Abstract
Background
Cystic fibrosis (CF) is characterized by airways infection and inflammation resulting in respiratory complications including hemoptysis. The objectives of this study were to characterize risk of hemoptysis attributable to the underlying disease and in the presence of standard of care therapy.
Methods
This retrospective cohort study estimated hemoptysis rates overall and by relevant risk factors utilizing adverse event data from longitudinal prospective CF clinical trials.
Results
Of the 1008 participants, 73% were ≤18 years old; of 929 with available spirometry, 27% had an FEV1 < 70% predicted. During the average 8.2 months of follow-up, 8% experienced ≥1 hemoptysis events (95% CI: 6%, 10%). Of the 125 events, 76% were mild in severity and only 9% were serious. Hemoptysis rates were greater among adults than children, those with FEV1 < 70% predicted, and participants infected with P. aeruginosa but not with S. aureus.
Conclusions
Hemoptysis is a common adverse event among CF clinical trial participants, and particularly in adults with more severe lung disease. These results can be used to predict event occurrence in future clinical trials.
Keywords: cystic fibrosis, clinical trial, hemoptysis
Introduction
Cystic fibrosis (CF) is a genetic condition associated with deterioration of lung function due to impaired clearance of airway mucus, resulting in chronic infection and inflammation.[1] The natural history of the lung disease is marked by an exaggerated inflammatory response [2, 3] causing injury to the airways and progressive airways obstruction. There are acute complications attributable to the airways infection and inflammation, including hemoptysis, which can range from minor streaking of blood in the sputum to massive bleeding that can be life-threatening.[4] The pathogenesis of hemoptysis is multi-factorial [5], although there are risk factors shown to be associated with massive hemoptysis, such as older age, more severe pulmonary impairment, and the presence of Staphylococcus aureus in sputum cultures.[6]
There are published guidelines on the management of hemoptysis in individuals with CF.[4] In the setting of massive hemoptysis, it is recommended to withhold inhaled medications under the assumption they could aggravate hemoptysis. It is understandable how one might attribute hemoptysis events to an inhaled medication if the event occurred temporally to its use. However, tobramycin and dornase alfa are aerosolized medications currently administered as a part of standard of care and neither of which have been identified as risk factors for hemoptysis; rather they have been associated with a lower incidence of massive hemoptysis.[6] Although hemoptysis is thought to be a common event, there are few published studies reporting rates of hemoptysis in the CF population. The CF Patient Registry has long tracked the occurrence of massive hemoptysis with an annual incidence of approximately 4% [6] but it has not tracked all hemoptysis events. In a retrospective study of 440 Israeli individuals with CF, there was an overall 9.1% incidence of hemoptysis events recorded in the medical charts during a 5-year observation period; 25% of these participants were younger than 13 years of age.[7] A few clinical trials have reported the prevalence of hemoptysis in the placebo-treated participants (tobramycin inhaled solution TIS: 31% over 6 months [8], dornase alfa: 21% over 6 months [9], and ivacaftor: 22% over 12 months[10]).
Another analysis of the placebo-treated participants in clinical trials reported that hemoptysis events were rare in both children and adults in short-term trials (0–1 weeks) but not uncommon in long term trials (1–6 months), with adults nearly four times more likely to experience hemoptysis than children.[11] However, none of these analyses have assessed risk factors, other than age, that could further elucidate the expected rate of hemoptysis in the CF population. While it is realistic to anticipate that the rates increase in older patients with more advanced lung disease, being able to estimate expected occurrence of hemoptysis based on risk factors is of great value for assessing risk in future drug development.
