Abstract
Tissue engineering using mesenchymal stem cells holds great promise for regenerating critically sized bone defects. While the bone marrow-derived mesenchymal stem cell (MSC) is the most widely studied stromal/stem cell type for this application, its rarity within bone marrow and painful isolation procedure have motivated investigation of alternative cell sources. Adipose-derived stromal/stem cells (ASCs) are more abundant and more easily procured; furthermore, they also possess robust osteogenic potency. While these two cell types are widely considered very similar, there is a growing appreciation of possible innate differences in their biology and response to growth factors. In particular, reports indicate that their osteogenic response to platelet-derived growth factor BB (PDGF-BB) is markedly different: MSCs responded negatively or not at all to PDGF-BB while ASCs exhibited enhanced mineralization in response to physiological concentrations of PDGF-BB. In this study, we directly tested whether a fundamental difference existed between the osteogenic responses of MSCs and ASCs to PDGF-BB. MSCs and ASCs cultured under identical osteogenic conditions responded disparately to 20 ng/mL of PDGF-BB: MSCs exhibited no difference in mineralization while ASCs produced more calcium per cell. siRNA-mediated knockdown of PDGFRβ within ASCs abolished their ability to respond to PDGF-BB. Gene expression was also different; MSCs generally downregulated and ASCs generally upregulated osteogenic genes in response to PDGF-BB. ASCs transduced to produce PDGF-BB resulted in more regenerated bone within a critically sized murine calvarial defect compared to control ASCs, indicating PDGF-BB used specifically in conjunction with ASCs might enhance tissue engineering approaches for bone regeneration.
Keywords: Tissue engineering, platelet-derived growth factor, bone, stem cells
Introduction
It is estimated that over one million bone fractures requiring hard tissue transplantation occur annually in the United States, incurring an economic burden of $3 billion per year [1]. The demand for donor tissue greatly outstrips the supply of both allogeneic and autologous sources, underscoring the pressing need for alternative approaches to reconstruct bone. In recent years, tissue engineering (TE) has emerged as a promising method for producing bone grafts de novo. In the traditional TE paradigm, cells are housed inside biomaterial scaffolds and signaled with bioactive factors [2]; the scaffold provides mechanical and structural support while the bioactive factors guide the cells in regenerating tissue.
For bone regeneration specifically, the marrow-derived mesenchymal stem cell (MSC) is the most widely studied cell type. MSCs, isolated from bone marrow aspirate, have the ability to differentiate into the three classical mesenchymal lineages of bone, fat, and cartilage [3]; furthermore, their immunomodulatory characteristics suggest their potential in allogeneic transplantation [4]. The study of MSC-based therapies for bone regeneration has reached clinical trials for applications in, but not limited to, osteonecrosis [5], non-union repair [6], and spinal fusion [7]. MSCs, however, are a fairly rare population within bone marrow, comprising less than 0.01% of the nucleated population. To address this shortcoming, recent studies have investigated the presence of similar cells in other tissues of the body, most notably within fat. These adipose-derived stem cells (ASCs) also have the capability to differentiate down the classical mesenchymal lineages and represent a larger population within adipose tissue, accounting for up to about 5% of nucleated cells in the collagenase-released stromal vascular fraction [8, 9]. While it is generally recognized that MSCs and ASCs exhibit similar surface immunophenotypes and multilineage differentiation characteristics, recent studies have called into question the extent of their similarities [10].
In particular, studies into the osteoinductive potential of platelet-derived growth factor BB (PDGF-BB), prompted by the observation of heightened PDGF-BB levels within bone fracture microenvironments [11, 12] have largely determined that PDGF-BB is not osteoinductive when signaling MSCs. In fact, PDGF-BB was shown to inhibit mineralization [13–16] and when the beta receptor for PDGF, PDGFRβ, was deleted using Cre-LoxP recombination, mineralization of MSCs was restored even in the presence of PDGF-BB [17]. In contrast, our group has recently shown a dose-dependent increase in calcification per cell in ASCs when signaled with PDGF-BB [18]. More recent studies have exploited this, using ASCs in fibrin matrices incorporating PDGF-BB [19]; however, to date no direct comparison of MSCs and ASCs in their osteogenic response to PDGF-BB has been performed to resolve the apparent contradiction.
A potential difference between MSCs and ASCs is of high importance in the use of TE approaches to treat bone defects. PDGF-BB is a known mitogen [20] and chemoattractant [21] and it has been observed that injection of PDGF-BB into fracture sites accelerates bone healing [22]. Given the in vitro observations that PDGF-BB does not directly promote osteogenesis in MSCs, it is thought that PDGF-BB in this case is largely acting through recruitment of endogenous repair cells. The notion that PDGF-BB can directly enhance ASC mineralization, however, presents the possibility that the use of ASCs in conjunction with PDGF-BB for bone repair can more efficiently make use of both the cellular and biomolecular components.
In the current study, we hypothesize that the osteogenic response of MSCs and ASCs to PDGF-BB is different at a fundamental genetic level. To test this hypothesis, the objectives of this study are (1) to investigate the differences in osteogenic response of MSCs and ASCs at a cellular and genetic level, (2) to utilize siRNA-mediated knockdown of PDGFRβ for loss-of-function evidence that specifically PDGF-BB leads to enhanced mineralization of ASCs but not of MSCs, and (3) to demonstrate the application of this finding using ASCs overexpressing PDGFB in an in vivo murine calvarial defect model.
Materials and Methods
Isolation and source of cells
All tissues obtained for this study were obtained under Institutional Review Board approved protocols with patient consent. To ensure the observed phenomena are cell-type specific rather than donor-dependent, the initial characterization study was performed using three donors for MSCs, denoted M1, M2, and M3; and three donors for ASCs, denoted A1, A2, and A3. Donor M1 (late 20s, male) was commercially obtained from Lonza (Basel, Switzerland), while Donors M2 (32-year-old male) and M3 (27-year-old male) were isolated at Case Western Reserve University following established marrow isolation procedures [23–25]. Briefly, aspirated iliac crest bone marrow was mixed with culture medium and centrifuged to remove adipocytes. MSCs were isolated from the resulting cell pellet via centrifugation in a Percoll gradient and the MSC-enriched fraction was plated. Donors A1 (54-year-old female) and A2 (50-year-old female) were isolated from lipoaspirate using established protocols [26, 27] at Johns Hopkins Medical Institutions, while Donor A3 (47-year-old female) was isolated at Tulane University School of Medicine. Briefly, harvested lipoaspirate tissue was digested in 1 mg/mL collagenase type I (Worthington Biochemical Corporation, Lakewood, NJ) for 1 hour at 37 degrees Celsius. The released cells were then centrifuged to obtain the stromal vascular fraction (SVF) pellet; the pellet was then resuspended and plated to obtain passage 0 ASCs. Cells from all six donors were characterized via flow cytometry for surface expression of CD31, CD34, CD73, CD90, and PDGFRβ as previously described [27]. In this study, PDGFRβ was studied specifically as it preferentially binds PDGF-BB.
