard ratio plot of multivariable Cox proportional hazard regression for transplant/mortality and VT recurrence.
ABBREVIATIONS
EF- ejection fraction
ICD- implantable cardioverter-defibrillator
CRT- cardiac resynchronization therapy
AAD- anti-arrhythmic drugs
NYHA- New York Heart Association
Epi- epicardial
Endo- endocardial
NI-noninduci
Predictors Of Transplant and Mortality
Patients who died or underwent transplant were older and had higher rates of hyperlipidemia, diabetes mellitus, atrial fibrillation, chronic kidney disease, advanced heart failure, ICD, CRT, lower EF, electrical storm, shocks, amiodarone, and ≥2 antiarrhythmic drugs. (TABLE 1) During the procedure, patients who died were more likely to have hemodynamic support devices used, a greater number of VTs induced, procedural complications, and longer procedural times. A higher rate of mappable VTs and noninducibility post-ablation was observed in patients who survived. (TABLE 2) Univariate Cox analysis is shown in Supplemental Table 4.
In the Cox multiple regression frailty analysis, transplant or death was associated with older age, NYHA class III and IV, chronic kidney disease, electrical storm, and use of hemodynamic support devices. While deferred post-ablation testing (HR 1.972 (1.248-3.116); p=0.004) and inducibility of any sustained monomorphic VT post-ablation (HR 1.994 (1.480-2.687); p<0.001) were associated with a higher probability of transplant or death, recurrence of VT after ablation was associated with the highest risk (HR 6.901 (5.282-9.017); p<0.001). Higher EF was associated with a lower probability for transplant or death. (FIGURE 4) Complete-case analysis showed a higher hazard ratio (HR 9.796 (6.137-15.636); p<0.001) for mortality with VT recurrence as compared to multiple imputation analysis. (Supplemental FIGURE 1)
Interaction of recurrence, HF status, and EF with mortality
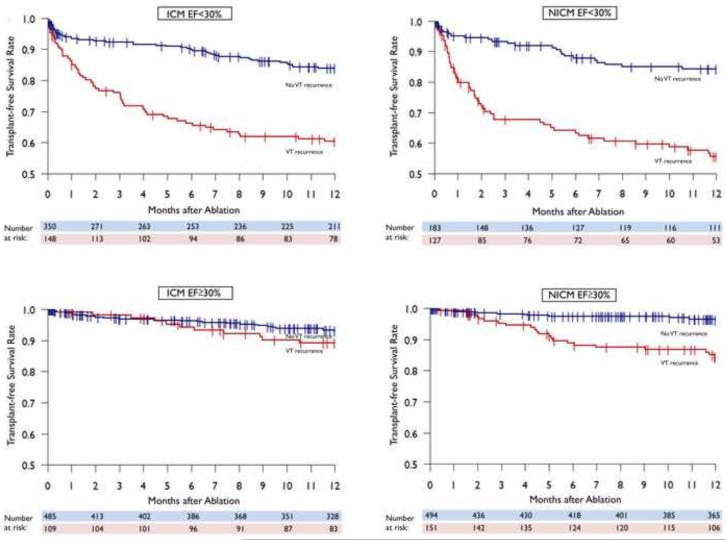
On both univariate and multivariable analysis, higher rates of both VT recurrence and mortality were observed in patients with lower EF and higher NYHA status. Subgroup analysis of patients with EF <30% demonstrated that for both ICM and NICM, patients without VT recurrence had improved transplant-free survival compared to those with VT recurrence (83% vs. 59%, adjusted HR 8.345 (5.607-12.422); p<0.001 for VT recurrence) and (81% vs. 53%, adjusted HR of 6.746 (4.211-10.807); p<0.001 for VT recurrence), respectively. The same trend was seen in patients with EF≥30 in ICM patients (93% vs. 89%, adjusted HR 3.190 (1.517-6.707); p=0.002 for VT recurrence) and NICM patients (96% vs. 84%, adjusted HR 9.293 (4.867-17.743); p<0.001 for VT recurrence). (FIGURE 5)
FIGURE 5.
Kaplan-Meier display of transplant-free survival by VT recurrence in patients with EF greater and less than 30%.