Because hemoptysis is an expected adverse event in CF clinical trials, understanding the anticipated rates of hemoptysis due to the underlying disease and in the presence of standard of care is important for assessing safety of new therapies in CF clinical trials, particularly for the pediatric population in whom the even rate is not well established. Also as CF therapies have evolved over the last decade with the introduction of more therapies to standard of care, there is a need to evaluate the incidence of hemoptysis using data from a more contemporary cohort than has previously been studied. Utilizing a diverse cohort of children and adults with CF who underwent comprehensive longitudinal monitoring for adverse events, the objectives of this study were (1) to obtain estimates of the frequency and rate of hemoptysis events in the presence of standard of care therapy, and (2) to characterize hemoptysis rates by possible risk factors include age, disease severity as defined by lung function, and microbiologic results.
Methods
Study Design
Recently completed placebo controlled trials were selected for this retrospective cohort study. It utilized existing data from eight completed prospective, longitudinal randomized trials in CF ranging from 2 to 18 months duration between 2000 and 2012. Trial therapies and key eligibility criteria, along with trial duration, visits, and key clinical and safety outcomes are summarized in the Online Supplement (Table E1). For 5 of 8 [12–16] trials, only subjects randomized to placebo were included in the analyses. Pooled data from the treatment and placebo arms were included for 2 trials of azithromycin which led to the adoption of azithromycin into standard of care [17–19], and because comparable hemoptysis rates were observed between treatment arms. The final trial included in our study for treatment of new onset Pseudomonas aeruginosa [20] included all participants receiving tobramycin inhalation solution (TIS) monotherapy and not in combination with oral ciprofloxacin. This analysis was approved by the Institutional Review Board at Seattle Children’s Hospital, Seattle, Washington.
All trials collected adverse event (AE) data, which was standardized using MedDRA or COSTART coding. Follow-up time used for computation of event rates was defined as the date of randomization or the date of first dose of study drug (denoted “baseline”) through the end of follow-up. Adverse event severity grades and treatment outcomes were collected in all trials (Online Supplement, Table E1). Treatment outcomes of the AEs were reported differently across trials; thus, for this study a hemoptysis event was considered treated if any of the following treatment options were selected on the trial case report forms: concomitant medications, non-drug therapies, concomitant medications in combination with non-drug therapies, or hospitalization.
Baseline FEV1 % predicted was calculated for subjects ≥6 years using either the Wang [21] (females ≤16, males ≤18 years old) or Hankinson [22] (females >16, males >18 years old) equations. Three of the eight trials did not collect respiratory microbiology cultures or history of infection at baseline (Online Supplement, Table E1) and were therefore excluded from analyses of the association between microbiology parameters and hemoptysis.
Statistical Analyses
Demographic and baseline characteristics were descriptively summarized by trial and overall. Negative binomial regression models adjusted for follow-up months were used to compute hemoptysis rates and corresponding 95% confidence intervals, as well as to compute rate ratios comparing event rates by age group and disease severity. Sensitivity analyses were performed where appropriate by adjusting for additional covariates. SAS version 9.2 (SAS Institute, Cary, NC) and R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
Results
Baseline Characteristics
A total of 1008 subjects were included in the study cohort, with the majority (73%) of the cohort 18 years of age or younger, and a mean age of 16.1 years (SD = 10.5). Table 1 shows baseline demographic and clinical characteristics for the cohort. While the gender representation was well balanced overall, there were more males (59%) in the adult (>18 years) group. The pediatric cohort (≤18) was concentrated in the 7–12 age group (34% overall) and 13–18 age group (26%), with a more sparse representation of children 6 years old and younger (13%). Among those with available baseline spirometry (92%), the baseline mean FEV1 was 83% of predicted (SD = 24) overall (91% and 64% among pediatric and adult cohorts, respectively). Because this is a young cohort, the majority (73%) of the participants had FEV1 ≥ 70%. Approximately two-thirds of the pediatric and one-third of the adult participants had baseline microbiology results. Among those, P. aeruginosa infection was more predominant in adults (96%) than in children (50%), whereas S. aureus was more common in children (65%) than in adults (52%).
Table 1.