Culture conditions
For all experiments, cells were expanded for use at passage 2. Expansion medium consisted of Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/L glucose (Life Technologies, Frederick, MD) supplemented with 10% v/v fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA), 100 U/mL penicillin and 100 μg/mL streptomycin (Cellgro, Manassas, VA), and 1 ng/mL basic fibroblast growth factor (bFGF; PeproTech, Rocky Hill, NJ). Subsequent to expansion, cells were cultured in one of four conditions: namely, the control (−), control (+), osteogenic (−), and osteogenic (+) conditions. The control (−) medium consisted of DMEM with 1 g/L glucose, 100 U/mL penicillin and 100 μg/mL streptomycin, and 6% v/v FBS. Control (+) medium consisted of control (−) medium with the addition of 20 ng/mL recombinant human PDGF-BB (PeproTech), a concentration determined based on our previous work [18]. The osteogenic (−) medium consisted of control (−) medium with 10 mM β-glycerophosphate (Sigma Aldrich, St. Louis, MO) and 50 μM ascorbic acid (Sigma Aldrich). Finally, osteogenic (+) medium consisted of osteogenic (−) medium with 20 ng/mL PDGF-BB. For all conditions, PDGF-BB was replenished twice a week. These culture conditions were established from our previous studies [18]. Unless otherwise noted, all osteogenic cultures were carried out for three weeks.
Characterization of mineralization response to PDGF-BB
MSCs and ASCs were cultured under control (−), control (+), osteogenic (−), and osteogenic (+) conditions for three weeks and then subjected to Alizarin Red S (Sigma Aldrich) or von Kossa (silver nitrate and sodium thiosulfate both from Sigma Aldrich) staining for qualitative assessments. Quantitatively, samples were subject to the Quant-It PicoGreen dsDNA assay (Invitrogen) and the Stanbio LiquiColor calcium assay (Stanbio, Boerne, TX) to determine calcium content normalized to cell number. DNA content was converted to cell number using 6.24 pg/MSC and 7.23 pg/ASC, determined by performing the DNA assay on known numbers of the cells specifically used in this study (data not shown).
Real-time polymerase chain reaction (RT-PCR)
To investigate the genetic expression of MSCs and ASCs under the four conditions, RT-PCR was performed at 1, 2, and 3 weeks of culture for β-Actin (BA), Runx2, osteocalcin (OCN), osteonectin (OSN), and collagen I (Col-I). Cells were digested using TRIzol reagent (Life Technologies) and the mRNA isolated with chloroform washes. The mRNA was further purified and concentrated using isopropanol and ethanol washes and used to produce cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). cDNA was then subject to RT-PCR using custom-designed primers. The primer sequences for all primers used in this study can be found in Table 1. For all analysis, the delta-delta Ct method was used in which the housekeeping gene (BA) and the control (−) group were subtracted from all other Ct readings.
Table 1.
Primer sequences used for real-time polymerase chain reaction.
| Gene Name | Primer Sequence (5’ – 3’) | |
|---|---|---|
| β-Actin |
Fwd.
Rev. |
AGTTGCGTTACACCCTTTCTTG TCACCTTCACCGTTCCAGTTT |
| RUNX-2 |
Fwd.
Rev. |
GTCTCACTGCCTCTCACTTG CACACATCTCCTCCCTTCTG |
| OCN |
Fwd.
Rev. |
GTGACGAGTTGGCTGACC TGGAGAGGAGCAGAACTGG |
| OSN |
Fwd.
Rev. |
TCGGCATCAAGCAGAAGGATA CCAGGCAGAACAACAAACCAT |
| Col-1 |
Fwd.
Rev. |
GAGAGGAAGGAAAGCGAGGAG GGGACCAGCAACACCATCT |
| PDGF-B |
Fwd.
Rev. |
GTTGAGGTGGCTGTAGATGGT AGGGTGGAGGTAGAGAGATGAA |
| PDGFR-β |
Fwd.
Rev. |
TGAGGCTTTGGAGGAATC CCTTGCTTCATCTGGACA |
Monomer synthesis
Bioreducible monomer 2,2’-disulfanediylbis(ethane-2,1-diyl) (BR6) was synthesized as previously described [28, 29]. Briefly, bis(2-hydroxyethyl) disulfide (10 mmol) was acrylated with acryloyl chloride (300 mmol) in the presence of triethylamine (TEA; 300 mmol) in anyhydrous tetrahydrofuran (THF) for 24 hours. TEA HCl precipitate was removed via filtration, and THF was removed via rotary evaporation. The product was further purified by dissolving it in dichloromethane (DCM) and washing five times with a 0.2 M solution of Na2CO3 and three times with water. The organic phase was dried with Na2SO4 and DCM was removed via rotary evaporation. BR6 structure and purity was verified by 1H-NMR [28].
Polymer synthesis
To obtain loss-of-function evidence, siRNA against PDGFRβ was delivered to MSCs and ASCs using bioreducible poly(β-amino ester) (PBAE)-based nanoparticles. Bioreducible PBAEs were synthesized in a method similar to Kozielski et al. [28]. Base monomer BR6 was polymerized with side chain monomer 4-amino-1-butanol (S4) at a ratio of 1.05:1 at 500 mg/mL in anhydrous tehtrahydrofuran (THF) at 60°C for 24 hours while stirring. Polymers were endcapped at a concentration of 100 mg/mL in THF with either 2-(3-(aminopropyl)amino)methanol (E6) or 1-(3-aminopropyl)-4-methylpiperazine (E7) at 0.2 M for 1 hour at room temperature while stirring. Polymers were precipitated in diethyl ether to remove unreacted monomer and THF. The precipitate was recovered by centrifugation and solvent decanting. The polymer was washed and isolated a second time, and residual ether was removed under vacuum for 48 hours. The resulting polymers BR6-S4-E6 (R646) and BR6-S4-E7 (R647) were stored in dimethyl sulfoxide (DMSO) at 100 mg/mL at –20°C.