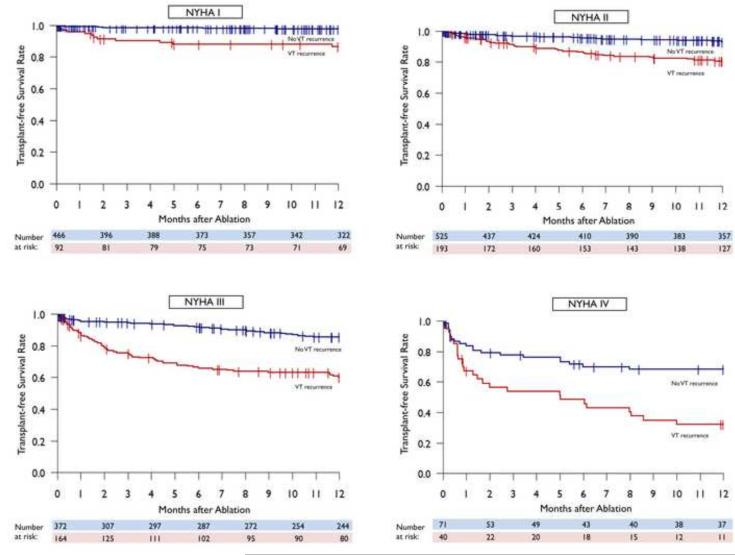
Analysis of mortality by heart failure status demonstrated a consistent improvement in transplant-free survival in patients without VT recurrence compared to those with recurrence across NYHA I (97% vs. 85%, adjusted HR 8.338 (3.616-19.229); p<0.001 for VT recurrence), NYHA II (92% vs. 79%, adjusted HR 6.842 (4.196-11.157); p<0.001 for VT recurrence), NYHA III (85% vs. 59%, adjusted HR 6.681 (4.466-9.994); p<0.001 for VT recurrence) and NYHA IV (65% vs. 31%, adjusted HR 6.911 (3.959-12.064); p<0.001 for VT recurrence) functional classes with a larger differential risk for mortality seen with increasing heart failure severity. (FIGURE 6)
FIGURE 6.
Kaplan-Meier display of transplant-free survival by VT recurrence in in patients by NYHA class.
Kaplan-Meier estimates suggest that NYHA IV patients without recurrence had comparable transplant-free survival compared to NYHA III patients with recurrent VT (65% vs. 59%) and NYHA III patients without recurrence had comparable transplant-free survival compared to NYHA II patients with VT recurrence (85% vs. 79%). NYHA II patients without recurrence seemed to have improved statistically significant transplant-free survival compared to NYHA I patients with recurrent VT (92% vs. 85%)
DISCUSSION
The present study draws upon some of the most experienced centers around the world and demonstrates that catheter ablation of scar-related VT results in a 70% freedom from VT recurrence, transplant, and mortality at one year. Patients referred for VT ablation have a transplant/mortality rate of 15% at one year. Freedom from recurrent VT after catheter ablation is strongly associated with a significant reduction in all-cause mortality, independent of EF and heart failure status.
Catheter ablation has been shown to decrease VT recurrence in patients presenting with ICD shocks and electrical storm across observational cohort studies and randomized trials.2,4,8-11,17,18 However, freedom from VT recurrence has been associated with improved survival only in a few single-center studies specializing in VT ablation 19-21, limiting the generalizability of these results. This present data represents the largest study to date to assess the outcomes, and in particular, survival of patients with catheter ablation of VT using data from multiple centers.
Large sample sizes are required to assess for incremental mortality benefit of catheter ablation beyond ICD implantation. Recently, ICD shocks and therapy have been shown to be predictive of increased mortality22,23, highlighting the biologic plausibility for improvement in survival with successful catheter ablation. Shocks have the potential to be deleterious possibly by worsening heart failure, where ICDs appeared to shift the mode of death towards an increase in non-arrhythmic mortality, offsetting arrhythmic mortality benefit in the immediate post-infarct period.24,25 Alternatively, ventricular arrhythmias that prompt ICD therapy may reflect more advanced disease. Goldenberg et al. highlighted a U-shaped relationship between the severity of heart failure and mortality benefit from ICD therapy.26 Regardless of the mechanisms underlying worsened outcomes with ICD therapies, the present analysis demonstrates that the association of improved mortality in patients without recurrence after ablation was seen across all NYHA classes, with a greater hazard ratio in patients with lower EF and more advanced heart failure. Patients without VT recurrence after ablation with NYHA IV and NYHA III status had similar transplant-free survival compared to patients with recurrent VT in NYHA III and NYHA II, respectively, suggesting that catheter ablation therapy may not have a U-shaped therapeutic curve, but rather an increasing benefit with advancing heart failure severity.