Demographic Characteristics
| ≤ 18 y.o. (N=733) | > 18 y.o. (N=275) | Total (N=1008) | |
|---|---|---|---|
| n (%) | |||
|
| |||
| Sex (Female) | 352 (48.0) | 113 (41.1) | 465 (46.1) |
| Race | |||
| Caucasian | 671 (91.5) | 227 (82.5) | 898 (89.1) |
| Hispanic | 11 (1.5) | 1 (0.4) | 12 (1.2) |
| African-American | 15 (2.0) | 7 (2.5) | 22 (2.2) |
| Unknown/Other | 36 (4.9) | 40 (14.5) | 76 (7.5) |
| Genotypea | |||
| Delta F508 Homozygous | 327 (44.6) | 104 (37.8) | 431 (42.8) |
| Delta F508 Heterozygous | 271 (37.0) | 99 (36.0) | 370 (36.7) |
| Other | 61 (8.3) | 30 (10.9) | 91 (9.0) |
| Not Done | 74 (10.1) | 42 (15.3) | 116 (11.5) |
| Age Group | |||
| 0– ≤6 | 133 (18.1) | 0 (0) | 133 (13.2) |
| 7–≤12 | 342 (46.7) | 0 (0) | 342 (33.9) |
| 13–≤18 | 258 (35.2) | 0 (0) | 258 (25.6) |
| >18 | 0 (0) | 275 (100) | 275 (27.3) |
| FEV1 % Predicted Distributionb | |||
| <40% | 17 (2.6) | 39 (14.2) | 56 (6.0) |
| 40% – <70% | 79 (12.1) | 118 (42.9) | 197 (21.2) |
| 70% – <90% | 163 (24.9) | 93 (33.8) | 256 (27.6) |
| ≥90% | 395 (60.4) | 25 (9.1) | 420 (45.2) |
| Positive for Pac | 262 (49.6) | 94 (95.9) | 356 (56.9) |
| Positive for S. aureusd | 343 (65.1) | 50 (51.5) | 939 (63.0) |
|
| |||
| Mean (SD) | |||
|
| |||
| Age (years) | 11.0 (4.4) | 29.9 (9.6) | 16.1 (10.5) |
| FEV1 % Predictedb | 91.2 (20.4) | 64.2 (20.1) | 83.2 (23.8) |
| FEV1 (L) | 2.1 (0.8) | 2.4 (0.9) | 2.2 (0.9) |
| Height (cm) | 138.8 (24.2) | 168.2 (9.0) | 146.9 (24.9) |
| Weight (kg) | 36.6 (16.3) | 63.2 (12.2) | 43.9 (19.3) |
| Follow-up from Randomization (months) | 9.1 (4.6) | 5.8 (2.4) | 8.2 (4.4) |
Other refers to participants with at least 1 known, non-Delta F508 CF mutation.
FEV1 % predicted was available for 654 pediatric participants and 275 adults.
P. aeruginosa culture result was available for 528 pediatric participants and 98 for adults.
S. aureus culture result was available for 527 pediatric participants and 97 for adults.
The mean follow-up time of the cohort was 8.2 months with pediatric participants followed slightly longer for an average of 9.1 months, while adults were followed for an average of 5.8 months. Detailed baseline demographics by trial can be found in the Online Supplement (Online Supplement, Table E2).
Hemoptysis Rates by Age
Fifty-four (20%) of the 275 adults and 26 (4%) of the 733 children experienced at least one hemoptysis event during the follow-up period, for a combined incidence of 8% of all participants in the cohort. The overall event rate was 0.019 per month (95% confidence interval [CI]: 0.015, 0.026). The rates were 0.057 (95% CI: 0.042, 0.077) and 0.007 (95% CI: 0.004, 0.012) per month for adults (>18) and all children (≤18), respectively (Table 2). As anticipated, adult participants were more likely to experience a hemoptysis event as compared to children (Rate Ratio [RR] 9.44, 95% CI: 5.65, 15.96, p<0.001), even after adjusting for and FEV1 category (RR 4.92, 95% CI: 2.82, 8.65, p<0.001) (Online Supplement, Table E3). A sensitivity analysis was performed by also adjusting for P. aeruginosa status among participants with culture status available. Further, because prevalence of P. aeruginosa is known to increase with age [23], another sensitivity analysis was performed where adult participants with missing culture results were presumed to be positive for P. aeruginosa (Online Supplement, Table E3).