Nanoparticle screen using green fluorescent protein (GFP)
Two polymers, R646 and R647, were used to deliver siRNA against GFP (siGFP) to MSCs transduced with GFP by lentivirus, a procedure we have used previously to screen nanoparticles [30]. Briefly, lentiviral production was produced using 293T cells and the ViraSafe Lentiviral Packaging System (Cell Biolabs, San Diego, CA). MSCs were seeded at 6000 cells/cm2, allowed to adhere for one day, and given virus at 8 x 107 viral particles/mL for four hours (an approximate multiplicity of infection of 80) under serum-free conditions. Expansion medium was added after the viral incubation step and transduction was allowed to continue for 72 more hours, after which medium was changed to clean expansion medium and the cells were allowed to proliferate to confluence. The efficiency of transduction was approximately 86% as determined by fluorescence-activated cell sorting (FACS) [30].
GFP+ MSCs were screened for polymeric siRNA delivery using Ambion® Silencer® siRNA targeting GFP (siGFP) with sequence 5’-CAAGCUGACCCUGAAGUUCTT (sense) and 3’-GAACUUCAGGGUCAGCUUGCC (antisense), or Ambion® Silencer® Negative Control #1 siRNA (scrRNA) with sequence 5’-AGUACUGCUUACGAUACGGTT (sense) and 3’-CCGUAUCGUAAGCAGUACUTT (anti-sense) (Life Technologies). Nanoparticles were formed by dissolving siRNA and polymers separately in 25 mM sodium acetate (NaAc), mixing the siRNA and polymer solutions, and allowing nanoparticles to self-assemble for 10 minutes. The cell culture medium was removed and replaced with 100 μL of serum-free medium, then 20 μL of nanoparticle solutions were added directly to the cell culture media. Polymers R646 and R647 were used at final concentrations of 360, 270, or 180 μg/mL, and siRNA were at final concentrations of 80, 40, or 20 nM. Following a 2-hour incubation with cells, the nanoparticle-containing media was removed and replaced with fresh, complete cell culture medium.
At 24 hours post-transfection, viability was assessed using a Cell Titer 96® AQueous One MTS Cell Proliferation assay (Promega, Madison, WI) following manufacturer’s instructions. Absorbance at 490 nm was read using a BioTek Synergy 2 Microplate Reader and viability of cells in treated wells was calculated by normalizing to absorbance values of cells in untreated wells.
GFP knockdown was measured every day for 3.5 weeks using a BioTek Synergy 2 Microplate Reader by reading the total fluorescence of each well at 485 ± 10 nm excitation and 520 ± 10 nm emission. In previous work, we have found that this method of measuring GFP expression correlates well with data acquired via flow cytometry [30] while enabling facile tracking of GFP knockdown over time. Fluorescence values from wells treated with siRNA were normalized to values from wells treated with scrRNA and subtracted from 1 to determine knockdown.
Knockdown of PDGFRβ
siRNA against PDGFRβ (siPDGFRβ) was delivered to both MSCs and ASCs using the transfection protocol above. At 1, 2, and 3 weeks, knockdown of PDGFRβ was quantified using both RT-PCR of PDGFRβ and flow cytometry via an antibody against the receptor (Santa Cruz, Santa Cruz, CA).
Separately, siPDGFRβ was delivered to MSCs and ASCs and cells were cultured under control (−), osteogenic (−), and osteogenic (+) conditions for three weeks. After the culture period, calcium and DNA content was quantified as outlined above.
Murine critically sized calvarial defect model
For the final portion of the study, two different cell groups were created by lentiviral transduction: (1) ASCs transduced with PDGFB (DNASU plasmid HsCD00437330 [31]) and (2) ASCs transduced with the fluorescent protein mCherry (plasmid kindly provided by Don Zack’s laboratory). Transduction was performed following the protocol above and PDGFB-transduced ASCs were verified by RT-PCR and enzyme-linked immunosorbent assay (ELISA, PeproTech). In addition, cells were cultured under control (−) and osteogenic (−) conditions for three weeks and then subject to DNA and calcium assays as outlined above to determine whether the overexpressed PDGF-BB was having a mitogenic and mineralization effect.
For the in vivo study, ASCs were encapsulated at 2 x 107 cells/mL in fibrin gels containing final concentrations of 8 mg/mL fibrinogen and 2 U/mL thrombin. Cells in fibrin were seeded into porous polycaprolactone (PCL) scaffolds (diameter: 4 mm, height: 644 μm, porosity 60% by volume) mixed with mineralized particles printed with a custom 3D printer [32]. This geometry was chosen to match the geometry of the murine calvarial defect described below. In addition, a third group was produced with the same scaffolds and fibrin, but containing no cells. For seeding, cells were suspended in fibrinogen and thrombin was added at the proper ratio. Prior to gelation, the mixture was pipetted into the pore spaces of the scaffold and subsequent gelation held the cells in place within the pore spaces.
Eight 8-week-old male FOXN1-knockout mice (Jackson Laboratories, Bar Harbor, ME) were operated on, resulting in 16 sites with n = 4 for ASCs overexpressing PDGFB, n = 4 for ASCs transduced with mCherry, n = 4 for acellular controls, and n = 4 unoperated controls. In all cases, IACUC-approved procedures were followed. For creation of the defect, previously established methods were adapted [33, 34]. Briefly, a 4-mm circular knife (Medicon, Tuttlingen, Germany) was used to excise 4-mm pieces of calvaria, with special care made to avoid damaging the underlying dura mater. The location of the defect was kept consistent from animal to animal by placement between the coronal and lambdoid sutures and approximately 1 mm lateral to the sagittal suture.
Mice were imaged using computerized tomography (CT) at 8 weeks post-implantation, sacrificed, and calvariae were excised for histological analysis. Imaging was performed on a Gamma Medica X-SPECT small animal system (Gamma Medica, Salem, NH) with 80 kV peak voltage and 600 µA current. Reconstruction was performed with voxel size 100 µm and threshold 15300/65535. For sectioning, samples were fixed in 3.7% formalin overnight and fixed samples were infiltrated with 30% sucrose, frozen in Tissue Tek OCT medium, and cut into 10 μm-thick sections. Cryosections were mounted and dried on Superfrost Plus slides, followed by rehydration in water before staining with either Haematoxylin and Eosin (H&E; Sigma) or immunohistochemistry. Immunohistochemistry was performed by blocking for 30 minutes (10% normal serum / 0.2% Triton X), followed by overnight incubation with primary antibody (0.5 μg/mL mouse anti-human Lamin A/C; Abcam, Cambridge, Britain) at 4 degrees Celsius, 1 hour incubation with Cy3-conjugated donkey anti-mouse (Jackson ImmunoResearch, West Grove, PA) at room temperature, and nuclear counterstain for 4’-6-diamidino-2-phenylindole (DAPI; Sigma). Cryosections were imaged using an inverted Zeiss Axio Observer microscope.