This multi-center experience demonstrates an overall improvement in the success rate of scar-related VT ablation (70%) compared with a ~50% freedom from VT previously reported8-10, which may contribute to the lower one-year mortality rate observed (10% vs. 18% in the Multicenter ThermoCool Ventricular Tachycardia Ablation Trial). As the field of catheter ablation has advanced over the past decade, the implementation of electroanatomic mapping, irrigated ablation technology9, epicardial mapping and ablation27,28, imaging29, improved identification of critical sites during sinus rhythm30, and more extensive ablation aimed to homogenize scar5,6,31 have likely improved efficacy.
Consistent with previous studies, lower EF, advanced NYHA, and multiple VT morphologies are associated with higher recurrence rates.9,19,32 The observed increase in mortality associated with use of hemodynamic support devices may reflect their discretionary implementation as a marker of disease severity. Patients with NICM have been shown to have inferior outcomes relative to ICM due to the heterogeneous nature of disease and differences in scar biology which may reduce ablation targets and increase VT recurrence.33 As NICM is a heterogeneous and frequently idiopathic condition, we chose to include multiple etiologies with the requirement that VT was related to scar identified on electroanatomic mapping.
Acute procedural success has been shown to have prognostic implications for mortality in single-center studies and a recent meta-analysis.4,19,21,34 In the present analysis, success after catheter ablation was defined as freedom from VT recurrence. Assessing acute procedural success with programmed stimulation has three major limitations: extrastimulus testing has been shown to have variable reproducibility 8,35, some patients are noninducible prior to ablation, and VT induction is frequently less aggressive or deferred post-ablation due to concerns of prolonged procedure time and hemodynamic instability. The results of the present study underscore these limitations as 12% were noninducible pre-ablation, 5% were not tested post-ablation, and 58% of patients rendered noninducible for any monomorphic VT post-ablation had recurrence at follow-up. Despite these limitations, noninducibility still serves as the most commonly employed procedural endpoint and was predictive of both VT recurrence and mortality in this study.
LIMITATIONS
The present analysis is retrospective and represents outcomes at specialized tertiary referral centers. Given the expertise of the centers in catheter ablation of VT, the results may not be completely applicable to centers that do not perform these procedures as frequently. Further, many ablation procedures performed at referral centers are after initial attempts fail. Although the approach to catheter ablation of VT is similar, there is inevitably individual practice variability across centers, which prompted frailty analysis. Due to the retrospective multi-center nature of this study, some clinical and procedural characteristics were not available for analysis. Therefore, multivariable analysis was performed by two methods, using multiple imputation techniques and complete-case analysis. The cohort with incomplete variables was sicker than those with complete data, which lead to a conservative estimate of the association between VT recurrence and mortality using imputation. The hazard ratio for VT recurrence when using the complete cases was even higher than that derived by the multiple imputation analysis, suggesting that the multiple imputation hazard ratio is a more conservative estimate of the actual risk. Nonetheless, this data comprises the largest collection of outcomes after VT ablation in patients with structural heart disease.
A causal relationship between VT recurrence and mortality cannot be concluded based on this analysis as clinical variables not accounted for may influence both the propensity for VT recurrence and mortality. There is a possibility that patients who were referred for ablation were inherently different from patients who were not. Additionally, ICD programming was not uniform across patients. This study highlights the need for prospective randomized clinical trials to examine the impact of ablation on survival as VT ablation is still perceived as a palliative therapy of last resort.
CONCLUSIONS
Catheter ablation of VT in patients with structural heart disease results in a 70% freedom from recurrence, with an overall combined transplant and mortality rate of 15% at one year. Freedom from VT recurrence after catheter ablation is strongly associated with improved transplant-free survival, independent of heart failure severity. Successful VT ablation may have benefit beyond arrhythmia control, supporting a shift from its current role as a therapy of last resort.