Table 2.
Hemoptysis Rates (Events per Month) by Age and Disease Severity
| Participantsa | Events | |||||
|---|---|---|---|---|---|---|
| Age | N | n (%) | n | Rate (95% CI) | Rate Ratiob (95% CI) | p-value |
| ≤ 18 years oldc | 733 | 26 (4) | 36 | 0.007 (0.004, 0.012) | ||
| > 18 years old | 275 | 54 (20) | 59 | 0.057 (0.042, 0.077) | 9.44 (5.65, 15.96) | <0.001 |
|
| ||||||
| FEV1 ≥ 70% | 676 | 27 (4) | 45 | 0.010 (0.006, 0.015) | ||
| FEV1 < 70% | 253 | 42 (17) | 80 | 0.054 (0.038, 0.076) | 5.89 (3.46, 10.16) | <0.001 |
|
| ||||||
| Pa − | 270 | 6 (2) | 7 | 0.003 (0.001, 0.007) | ||
| Pa + | 356 | 44 (12) | 78 | 0.035 (0.009, 0.030) | 11.23 (4.41, 31.80) | <0.001 |
|
| ||||||
| S. aureus − | 231 | 26 (11) | 45 | 0.030 (0.018, 0.054) | ||
| S. aureus + | 393 | 24 (6) | 40 | 0.016 (0.009, 0.029) | 0.48 (0.21, 1.07) | 0.072 |
|
| ||||||
| Total | 1008 | 80 (8) | 125 | 0.019 (0.015, 0.026) | ||
Participants with at least one hemoptysis event.
Rates, rate ratios, 95% confidence intervals, and corresponding p-values are estimated using negative binomial regression adjusting for follow-up time.
In the pediatric cohort, there were 1 (1%), 8 (2%), and 17 (7%) participants with at least one hemoptysis event in the 0–6, 7–12, and 13–18 age groups, respectively. The rates were 0.001 (95% CI: 0.000, 0.014), 0.003 (95% CI: 0.001, 0.006), and 0.016 (95% CI: 0.009, 0.030) events per month in these age groups. The combined rate in 7–18 age group was 0.009 per month (95% CI: 0.005, 0.014).
Hemoptysis Rates by Disease Severity
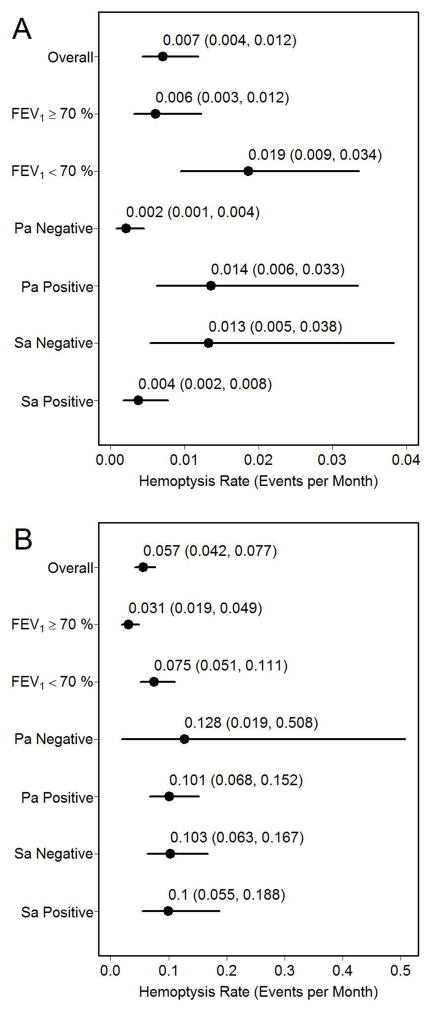
Hemoptysis rates were higher among participants with FEV1 < 70% predicted as compared to those with FEV1 ≥ 70% predicted (RR 5.89, 95% CI: 3.46, 10.16, p<0.001) (Table 2). A higher rate of hemoptysis was associated with presence of a P. aeruginosa positive culture (RR 11.23, 95% CI: 4.41, 31.80, p<0.001), but not associated with S. aureus positivity (RR 0.48, 95% CI: 0.21, 1.07, p=0.072). In the pediatric subgroup, the rates were lowest among P. aeruginosa negative and S. aureus positive participants, and among those with FEV1 ≥ 70% predicted (Figure 1A). In the adult subgroup, participants with FEV1 ≥ 70% predicted experienced the lowest hemoptysis rates (Figure 1B).