Statistics
Unless otherwise noted, statistical comparisons utilized the two-tailed Student’s t-test at α level 0.05.
Results
Cell characterization
Surface marker characterization matched well-documented profiles for MSCs/ASCs: all cells were negative for CD31, the MSCs negative for CD34, the ASCs weakly positive for CD34, and all cells were positive for both CD73 and CD90 (Supplemental Fig. S1, bottom).
Osteogenic response of MSCs and ASCs to PDGF-BB
After three weeks of culture, MSCs and ASCs were stained with Alizarin Red S and von Kossa for a qualitative assessment of mineralization. MSCs and ASCs stained negatively under both control (−) and control (+) conditions (Fig. 1, a-h), as no calcium phosphate source was present in these conditions. Under osteogenic (−) conditions, both cell types stained positively for mineralization (Fig. 1, i-l); the ASC group stained more intensely positive under osteogenic (+) conditions (Fig. 1, o-p). MSCs under osteogenic (+) conditions stained with similar intensity to MSCs under osteogenic (−) conditions (Fig. 1, m-n). Because PDGF-BB is a mitogen, we considered the possibility that the more intense staining with ASCs was simply due to the presence of more cells. To address this, calcium content was quantified and normalized to cell counts. ASCs under osteogenic (+) conditions displayed significantly higher calcium per cell than did ASCs under osteogenic (−) conditions, an observation that did not hold for MSCs; there was no difference in calcium per cell between MSCs under osteogenic (−) conditions versus MSCs under osteogenic (+) conditions (Fig. 1, bottom). In particular, this held for cells across all donors examined. Also of note, in all cases PDGF-BB acted as a mitogen, with osteogenic (+) groups displaying higher cell counts at the end of three weeks compared to cells under osteogenic (−) conditions; this held regardless of cell type and donor (Supplemental Fig. S1, top), confirming that the MSCs were able to respond to the PDGF-BB, just not in an osteogenic manner.
Figure 1.
MSC and ASC mineralization under the effect of exogenous PDGF-BB. MSCs and ASCs were cultured under control (−), control (+), osteogenic (−), and osteogenic (+) conditions for three weeks. Staining after three weeks of culture revealed no mineralization under either control condition (a-h) and an enhancement of mineralization under the presence of PDGF-BB in ASCs (o-p vs. k-l) but not in MSCs (m-n vs. i-j). Quantitative calcium per cell analysis revealed the increased mineralization was on a per-cell basis and the ASC-specific phenomenon was a donor-independent effect over six donors (bottom). Scalebar: 100 μm. *p<0.05.
To determine whether there were a genetic mechanism underlying this data, RT-PCR was performed at 1, 2, and 3 weeks of culture. Cells cultured under control (+) conditions, despite being unable to form mineral, displayed a genetic response: MSCs generally downregulated osteogenic genes compared to MSCs under control (−) conditions, whereas ASCs generally upregulated those genes (Fig. 2, left). With the addition of osteogenic factors, the same trend held, with ASC expression of the same genes upregulated under osteogenic (+) conditions compared to expression levels under osteogenic (−) conditions and the opposite true for MSCs (Fig. 2, right).
Figure 2.
Gene expression of MSCs and ASCs under the effect of exogenous PDGF-BB. Gene expression analysis of the osteogenic genes Runx2, osteocalcin, osteonectin, and collagen-I via real-time polymerase chain reaction showed that exogenous PDGF-BB under control (+) conditions tended to downregulate genes in MSCs while upregulating them in ASCs (left; normalized to expression under control medium conditions without PDGF-BB, variation shown by dotted lines). The same observations held when considering osteogenic conditions (right; normalized to expression under osteogenic medium conditions without PDGF-BB, variation shown by dotted lines). All expression quantities are relative to β-actin as housekeeping gene. Red asterisk denotes downregulation compared to (−) conditions while green asterisk denotes upregulation compared to (−) conditions, p<0.05.
Loss-of-function effect on MSC and ASC osteogenic response to PDGF-BB
The polymer screen indicated that both R646 and R647 were able to knock down GFP, with R646 slightly outperforming R647. Increasing siRNA concentration up to 40 nM enhanced knockdown, while increasing polymer concentration beyond 180 μg/mL either had no effect on or actually reduced the extent of knockdown. Both polymers displayed similar cytotoxicity levels at 24 hours post-transfection. Based on these results (Supplemental Fig. S2), polymer R646 at 180 μg/mL and siRNA concentration of 40 nM was selected for subsequent studies.
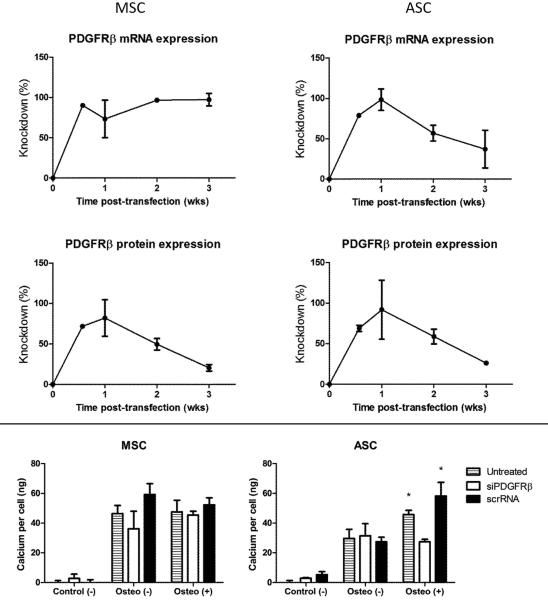
R646 knocked down PDGFRβ well, achieving a peak knockdown approaching 100% at 1 week post-transfection and declining afterwards to ~30% at 3 weeks post-transfection (Fig. 3, top). After three weeks of culture under control (−), osteogenic (−), and osteogenic (+) conditions (the control (+) group was omitted in this experiment since no mineralization occurred in the absence of osteogenic medium), cells were subjected to calcium per cell quantification to determine the effect of knocking down the receptor. MSCs produced similar levels of calcium per cell irrespective of the presence of PDGF-BB or whether cells were treated with siRNA, reinforcing the notion that PDGF-BB did not directly affect MSC mineralization (Fig. 3, bottom). In contrast, while the silenced ASCs showed no statistically significant difference between osteogenic (−) and osteogenic (+) groups, untreated ASCs and ASCs given scrRNA retained a statistically higher calcium per cell reading in the osteogenic (+) groups as compared to the osteogenic (−) groups via a two-way ANOVA with p<0.05 (Fig. 3, bottom) despite the knockdown being less pronounced in ASCs at the mRNA level at later time points. This loss-of-function data further supports the observations that ASCs upregulate calcium production on a per-cell basis when signaled with PDGF-BB, whereas MSCs do not.