Supplementary Material
CLINICAL PERSPECTIVE.
Catheter ablation has been shown to decrease recurrence of VT and ICD shocks. In clinical practice, it has historically been implemented as a last resort strategy. Given that ICD shocks have been strongly correlated with increased risk for mortality in patients with heart failure, it is plausible that successful catheter ablation may improve survival. As the majority of VT ablation studies are limited by sample size and power due to the specialized nature of the procedure, the present multi-center retrospective study is the largest to date to examine the relationship between VT recurrence and mortality. The major findings of this present analysis are that freedom from VT recurrence after catheter ablation is strongly associated with improved transplant-free survival, independent of ejection fraction and heart failure severity. This signal supports the transition of catheter ablation to a more preemptive strategy in the management of patients with structural heart disease and VT. Prospective clinical trials are necessary and ongoing to examine the potential mortality impact of catheter ablation.
TABLE 3.
Univariate Cox analysis for VT recurrence
| HR (95% CI) | P value | |
|---|---|---|
| Age (+5y) | 1.033 (1.000, 1.068) | 0.051 |
| Female | 1.306 (1.031, 1.654) | 0.027 |
| ICM | 0.810 (0.684, 0.959) | 0.015 |
| EF Pre-Ablation (+5%) | 0.871 (0.841, 0.902) | <0.001 |
| NYHA (Ref=I) | ||
| II | 1.721 (1.343, 2.206) | <0.001 |
| III | 2.077 (1.609, 2.682) | <0.001 |
| IV | 2.811 (1.939, 4.075) | <0.001 |
| ICD | 2.361 (1.697, 3.285) | <0.001 |
| CRT | 1.524 (1.268, 1.833) | <0.001 |
| Electrical Storm | 1.583 (1.327, 1.888) | <0.001 |
| ICD Shocks | 1.714 (1.400, 2.099) | <0.001 |
| Syncope | 1.064 (0.779, 1.453) | 0.697 |
| Prior VT Ablation | 1.159 (1.065, 1.261) | <0.001 |
| Prior Heart Surgery | 1.023 (0.842, 1.243) | 0.820 |
| Hypertension | 1.123 (0.937, 1.346) | 0.208 |
| Hyperlipidemia | 1.224 (1.019, 1.470) | 0.030 |
| Atrial Fibrillation | 1.209 (1.002, 1.460) | 0.048 |
| Diabetes Mellitus | 1.343 (1.100, 1.640) | 0.004 |
| Chronic Kidney Disease | 1.308 (1.089, 1.570) | 0.004 |
| Baseline Creatinine | 1.165 (1.052, 1.290) | 0.003 |
| Amiodarone | 1.237 (1.031, 1.484) | 0.022 |
| ≥2 AAD | 1.522 (1.230, 1.883) | <0.001 |
| Beta Blocker | 1.251 (1.005, 1.558) | 0.045 |
| Hemodynamic Support Device | 1.727 (1.203, 2.478) | 0.003 |
| Epi Access | 1.316 (1.093, 1.585) | 0.004 |
| Ablation (Ref=Endo) | ||
| None | 2.272 (0.940, 5.490) | 0.068 |
| Epi | 0.761 (0.503, 1.151) | 0.196 |
| Endo+Epi | 1.237 (0.995, 1.537) | 0.055 |
| Number of VTs Induced | ||
| 1 | 1.031 (0.719, 1.476) | 0.870 |
| 2 | 1.867 (1.310, 2.660) | <0.001 |
| ≥3 | 2.226 (1.582, 3.133) | <0.001 |
| VT Mappability (Ref=All Unmappable) | ||
| Both Unmappable and Mappable | 1.291 (0.972, 1.716) | 0.078 |
| All Mappable | 0.765 (0.610, 0.959) | 0.021 |
| Total Lesion Time | 1.000 (0.997, 1.004) | 0.832 |
| Procedure Time (+15m) | 1.031 (1.020, 1.043) | <0.001 |
| Acute Outcome | ||
| Not Tested | 2.400 (1.689, 3.410) | <0.001 |
| Partial/Failure | 1.845 (1.529, 2.227) | <0.001 |
| Procedural Complications | 1.098 (0.759, 1.590) | 0.619 |
TABLE 4.