Figure 1. Hemoptysis Rates by Disease Severity.
Hemoptysis rates (events per month) by disease severity for participants ≤18 years of age (A) and >18 years of age (B).
Severity of Hemoptysis Events
Of the 125 hemoptysis events, the majority (76%) were deemed to be mild in severity (Table 3). There were a total of 11 (9%) adverse events classified as serious: 6 of 36 (17%) of hemoptysis events in the pediatric subgroup, and 5 of 89 (6%) in the adult subgroup. Participants with FEV1 < 70% predicted experienced a slightly higher proportion of moderate and severe events (22 of 80 events [28%]) than those with FEV1 ≥ 70% predicted (8 of 45 events [18%]) (Table 3). Hemoptysis events were more frequently treated in children than in adults (47% of the 36 events in the pediatric subgroup were treated and 25% of the 89 events in the adult subgroup). Hemoptysis events were treated more frequently in participants with FEV1 ≥ 70% predicted (17 of 45 events [38%]) than in those with FEV1 < 70% predicted (22 of 80 events [28%]).
Table 3.
Hemoptysis Event Severity Grading and Treatment Outcomes
| ≤ 18 y.o. Nevents = 36 |
> 18 y.o. Nevents = 89 |
FEV1 ≥ 70% Nevents = 45 |
FEV1 < 70% Nevents = 80 |
Total Nevents = 125 |
||
|---|---|---|---|---|---|---|
|
| ||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| None/Milda | 25 (69) | 70 (79) | 37 (82) | 58 (72) | 95 (76) | |
| Moderate | 9 (25) | 17 (19) | 6 (13) | 20 (25) | 26 (21) | |
| Severe | 2 (6) | 2 (2) | 2 (4) | 2 (2) | 4 (3) | |
|
| ||||||
| Non-Serious | 30 (83) | 84 (94) | 42 (93) | 72 (90) | 114 (91) | |
| Seriousb | 6 (17) | 5 (6) | 3 (7) | 8 (10) | 11 (9) | |
|
| ||||||
| Not Treated | 19 (53) | 67 (75) | 28 (62) | 58 (72) | 86 (69) | |
| Treatedc | 17 (47) | 22 (25) | 17 (38) | 22 (28) | 39 (31) | |
While severity grade definitions vary somewhat across study protocols, an event is considered “mild” if signs and symptoms are easily tolerated and require no intervention; “moderate” if discomfort is enough to cause interference with usual activity and may require minimal medical intervention; “severe” if there is marked limitation in activity and medical intervention is required; “life-threatening” if there is an immediate risk of death.
A serious adverse event is any event that is fatal, life-threatening, significantly or permanently disabling, requires or prolongs hospitalization, is a congenital anomaly/birth defect, or other important medical event that may require an intervention to prevent any of the aforementioned outcomes.