Figure 3.
Loss-of-function experiment for the effect of exogenous PDGF-BB. siRNA against the receptor PDGFRβ was delivered to MSCs and ASCs using a reducible poly[β-amino ester] vehicle. Knockdown of receptor relative to a scrambled control was evident over three weeks via both real-time polymerase chain reaction and antibody-based flow cytometry (top). While MSC mineralization at three weeks post-transfection was unaffected irrespective of treatment or the presence of PDGF-BB, silenced ASCs lost the enhancement of mineralization under osteogenic (+) conditions in contrast to untreated ASCs or ASCs given the scrambled control. *p<0.05 compared to corresponding osteogenic (−) quantities via two-way ANOVA.
Transduction of PDGFB into ASCs and effect on murine calvarial defect
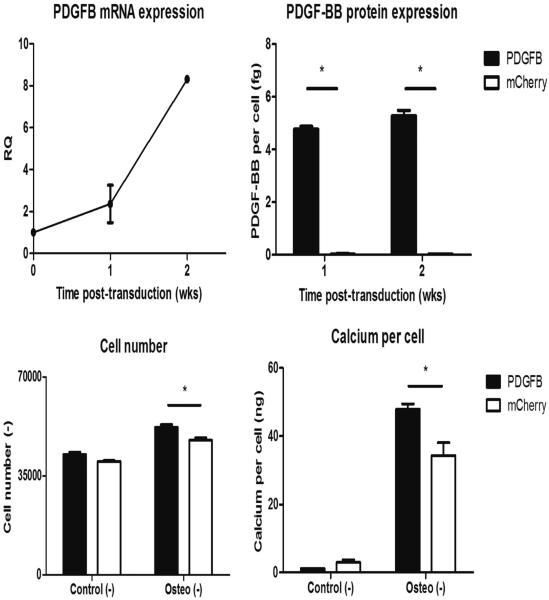
Both RT-PCR and PDGF-BB ELISA confirmed the efficacy of transduction, with the PDGFB mRNA and protein greatly upregulated compared to cells transduced with mCherry by 2 weeks post-transduction (Fig. 4, top). PDGFB-transduced cells also proliferated more under osteogenic conditions (Fig. 4, bottom left) and produced more calcium per cell (Fig. 4, bottom right) compared to mCherry-transduced cells, indicating the overexpressed PDGFB gene was having a functional effect on ASCs. PDGFB-transduced MSCs displayed similar increases in proliferation (Fig. S5, bottom left), but the transduction did not have an effect on MSC calcium per cell (Fig. S5, bottom right).
Figure 4.
Verification of lentiviral transduction. Lentivirus containing the gene PDGFB or mCherry was used to transduce ASCs. PDGFB-transduced ASCs overexpressed the gene and produced more protein compared to mCherry-transduced controls as determined by real-time polymerase chain reaction and enzyme-linked immunosorbent assay (top). The produced protein had a functional effect, increasing cell proliferation and calcium-per-cell content as evidenced by DNA and calcium assays (bottom). *p<0.05.
Transduced cells were encapsulated into fibrin gels and seeded into custom-printed scaffolds and implanted for eight weeks within the murine calvarial defect. CT imaging of murine calvariae at eight weeks post-implantation (Fig. 5, a-b) showed a significantly higher volume of regenerated bone within the PDGFB-transduced groups compared to both other groups via one-way ANOVA both when considering absolute bone volume (Fig. 5, c) or when normalizing bone volume to unoperated contralateral controls (Fig. 5, d). In particular, the higher mineral content was observed throughout the scaffolds (both outer and inner regions), strongly suggesting that transplanted ASCs themselves were being signaled by the elevated PDGF-BB concentrations to undergo osteogenesis.
Figure 5.
Computed tomography analysis of in vivo regeneration. Scaffolds seeded with mCherry-transduced ASCs (a, right defect), PDGFB-transduced ASCs (b, right defect), or empty fibrin (a-b, left defects) were implanted in critically sized 4-mm-diameter murine calvarial defects for eight weeks. Computed tomography reconstructions (a-b) were used for quantification of bone volume within the defect. In terms of both absolute bone volume (c) and bone volume normalized to unoperated values (d), the PDGFB-transduced ASCs produced significantly more bone volume beginning at a 2-mm radius within the defect compared to both other groups. *p<0.05 via one-way ANOVA.
To investigate this further, we tested whether human ASCs remained at the defect site. Excised scaffolds were sectioned and stained with human-specific Lamin A/C with DAPI counterstain for retention of human cells (Fig. 6, left), H&E for general scaffold cellularity and tissue formation (Fig. 6, middle; Fig. S3, b-c), and von Kossa/van Gieson for bone formation (Fig. 6, right). All scaffolds were populated with cells and matrix as evidenced by DAPI and H&E stains. While a small amount of von Kossa staining occurred in the mCherry-transduced group with some surrounding osteoid, there was much more mineralized tissue in the PDGFB-transduced group. Of particular note, positive human-specific staining was apparent in both mCherry-transduced and PDGFB-transduced groups, indicating the human cells were still present eight weeks post-implantation and were potentially contributing to the tissue formation within the scaffold. As a control, the acellular scaffolds, while showing DAPI staining, had no human-specific staining, indicating the resident cells were of murine origin.
Figure 6.
Histological analysis of in vivo regeneration. Eight weeks post-implantation, mice were sacrificed and scaffolds excised. Immunohistochemistry for human-specific Lamin A/C (left), Haematoxylin and Eosin (middle), and von Kossa/van Gieson (right) were performed to assess retention of human cells, scaffold cellularity, and bone formation, respectively. The majority of mineralization (von Kossa staining, black) occurred PDGFB-transduced group. The implanted human cells (human Lamin A/C, green) were retained within all scaffolds, with positive staining evident in all groups except for the acellular group, where no human cells were implanted. Scalebar: 200 μm.