Univariate Cox analysis for transplant/mortality
| HR (95% CI) | P value | |
|---|---|---|
| Age (+5y) | 1.153 (1.094, 1.216) | <0.001 |
| Female | 0.997 (0.692, 1.437) | 0.989 |
| ICM | 1.139 (0.891, 1.455) | 0.298 |
| EF Pre-Ablation (+5%) | 0.696 (0.655, 0.739) | <0.001 |
| NYHA (Ref=I) | ||
| II | 2.361 (1.457, 3.824) | <0.001 |
| III | 5.483 (3.466, 8.672) | <0.001 |
| IV | 13.525 (8.149, | <0.001 |
| ICD | 2.983 (1.741, 5.113) | <0.001 |
| CRT | 2.192 (1.705, 2.818) | <0.001 |
| Electrical Storm | 2.639 (2.047, 3.403) | <0.001 |
| ICD Shocks | 2.008 (1.477, 2.731) | <0.001 |
| Syncope | 1.244 (0.806, 1.921) | 0.324 |
| Prior VT Ablation | 1.214 (1.087, 1.355) | <0.001 |
| Prior Heart Surgery | 1.188 (0.906, 1.558) | 0.212 |
| Hypertension | 1.093 (0.839, 1.425) | 0.510 |
| Hyperlipidemia | 1.426 (1.080, 1.882) | 0.012 |
| Atrial Fibrillation | 1.474 (1.131, 1.920) | 0.004 |
| Diabetes Mellitus | 2.186 (1.682, 2.841) | <0.001 |
| Chronic Kidney Disease | 2.589 (2.027, 3.308) | <0.001 |
| Baseline Creatinine | 1.382 (1.271, 1.503) | <0.001 |
| Amiodarone | 2.125 (1.599, 2.823) | <0.001 |
| ≥2 AAD | 1.822 (1.364, 2.434) | <0.001 |
| Beta Blocker | 1.340 (0.969, 1.854) | 0.077 |
| Hemodynamic Support Device | 4.847 (3.399, 6.913) | <0.001 |
| Epi Access | 1.104 (0.843, 1.446) | 0.474 |
| Ablation (Ref=Endo) | ||
| None | 1.366 (0.339, 5.506) | 0.661 |
| Epi | 0.569 (0.291, 1.111) | 0.098 |
| Endo+Epi | 1.151 (0.843, 1.571) | 0.376 |
| Number of VTs Induced | ||
| 1 | 1.218 (0.685, 2.165) | 0.503 |
| 2 | 2.535 (1.452, 4.429) | 0.001 |
| ≥3 | 2.936 (1.706, 5.052) | <0.001 |
| VT Mappability (Ref=All Unmappable) | ||
| Both Unmappable and Mappable | 1.336 (0.933, 1.913) | 0.114 |
| All Mappable | 0.542 (0.391, 0.751) | <0.001 |
| Total Lesion Time | 1.004 (1.000, 1.008) | 0.077 |
| Procedure Time (+15m) | 1.033 (1.016, 1.049) | <0.001 |
| Acute Outcome | ||
| Not Tested | 4.272 (2.740, 6.660) | <0.001 |
| Partial/Failure | 3.028 (2.318, 3.955) | <0.001 |
| Procedural Complications | 2.300 (1.559, 3.393) | <0.001 |
ACKNOWLEDGMENTS
None
FUNDING SOURCES:
The study was an unfunded, investigator-initiated collaborative study. Dr. Shivkumar was supported by the NHLBI (R01HL084261)
Abbreviations
- VT
ventricular tachycardia
- ICD
implantable cardioverter defibrillator
- EF
ejection fraction
- NYHA
New York Heart Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST:
Roderick Tung- none
Marmar Vaseghi- none
David S. Frankel- none
Pasquale Vergara- none
Luigi Di Biase- Consultant: Biosense Webster, St. Jude Medical, Hansen Medical
Koichi Nagashima- Medtronic Japan Fellowship
Ricky Yu- none
Sitaram Vangala-none
Chi-Hong Tseng- none
Eue-Keun Choi- none
Shaan Khurshid- none
Mehul Patel MD- none
Nilesh Mathuria- none
Shiro Nakahara- none
Wendy Tzou- none
William Sauer- none
Kairav Vakil- none
Usha Tedrow- Honoraria: Medtronic (minor), Boston Scientific (minor), St. Jude Medical (minor); Research grant:: Biosense Webster (minor), St Jude Medical (minor)
David Burkhardt- Consultant: Biosense Webster
Venkat Tholakanahalli- Grants: SJM foundation, Consultant : Biosense Webster and St. Jude Medical
Anastasios Saliaris- none
Timm Dickfeld- Research grant: Biosense Webster (major), Consultant: Biosense (minor)
J. Peter Weiss- Consultant: Stereotaxis
T. Jared Bunch- Consultant: Boston Scientific, (minor)
Madhu Reddy- none
Arun Kanmanthareddy- none
David Callans-none
Dhanunjaya Lakkirreddy- none
Andrea Natale- none
Francis Marchlinski- none
William G. Stevenson- patent for needle ablation consigned to Brigham and Women’s Hospital
Paolo Della Bella- Consultant: St. Jude Medical; Honoraria for lectures: Biosense Webster, St Jude Medical and Biotronik.