A hemoptysis event is considered treated if any of the following were selected on the trial adverse event CRF: concomitant medication, non-drug therapies, concomitant medications and non-drug therapies, or hospitalization.
Discussion
These data confirm that hemoptysis is a common complication of CF, occurring even in younger and healthier individuals. Factors associated with an increased risk of hemoptysis include older age, more advanced lung disease, and the presence of P. aeruginosa in the airways. Although these conclusions may seem obvious, there is now a greater understanding of and an ability to thoroughly quantify the natural history of CF disease with respect to hemoptysis than has previously been done. Regulatory agencies need robust natural history data for which to compare adverse event data during a trial when reviewing a new drug application. This study, albeit more descriptive than confirmatory for testing novel hypotheses, fills an important gap in the literature that can serve as a resource for future intervention trials.
Our study indicates that hemoptysis is a common adverse event captured in CF clinical trials, and in many cases this complication may not be related to initiation of a new intervention. The rates and confidence intervals summarized in this paper can be used to derive expected number of hemoptysis events in a clinical trial setting, given that the population in question has similar characteristics to the cohort described here. For example, for a trial of 100 participants with CF followed for 6 months, one would expect an average of 4 hemoptysis events in a pediatric population (95% CI 2, 7) and 34 events in an adult population (95% CI 25, 46) attributable to the natural history of the disease (Table 4). Note that the number of hemoptysis events is likely greater than the expected number of participants in the adult group, as 20% of adult participants had hemoptysis in our study as compared to 4% of the pediatric participants.
Table 4.
Hypothetical Trial Scenarios and Corresponding Hemoptysis Rates
| Trial Participants | N | Follow-up | Expected Number of Events (95% CI) |
|---|---|---|---|
| CF participants, children or adults | 100 | 6 months | Children: 4 (2, 7) Adults: 34 (25, 46) |
| Pa positive CF participants, children or adults | 130 | 6 months | Children: 11 (5, 26) Adults: 79 (53, 119) |
| CF participants with baseline FEV1 < 70% predicted, children or adults | 100 | 6 months | Children: 11 (5, 20) Adults: 45 (31, 67) |
We can compare these findings to results reported in placebo arms of clinical trials of TIS [8], dornase alfa [9], and ivacaftor.[10] The cohort in the TIS study included 49% <18 years of age, and all patients were required to have Pseudomonas present in cultures.[8] Using the event rate based on age and the presence of Pseudomonas, we would predict there would be 92 events over the 6 month duration of the trial. Hemoptysis was reported to occur in 31% of the patients treated with placebo (81 patients).[8] The cohort in the dornase alfa study had 52% <17 years of age.[9] Using the event rate based on age alone, we would predict there would be 61 events over the 6 month duration of the trial. The reported hemoptysis rate was 21%, or approximately 68 of the patients treated with placebo.[9] Since it would also be expected that some patients may have had more than one event during the course of the study (see above), it is highly likely that the number of events (not reported) in these two trials was greater than our predicted event rate. This is most likely because the patients in these trials were different that those included in our more contemporary analysis. The average FEV1 for the TIS study was 51.2% of predicted and for the dornase study the mean FEV1 was 61% of predicted suggesting they had more advanced lung disease than our contemporary cohort. More recently, the cohort in the ivacaftor study had only 22% <18 years of age and the mean FEV1 of 63.7% of predicted.[10] This degree of lung impairment is similar to the patients used in our analysis given the high number of adult patients. Again using the event rate based on age alone, we would predict there would be 43 events over the 12 month duration of the trial. The study reported that 22% (17 patients) had a hemoptysis event. Although it would seem that we have overestimated the event rate, we again note that the ivacaftor paper did not report the number of events, which are presumed to be greater than the number of patients with hemoptysis, but it could be concluded from their analysis that hemoptysis was not an event that could be attributed to the study drug since the actual events did not exceed the predicted.