Discussion
In the current study, MSCs and ASCs were compared directly in their osteogenic responses to PDGF-BB. The findings reported in the current study simultaneously confirm previous research showing that PDGF-BB is not directly osteoinductive on MSCs [13–17] while also confirming our more recent findings that PDGF-BB can directly enhance ASC osteogenesis [18, 27]. Both correlative (Fig. 1) and loss-of-function (Fig. 3) evidence support the observations that the divergent mineralization responses to PDGF-BB is marked. Since we have shown previously [18] that dexamethasone is not essential for mineralization of ASCs, we omitted it from the current study; however, due to the prevalence of dexamethasone in MSC osteogenic culture, we also compared the mineralization response of MSCs and ASCs to PDGF-BB in the presence of 100 nM dexamethasone. While the addition of dexamethasone resulted in an two-fold increase of calcium per cell for MSCs and three-fold increase of calcium per cell for ASCs (Supplemental Fig. S4), the mineralization of MSCs was still unaffected by the addition of PDGF-BB, while it was enhanced in ASCs. In particular, a key finding in this study is the differential response between MSCs and ASCs when examining the gene expression of osteogenic genes Runx2, OCN, and OSN (Fig. 2), indicating that the observed differences in mineralization arise from fundamental genetic differences in these two cell populations. Indeed, ASC expression of Runx2 and OCN was enhanced in the presence of PDGF-BB even in the absence of osteogenic factors, indicating that PDGF-BB itself is osteoinductive to ASCs but not to MSCs.
The impetus for hypothesizing a fundamental difference between MSCs and ASCs is not new and arises from subtleties observed in the literature. For example, the generally accepted surface marker profile for ASCs includes a weakly positive CD34 population [35, 36], while MSCs are traditionally reported to be negative for CD34 [3, 35], an observation supported by the present study (Supplemental Fig. S1, bottom). In addition, ASCs possess more proliferative potential than do MSCs [37]. Most importantly, there have been several studies demonstrating a difference in potency between MSCs and ASCs, with some groups suggesting an increased capacity for osteogenic differentiation of MSCs [38, 39] and a penchant for adipogenic differentiation in ASCs [39, 40]. Despite these observations, other studies have shown that by changing culture conditions (e.g. by the addition or subtraction of growth factors), differentiation potential can be modulated between the cell types [41], thus suggesting the innate biochemistries of MSCs and ASCs are different.
While differences in potency are not generally a subject of controversy, the mechanisms underlying these differences are still poorly understood. It has been shown that MSCs express a higher preponderance of genes associated with osteogenesis [38], while ASCs display higher expression of adipogenic genes [42]. The possibility of epigenetic mechanisms underlying differences in lineage-specific gene expression has been investigated, albeit not as extensively. For instance, osteo-specific genes such as osteoglycin and osteopontin have been shown to feature different levels of methylation in MSCs and ASCs [43, 44]. While delineating genetic and epigenetic mechanisms is outside the scope of the current study, the finding that gene expression of Runx2, OCN, and OSN differed between MSCs and ASCs with respect to the presence of PDGF-BB may be well-supported in this context. Taken together, previous studies into MSC and ASC stem cell biology and lineage potency provide ample motivation for rigorously delineating differences in response to growth factors.
The original impetus for investigating the role of PDGF-BB specifically in bone repair arises from its native presence in the fracture site [11, 12] and the clinical observation that injection of PDGF-BB accelerates bone regeneration [22]. Given previous results with MSCs, confirmed by the results of the current study, the idea that PDGF-BB in a fracture site enhances repair in an indirect fashion is well-supported. For instance, the role of PDGF-BB in recruiting vascular-stabilizing cells is well-studied: endothelial cells invading a region secrete PDGF-BB to attract pericytes that then wrap around the nascent vasculature, stabilizing the network [21, 45]. Given the observation that bone forms around a vascular template [12, 46–48], the vascular stabilization of PDGF-BB in a fracture site may be a possible mechanism for indirect enhancement of bone repair. Such indirect mechanisms would be the sole mechanisms in a TE approach using MSCs in conjunction with PDGF-BB; however, a TE approach using ASCs instead may take advantage of a second mechanism – that the PDGF-BB may directly enhance the osteogenesis of implanted ASCs while retaining its established vascular stabilizing properties. The potential for this additional mechanism underscores the importance of critically defining differences between cell populations such that a TE graft can take full advantage of both cellular and biomolecular components.
The clinical advantages of PDGF-BB itself are underscored when comparing to the current gold standard for growth factor-based bone regenerative therapies, bone morphogenic protein 2 (BMP2). BMP2 is known to be extremely osteoinductive [49, 50] and is approved for clinical use; however, achieving a clinical effect requires supraphysiological doses, on the order of milligrams [51, 52]. Such high doses result in high costs and numerous safety concerns [53]. We have shown here and previously [18] a robust enhancement of ASC mineralization in response to 20 ng/mL PDGF-BB, a concentration comparable to physiological levels within a fracture site [11, 54]. In particular, while ASCs cultured under osteogenic (−) conditions tended to produce less calcium per cell than did identically cultured MSCs, ASCs cultured under osteogenic (+) conditions not only produced more calcium per cell than did ASCs cultured under osteogenic (−) conditions, but also produced calcium levels at or above levels from MSCs cultured under osteogenic (−) conditions (Fig. 1 and Fig. 3, bottom). This observation held across the six donors investigated in this study, indicating a donor-independent phenomenon. Taken together, these considerations suggest that the use of PDGF-BB in clinical bone regenerative therapy in conjunction with ASCs may be an attractive option alongside more traditional approaches.
To illustrate the in vivo regenerative potential of TE constructs utilizing both ASCs and PDGF-BB, the murine calvarial defect model showed a marked difference in implanted ASCs overexpressing PDGF-BB compared to implanted ASCs without PDGF-BB. CT quantification of newly mineralized tissue was evidently higher from ASCs with PDGF-BB (Fig. 5) and positive von Kossa staining in the PDGFB-transduced groups confirmed this observation (Fig. 6). We considered the possibility that the regenerated bone was solely due to invading murine cells; however, the presence of human-specific staining within both PDGFB-transduced and mCherry-transduced groups (Fig. 6, left), the stark contrast of mineralized volume between the two, and the presence of mineralized tissue in the PDGFB-transduced scaffold center (Fig. 5, b and Fig. 6, right), suggests the implanted human cells directly contributed to increased bone regeneration. While there is previous data suggesting this [55], we have shown here that the contribution of implanted ASCs is greatly enhanced by the presence of PDGF-BB signaling. A more rigorous investigation on the exact contribution of implanted ASCs within an in vivo bone defect will be the subject of a future study.
Conclusion
Though MSCs can respond to PDGF-BB in a mitogenic manner, PDGF-BB does not directly induce mineralization of MSCs. In contrast, PDGF-BB directly enhances mineralization of ASCs. This difference suggests an increased efficacy for using ASCs in conjunction with PDGF-BB in TE-based approaches for bone repair and underscores the importance in delineating differences between stem cell types in their response to biomolecules.