Kalyanam Shivkumar-none
REFERENCES
- 1.Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6(6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101(11):1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 3.Soejima K, Suzuki M, Maisel WH, Brunckhorst CB, Delacretaz E, Blier L, Tung S, Khan H, Stevenson WG. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104(6):664–669. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 4.Della Bella P, Riva S, Fassini G, Giraldi F, Berti M, Klersy C, Trevisi N. Incidence and significance of pleomorphism in patients with postmyocardial infarction ventricular tachycardia. Acute and long-term outcome of radiofrequency catheter ablation. Eur Heart J. 2004;25(13):1127–1138. doi: 10.1016/j.ehj.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Di Biase L, Santangeli P, Burkhardt DJ, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60(2):132–141. doi: 10.1016/j.jacc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Jais P, Maury P, Khairy P, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125(18):2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 7.Sacher F, Tedrow UB, Field ME, Raymond JM, Koplan BA, Epstein LM, Stevenson WG. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol. 2008;1(3):153–161. doi: 10.1161/CIRCEP.108.769471. [DOI] [PubMed] [Google Scholar]
- 8.Tanner H, Hindricks G, Volkmer M, Furniss S, Kuhlkamp V, Lacroix D, Almendral J, Caponi D, Kuck KH, Kottkamp H. Catheter ablation of recurrent scar-related ventricular tachycardia using electroanatomical mapping and irrigated ablation technology: results of the prospective multicenter Euro-VT-study. J Cardiovasc Electrophysiol. 2010;21(1):47–53. doi: 10.1111/j.1540-8167.2009.01563.x. C DEC. [DOI] [PubMed] [Google Scholar]
- 9.Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008;118(25):2773–2782. doi: 10.1161/CIRCULATIONAHA.108.788604. [DOI] [PubMed] [Google Scholar]
- 10.Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz E, Pitschner HF, Kautzner J, Schumacher B, Hansen PS. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet. 2010;375(9708):31–40. doi: 10.1016/S0140-6736(09)61755-4. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, Kralovec S, Sediva L, Ruskin JN, Josephson ME. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med. 2007;357(26):2657–2665. doi: 10.1056/NEJMoa065457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7(6):531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 13.Soejima K, Couper G, Cooper JM, Sapp JL, Epstein LM, Stevenson WG. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110(10):1197–1201. doi: 10.1161/01.CIR.0000140725.42845.90. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson WG, Friedman PL, Sager PT, Saxon LA, Kocovic D, Harada T, Wiener I, Khan H. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29(6):1180–1189. doi: 10.1016/s0735-1097(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson WG, Sager PT, Natterson PD, Saxon LA, Middlekauff HR, Wiener I. Relation of pace mapping QRS configuration and conduction delay to ventricular tachycardia reentry circuits in human infarct scars. J Am Coll Cardiol. 1995;26(2):481–488. doi: 10.1016/0735-1097(95)80026-d. [DOI] [PubMed] [Google Scholar]
- 16.Arenal A, Glez-Torrecilla E, Ortiz M, Villacastin J, Fdez-Portales J, Sousa E, del Castillo S, Perez de Isla L, Jimenez J, Almendral J. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41(1):81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 17.Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G, Riva S, Moltrasio M, Cireddu M, Veglia F, Della Bella P. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117(4):462–469. doi: 10.1161/CIRCULATIONAHA.106.686534. [DOI] [PubMed] [Google Scholar]
- 18.Cesario DA, Vaseghi M, Boyle NG, Fishbein MC, Valderrabano M, Narasimhan C, Wiener I, Shivkumar K. Value of high-density endocardial and epicardial mapping for catheter ablation of hemodynamically unstable ventricular tachycardia. Heart Rhythm. 2006;3(1):1–10. doi: 10.1016/j.hrthm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Della Bella P, Baratto F, Tsiachris D, et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation. 2013;127(13):1359–1368. doi: 10.1161/CIRCULATIONAHA.112.000872. [DOI] [PubMed] [Google Scholar]