The other interesting observation is the reduced likelihood of hemoptysis in the presence of S. aureus, although this finding was not statistically significant. This is in contrast to an analysis of the CF National Patient Registry, where S. aureus was associated with massive hemoptysis.[6] We do not find these observations to be contradictory. Firstly, the patients enrolled in clinical trials are subject to specific exclusion criteria, which typically include recent episodes of hemoptysis, perhaps reducing the likelihood of recruiting patients who may have massive hemoptysis. This may create some selection bias, but these are also the patients who are likely to be enrolled in clinical trials, which is the intention of the analysis. Secondly, the prevalence of Pseudomonas is greater in older patients while there may be a greater preponderance of S. aureus in younger patients [23], and these factors may have a greater impact on the relative risk of hemoptysis.
There are some important limitations pertinent to our study. Eligibility criteria from the included trials dictated our trial cohort and many trials excluded participants with either an acute pulmonary exacerbation or history of hemoptysis. These exclusions may mean that our calculations are actually an underestimation of the normal rate of hemoptysis in the CF population. On the other hand, the monitoring for adverse events inherent to a clinical trial may lead to over-reporting; however, the rates presented in this study are intended for use in this same context. Furthermore, our analyses do not account for within subject correlation of hemoptysis events.
This study does however have several advantages in determining the natural rate of hemoptysis. It includes a large number of individuals with CF who were closely followed in a clinical trial setting with parallel source document monitoring to ensure accuracy of the reporting. Additionally, the 8.2 month average duration of follow up provides reasonable precision for estimation of monthly event rates. Finally, participants in these trials are receiving therapies still relevant to current standard of care.
In conclusion, hemoptysis is a common respiratory event in individuals with CF, and is observed more commonly in older individuals, those with more advanced lung disease, and in the presence of P. aeruginosa. These data can be used to predict the expected rates of hemoptysis in contemporary clinical trials to guide the safety evaluation of new therapies in CF.
Supplementary Material
Highlights.
We utilized adverse event data from longitudinal prospective CF clinical trials.
We estimated hemoptysis rates overall and by relevant risk factors.
Rates are higher among adults and participants with more advanced lung disease.
Rates are higher among participants infected with P. aeruginosa but not S. aureus.
These results can be used to predict event occurrence in future clinical trials.
Acknowledgments
Funding
Support for this study was provided by Pharmaxis. Additional support was provided by the NIH National Center for Advancing Translational Sciences through the Clinical and Translational Science Award Program (CTSA) grant number UL1TR000423 and P30 DK089507. This publication was also supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Number UL1TR000062. The funding sources had no role in the study design, analysis, interpretation of results, decision to publish, or preparation of the manuscript.
The authors thank the individuals with CF and study sites who participated in the contributing clinical trials and whose dedication to research made this study possible. The authors are grateful to the sponsors who have contributed data to this study, including the Cystic Fibrosis Foundation Therapeutics, the National Institute of Health (U01-HL080310, UL1TR000423, and P30 DK089507), Boehringer Ingleheim, Targeted Genetics, and Intermune.
Footnotes
Author Disclosures
Ms. Thompson and Ms. Kloster received grant support from Pharmaxis.
Dr. Hamblett receives grant support from the Cystic Fibrosis Foundation Therapeutics (CFFT) and National Institutes of Health (NIH), and is the principal investigator of investigator initiated grants supported by Novartis and Pharmaxis for which she receives salary support.
Dr. Bilton has received a research award from Insmed Incorporated; expects to receive a research award from Aradigm Corporation; and has served on the Advisory Committees for Gilead Sciences, Novartis Pharmaceutical Corporation, and Pharmaxis Ltd.
Dr. Flume receives grant support from the Cystic Fibrosis Foundation Therapeutics (CFFT) and National Institutes of Health (NIH), and is the principal investigator of multiple clinical trials including grants supported by Pharmaxis for which he receives salary support.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ms. V. Thompson, Email: valeria.thompson@seattlechildrens.org.