Supplementary Material
Supplemental Figure S1: Characterization of cells used in this study. To ascertain that all cells could respond to exogenous PDGF-BB, cell proliferation was quantified by DNA assay at three weeks of culture under osteogenic (+) conditions (top). Indeed, all six cell populations irrespective of type and donor showed increased cell numbers relative to osteogenic (−) controls. Surface marker characterization of all six donors revealed profiles in good agreement with established features of mesenchymal stem cells (bottom; data reported as mean and standard error of mean computed from all three donors for a given cell type).
Supplemental Figure S2: Nanoparticle screen for toxicity and knockdown. Polymer R646 and R647 were used to deliver siRNA against green fluorescent protein (GFP) in MSCs transduced with GFP. Polymer concentration in mg/mL and siRNA concentration in nM were varied. Toxicity via MTS assay was quantified relative to untreated controls at 24 hours post-transfection (left). Knockdown of GFP relative to scrambled controls was tracked for over three weeks via fluorescence readings on a plate reader (right). Toxicity was more pronounced in R647 and increased with both polymers as polymer concentration increased, while knockdown was either diminished or unaffected with increasing polymer concentration. No additional knockdown was observed by increasing siRNA concentration beyond 40 nM. With these observations, polymer R646 at 0.18 mg/mL with siRNA concentration of 40 nM was chosen for subsequent experiments.
Supplemental Figure S3: Low-magnification overview of haematoxylin & eosin stains. Scaffolds 4 mm in diameter consistently constructed with 4 center pores were imaged at the 4 center pores (a, green). Nodules of dense bone are visible in the PDGFB-transduced group (b) but not in the mCherry-transduced group (c). Scalebar: 500 μm.
Supplemental Figure S4: Effect of dexamethasone on MSC and ASC osteogenesis. To confirm that PDGF-BB enhances ASC, but not MSC, osteogenesis regardless of the presence of dexamethasone, 100 nM dexamethasone was added to all osteogenic medium conditions for both cell types and the calcium/cell experiment repeated for three weeks. Dexamethasone greatly increased calcium per cell for both MSCs and ASCs, but the PDGF-BB-dependent enhancement of mineralization remained specific to ASCs.
Supplemental Figure S5: Lentiviral transduction on MSCs. Similar to studies with ASCs (shown in Figure 4), MSCs were transduced with PDGFB or mCherry. Transduction was verified by increased mRNA and protein expression (top) as well by a mitogenic response (bottom left); however, the transduction had no effect on MSC calcium production per cell (bottom right). *p<0.05.
Significance Statement.
The findings of this study demonstrate that adipose-derived stem cells (ASCs) exhibit a fundamentally different osteogenic response to platelet-derived growth factor (PDGF) signaling from bone marrow-derived mesenchymal stem cells (MSCs). In the presence of physiological concentrations of PDGF-BB (20 ng/ml), ASCs increase the expression of osteogenic genes and, in the presence of a phosphate source, significantly upregulate mineral deposition. MSCs do not show a corresponding increase in either mineral deposition or gene expression. This finding suggests that while the two cell types exhibit significant similarities, they may possess intrinsically different biochemistries.
Acknowledgments
The authors thank Justin Morrissette-McAlmon for his technical assistance with histology. This project was funded by grants from the Johns Hopkins University Center for Musculoskeletal Research, the American Society for Bone and Moneral Research (2013CEA13), the NSF CAREER award (1350554), and the Maryland Stem Cell Research Fund (2014-MSCRFI-0699). AIC also thanks the E. Virginia and David Baldwin Research Fund for grant support. This work was also supported in part by the NIH (1R01EB016721). BPH thanks the NIH Ruth L Kirschtein National Research Service Award for fellowship support. KLK thanks the NIH Cancer Nanotechnology Training Center (R25CA153952) at the JHU Institute for Nanobiotechnology for fellowship support and CJB thanks the National Science Foundation for fellowship support.
Footnotes
Author contributions
BPH and DLH: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
KLK, CJB, and BN: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript
All other authors: conception and design, administrative support, data analysis and interpretation, final approval of manuscript
Disclosure of Potential Conflicts of interest
JMG is the co-founder, co-owner, and Chief Scientific Officer of LaCell LLC, a biotechnology company focusing on the clinical translation of stromal/stem cell science.
References
- 1.Desai BM. Osteobiologics. Am J Orthop. 2007;36:8–11. [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 5.Gangji V, Hauzeur JP, Matos C, et al. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–1160. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Liebergall M, Schroeder J, Mosheiff R, et al. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther. 2013;21:1631–1638. doi: 10.1038/mt.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart R, Komzak M, Okal F, et al. Allograft alone versus allograft with bone marrow concentrate for the healing of the instrumented posterolateral lumbar fusion. Spine J. 2014;14:1318–1324. doi: 10.1016/j.spinee.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strioga M, Viswanathan S, Darinskas A, et al. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21:2724–2752. doi: 10.1089/scd.2011.0722. [DOI] [PubMed] [Google Scholar]
- 11.Pountos I, Georgouli T, Henshaw K, et al. Release of growth factors and the effect of age, sex, and severity of injury after long bone fracture. A preliminary report. Acta Orthopaedica. 2013;84:65–70. doi: 10.3109/17453674.2013.765624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29:1795–1803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Hsieh SC, Bao W, Graves DT. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol. 1997;272:C1709–1716. doi: 10.1152/ajpcell.1997.272.5.C1709. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Kim KH, Shin SY, et al. Dual delivery of rhPDGF-BB and bone marrow mesenchymal stromal cells expressing the BMP2 gene enhance bone formation in a critical-sized defect model. Tissue Eng. 2013;19:2495–2505. doi: 10.1089/ten.tea.2012.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kratchmarova I, Blagoev B, Haack-Sorensen M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
- 16.Gruber R, Karreth F, Kandler B, et al. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets. 2004;15:29–35. doi: 10.1080/09537100310001643999. [DOI] [PubMed] [Google Scholar]
- 17.Tokunaga A, Oya T, Ishii Y, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519–1528. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 18.Hutton DL, Moore EM, Gimble JM, Grayson WL. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng. 2013;19:2076–2086. doi: 10.1089/ten.tea.2012.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vila OF, Martino MM, Nebuloni L, et al. Bioluminescent and micro-computed tomography imaging of bone repair induced by fibrin-binding growth factors. Acta Biomater. 2014;10:4377–4389. doi: 10.1016/j.actbio.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Hollinger JO, Hart CE, Hirsch SN, et al. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 21.Gehmert S, Hidayat M, Sultan M, et al. Angiogenesis: the role of PDGF-BB on adipose-tissue derived stem cells (ASCs) Clin Hemorheol Microcirc. 2011;48:5–13. doi: 10.3233/CH-2011-1397. [DOI] [PubMed] [Google Scholar]