- 20.Bunch TJ, Weiss JP, Crandall BG, Day JD, May HT, Bair TL, Osborn JS, Mallender C, Fischer A, Brunner KJ, Mahapatra S. Patients treated with catheter ablation for ventricular tachycardia after an ICD shock have lower long-term rates of death and heart failure hospitalization than do patients treated with medical management only. Heart Rhythm. 2014;11(4):533–540. doi: 10.1016/j.hrthm.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Tokuda M, Kojodjojo P, Tung S, Tedrow UB, Nof E, Inada K, Koplan BA, Michaud GF, John RM, Epstein LM, Stevenson WG. Acute failure of catheter ablation for ventricular tachycardia due to structural heart disease: causes and significance. J Am Heart Assoc. 2013;2(3):e000072. doi: 10.1161/JAHA.113.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367(24):2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
- 24.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ, Investigators D. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 25.Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361(15):1427–1436. doi: 10.1056/NEJMoa0901889. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51(3):288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 27.Sacher F, Roberts-Thomson K, Maury P, et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010;55(21):2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 28.Tung R, Michowitz Y, Yu R, et al. Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm. 2013;10(4):490–498. doi: 10.1016/j.hrthm.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 29.van Huls van Taxis CF, Wijnmaalen AP, Piers SR, van der Geest RJ, Schalij MJ, Zeppenfeld K. Real-time integration of MDCT-derived coronary anatomy and epicardial fat: impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6(1):42–52. doi: 10.1016/j.jcmg.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Tung R, Mathuria N, Michowitz Y, Yu R, Buch E, Bradfield J, Mandapati R, Wiener I, Boyle N, Shivkumar K. Functional pace-mapping responses for identification of targets for catheter ablation of scar-mediated ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5(2):264–272. doi: 10.1161/CIRCEP.111.967976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vergara P, Trevisi N, Ricco A, Petracca F, Baratto F, Cireddu M, Bisceglia C, Maccabelli G, Della Bella P. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23(6):621–627. doi: 10.1111/j.1540-8167.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 32.Tung R, Josephson ME, Reddy V, Reynolds MR, Investigators S-V. Influence of clinical and procedural predictors on ventricular tachycardia ablation outcomes: an analysis from the substrate mapping and ablation in Sinus Rhythm to Halt Ventricular Tachycardia Trial (SMASH-VT) J Cardiovasc Electrophysiol. 2010;21(7):799–803. doi: 10.1111/j.1540-8167.2009.01705.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakahara S, Tung R, Ramirez RJ, Michowitz Y, Vaseghi M, Buch E, Gima J, Wiener I, Mahajan A, Boyle NG, Shivkumar K. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55(21):2355–2365. doi: 10.1016/j.jacc.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghanbari H, Baser K, Yokokawa M, et al. Noninducibility in postinfarction ventricular tachycardia as an end point for ventricular tachycardia ablation and its effects on outcomes: a meta-analysis. Circ Arrhythm Electrophysiol. 2014;7(4):677–683. doi: 10.1161/CIRCEP.113.001404. [DOI] [PubMed] [Google Scholar]
- 35.McPherson CA, Rosenfeld LE, Batsford WP. Day-to-day reproducibility of responses to right ventricular programmed electrical stimulation: implications for serial drug testing. Am J Cardiol. 1985;55(6):689–695. doi: 10.1016/0002-9149(85)90138-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.