Dr. N. Mayer-Hamblett, Email: nicole.hamblett@seattlechildrens.org.
Ms. M. Kloster, Email: margaret.kloster@seattlechildrens.org.
Dr. D. Bilton, Email: d.bilton@rbht.nhs.uk.
Dr. P. A. Flume, Email: flumepa@musc.edu.
References
- 1.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ. 1995;310:1571–2. doi: 10.1136/bmj.310.6994.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birrer P, McElvaney NG, Rudeberg A, Sommer CW, Liechti-Gallati S, Kraemer R, et al. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:207–13. doi: 10.1164/ajrccm.150.1.7912987. [DOI] [PubMed] [Google Scholar]
- 3.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–54. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 4.Flume PA, Mogayzel PJ, Jr, Robinson KA, Rosenblatt RL, Quittell L, Marshall BC. Cystic fibrosis pulmonary guidelines: pulmonary complications: hemoptysis and pneumothorax. Am J Respir Crit Care Med. 2010;182:298–306. doi: 10.1164/rccm.201002-0157OC. [DOI] [PubMed] [Google Scholar]
- 5.Hurt K, Simmonds NJ. Cystic fibrosis: management of haemoptysis. Paediatr Respir Rev. 2012;13:200–5. doi: 10.1016/j.prrv.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Flume PA, Yankaskas JR, Ebeling M, Hulsey T, Clark LL. Massive hemoptysis in cystic fibrosis. Chest. 2005;128:729–38. doi: 10.1378/chest.128.2.729. [DOI] [PubMed] [Google Scholar]
- 7.Efrati O, Harash O, Rivlin J, Bibi H, Meir MZ, Blau H, et al. Hemoptysis in Israeli CF patients--prevalence, treatment, and clinical characteristics. J Cyst Fibros. 2008;7:301–6. doi: 10.1016/j.jcf.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sucharew H, Goss CH, Millard SP, Ramsey BW. Respiratory adverse event profiles in cystic fibrosis placebo subjects in short- and long-term inhaled therapy trials. Contemp Clin Trials. 2006;27:561–70. doi: 10.1016/j.cct.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 12.A Randomised, Double-blind, Placebo-controlled Parallel-group Trial to Confirm the Efficacy After 12 Weeks and the Safety of Tiotropium 5 Mcg Administered Once Daily Via the Respimat® Device in Patients With Cystic Fibrosis. http://clinicaltrialsgov/show/NCT01179347.
- 13.Safety and Efficacy of 12-wk Treatment With Two Doses of Tiotropium Respimat in Cystic Fibrosis. http://clinicaltrialsgov/show/NCT00737100.
- 14.Moss RB, Mayer-Hamblett N, Wagener J, Daines C, Hale K, Ahrens R, et al. Randomized, double-blind, placebo-controlled, dose-escalating study of aerosolized interferon gamma-1b in patients with mild to moderate cystic fibrosis lung disease. Pediatr Pulmonol. 2005;39:209–18. doi: 10.1002/ppul.20152. [DOI] [PubMed] [Google Scholar]
- 15.Moss RB, Milla C, Colombo J, Accurso F, Zeitlin PL, Clancy JP, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–32. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- 16.Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest. 2004;125:509–21. doi: 10.1378/chest.125.2.509. [DOI] [PubMed] [Google Scholar]
- 17.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 18.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 19.Saiman L, Mayer-Hamblett N, Anstead M, Lands LC, Kloster M, Goss CH, et al. Open-label, follow-on study of azithromycin in pediatric patients with CF uninfected with Pseudomonas aeruginosa. Pediatr Pulmonol. 2012;47:641–8. doi: 10.1002/ppul.21601. [DOI] [PubMed] [Google Scholar]
- 20.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med. 2011;165:847–56. doi: 10.1001/archpediatrics.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Cystic Fibrosis Foundation Patient Registry. 2012 Annual Data Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.