- 22.Graham S, Leonidou A, Lester M, et al. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs. 2009;18:1633–1654. doi: 10.1517/13543780903241607. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 24.Worster AA, Nixon AJ, Brower-Toland BD, Williams J. Effect of transforming growth factor beta1 on chondrogenic differentiation of cultured equine mesenchymal stem cells. Am J Vet Res. 2000;61:1003–1010. doi: 10.2460/ajvr.2000.61.1003. [DOI] [PubMed] [Google Scholar]
- 25.Hung BP, Babalola OM, Bonassar LJ. Quantitative characterization of mesenchymal stem cell adhesion to the articular cartilage surface. J Biomed Mater Res. 2013;101:3592–3598. doi: 10.1002/jbm.a.34647. [DOI] [PubMed] [Google Scholar]
- 26.Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutton DL, Kondragunta R, Moore EM, et al. Tumor Necrosis Factor improves vascularization in osteogenic grafts engineered with human adipose-derived stem/stromal cells. PLoS One. 2014;9:e107199. doi: 10.1371/journal.pone.0107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozielski KL, Tzeng SY, Green JJ. A bioreducible linear poly(beta-amino ester) for siRNA delivery. Chem Commun. 2013;49:5319–5321. doi: 10.1039/c3cc40718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozielski KL, Tzeng SY, De Mendoza BAH, Green JJ. Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano. 2014;8:3232–3241. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33:8142–8151. doi: 10.1016/j.biomaterials.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X, Boehm JS, Salehi-Ashtiani K, et al. A public genome-scale lentiviral expression library of human ORFs. Nat Methods. 2011;8:659–661. doi: 10.1038/nmeth.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temple JP, Hutton DL, Hung BP, et al. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res. 2014 doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 33.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 34.Gupta DM, Kwan MD, Slater BJ, et al. Applications of an athymic nude mouse model of nonhealing critical-sized calvarial defects. J Craniofac Surg. 2008;19:192–197. doi: 10.1097/scs.0b013e31815c93b7. [DOI] [PubMed] [Google Scholar]
- 35.Mosna F, Sensebe L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. 2010;19:1449–1470. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 36.Gronthos S, Franklin DM, Leddy HA, et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 37.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 38.Noel D, Caton D, Roche S, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues — Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 40.Pachon-Pena G, Yu G, Tucker A, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: greater doses of growth factor are necessary. J Orthop Res. 2009;27:612–619. doi: 10.1002/jor.20766. [DOI] [PubMed] [Google Scholar]
- 42.Liu TM, Martina M, Hutmacher DW, et al. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 43.Boquest AC, Noer A, Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2006;2:319–329. doi: 10.1007/BF02698059. [DOI] [PubMed] [Google Scholar]
- 44.Arnsdorf EJ, Tummala P, Castillo AB, et al. The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomech. 2010;43:2881–2886. doi: 10.1016/j.jbiomech.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindahl P, Hellstrom M, Kalen M, Betsholtz C. Endothelial-perivascular cell signaling in vascular development: lessons from knockout mice. Curr Opin Lipidol. 1998;9:407–411. doi: 10.1097/00041433-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discovery Today. 2003;8:980–989. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 49.Zegzula HD, Buck DC, Brekke J, et al. Bone formation with use of rhBMP-2 — (recombinant human bone morphogenetic protein-2) J Bone Joint Surg Am. 1997;79A:1778–1790. doi: 10.2106/00004623-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 51.Triplett RG, Nevins M, Marx RE, et al. Pivotal, randomized, parallel evaluation of recombinant human Bone Morphogenetic Protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67:1947–1960. doi: 10.1016/j.joms.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 52.Howell TH, Fiorellini J, Jones A, et al. A feasibility study evaluating rhBMP-2 absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997;17:125. [PubMed] [Google Scholar]
- 53.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 54.Giannoudis PV, Pountos I, Morley J, et al. Growth factor release following femoral nailing. Bone. 2008;42:751–757. doi: 10.1016/j.bone.2007.12.219. [DOI] [PubMed] [Google Scholar]
- 55.Allay JA, Dennis JE, Haynesworth SE, et al. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997;8:1417–1427. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Characterization of cells used in this study. To ascertain that all cells could respond to exogenous PDGF-BB, cell proliferation was quantified by DNA assay at three weeks of culture under osteogenic (+) conditions (top). Indeed, all six cell populations irrespective of type and donor showed increased cell numbers relative to osteogenic (−) controls. Surface marker characterization of all six donors revealed profiles in good agreement with established features of mesenchymal stem cells (bottom; data reported as mean and standard error of mean computed from all three donors for a given cell type).
Supplemental Figure S2: Nanoparticle screen for toxicity and knockdown. Polymer R646 and R647 were used to deliver siRNA against green fluorescent protein (GFP) in MSCs transduced with GFP. Polymer concentration in mg/mL and siRNA concentration in nM were varied. Toxicity via MTS assay was quantified relative to untreated controls at 24 hours post-transfection (left). Knockdown of GFP relative to scrambled controls was tracked for over three weeks via fluorescence readings on a plate reader (right). Toxicity was more pronounced in R647 and increased with both polymers as polymer concentration increased, while knockdown was either diminished or unaffected with increasing polymer concentration. No additional knockdown was observed by increasing siRNA concentration beyond 40 nM. With these observations, polymer R646 at 0.18 mg/mL with siRNA concentration of 40 nM was chosen for subsequent experiments.
Supplemental Figure S3: Low-magnification overview of haematoxylin & eosin stains. Scaffolds 4 mm in diameter consistently constructed with 4 center pores were imaged at the 4 center pores (a, green). Nodules of dense bone are visible in the PDGFB-transduced group (b) but not in the mCherry-transduced group (c). Scalebar: 500 μm.
Supplemental Figure S4: Effect of dexamethasone on MSC and ASC osteogenesis. To confirm that PDGF-BB enhances ASC, but not MSC, osteogenesis regardless of the presence of dexamethasone, 100 nM dexamethasone was added to all osteogenic medium conditions for both cell types and the calcium/cell experiment repeated for three weeks. Dexamethasone greatly increased calcium per cell for both MSCs and ASCs, but the PDGF-BB-dependent enhancement of mineralization remained specific to ASCs.
Supplemental Figure S5: Lentiviral transduction on MSCs. Similar to studies with ASCs (shown in Figure 4), MSCs were transduced with PDGFB or mCherry. Transduction was verified by increased mRNA and protein expression (top) as well by a mitogenic response (bottom left); however, the transduction had no effect on MSC calcium production per cell (bottom right). *p<0.05.