Abstract
Purpose/Objectives
Radium-223 is a first-in-class radiopharmaceutical recently approved for the treatment of castration-resistant prostate cancer (CRPC) in patients with symptomatic bone metastases. Initial studies investigating Radium-223 primarily utilized non-steroidal first-generation anti-androgens. Since that time, newer anti-androgen therapies have demonstrated improved survival in patients with CRPC. It has been suggested that the rational combination of these newly approved agents with Radium-223 may lead to improved response rates and clinical outcomes. Currently, there is lack of information regarding the safety of concurrent administration these agents with radiopharmaceuticals. Here, we report on hematologic toxicity findings from our institution in patients receiving concurrent Radium-223 and next generation anti-androgen therapies with either enzalutamide or abiraterone.
Materials/Methods
In a retrospective study, we analyzed patients who received Radium-223 as part of an early-access trial, and following FDA approval in May 2013, patients receiving Radium-223 as part of standard care. Radium-223 was given at standard dosing of 50 kBq/kg each month for 6 total cycles. Complete blood counts were performed prior to treatment monthly and following each injection. Blood counts from patients receiving Radium alone and concurrently with next-generation anti-androgens were compared. To date, 25 total patients were analyzed, with a median of five monthly doses received per patient. Fourteen patients received concurrent therapy during monthly Radium-223 with either enzalutamide (n=8) or abiraterone (n=6).
Results
Six patients expired due to disease progression. Two patients discontinued treatment due to grade 3 myelosuppression. For patients receiving either Radium alone and with concurrent next generation anti-androgen therapy, there did not appear to be any statistically significant differences between initial and nadir blood counts. Mean change from initial neutrophil count to nadir was 1.9 × 106/L in patients receiving Radium alone, vs. 2.3 × 106/L in patients receiving concurrent therapy (p= 0.77). Mean change from initial hemoglobin value to nadir was 1.5 g/L in patients receiving Radium alone, vs. 1.8 g/L in patients receiving concurrent therapy (p= 0.31). Mean change from initial platelet count to nadir was 52.3 ×109 cells/L in patients receiving Radium alone vs. 70.6 ×109 cells/L in patients receiving concurrent therapy (p= 0.39). Individual blood counts for each measured laboratory are included in the supplemental data. PSA was stable or decreased in 22% of patients receiving Radium alone vs. 35% of patients receiving combination treatment (p=0.24).
Conclusions
Concurrent administration of Radium-223 and next generation anti-androgen therapies appears to be well tolerated with similar toxicities to standard administration of Radium-223 alone. This particular cohort of patients represents a high-risk, heavily pretreated group of patients with advanced metastatic disease and significant marrow burden. Despite these risk factors, hematologic toxicity was modest and was in the range expected for this risk group based on previous trials. To date, this is the first study investigating the toxicity of combination treatment. Further studies investigating the safety and efficacy of combination treatments are warranted.
Introduction
Prostate cancer is the most common solid tumor in men with over 230,000 cases diagnosed per year in the United States1. Approximately 30,000 men will succumb to their disease annually due to metastatic spread of their cancer. The current standard of care following diagnosis of metastatic disease includes surgical or medical castration with luteinizing hormone releasing hormone (LHRH) analogs or antagonists, often with first generation anti-androgens such as bicalutamide. However, conversion to castrate resistance remains common2. Consensus guidelines regarding treatment after castrate resistance varies widely3,4. Over the last decade, the treatment landscape for patients with metastatic castrate resistant disease has drastically changed, with several novel agents demonstrating improved overall survival in several large, multi-institutional randomized trials. These new agents include the incorporation of cytotoxics5–7, next generation anti-androgens8–11, immunotherapeutics12, and radiopharmaceuticals13.
Among these newly approved agents include Radium-223, a first-in-class radiopharmaceutical approved for the treatment of castration-resistant prostate cancer (CRPC) in patients with symptomatic bone metastases14. Initial studies investigating Radium-223 primarily utilized non-steroidal first-generation anti-androgens or standard androgen suppression therapies 13,15,16. In the landmark ALSYMPCA trial, the usage of Radium-223 in this population demonstrated significantly improved overall survival when compared with placebo (median 14.9 months versus 11.3 months) and reduced the risk of death by 30% (hazard ratio [HR] 0.70; 95% CI 0.58–0.83; P<0.001)13. Patients previously receiving docetaxel were eligible for this trial, although approximately half of patients were not healthy enough or declined to receive it, or it was not available. Concurrent use of next generation anti-androgen use with either abiraterone or enzalutamide was not included.
Currently, there is limited data regarding the sequencing of newly approved therapeutic agents for use in patients with metastatic CRPC due to their novel availability17,18. Furthermore, it has been suggested that the rational use of combination treatment may lead to improved response rates and clinical outcomes with minimal increase in toxicity. Unlike traditional cytotoxic systemic treatments, several of these new agents demonstrate non-overlapping mechanisms of action with distinct toxicity profiles. Radium-223 in particular has been demonstrated to have a favorable side effect profile. In previous dose-escalation studies, there were no grade 3 adverse events in even the highest dose group16. In the phase III ALSYMPCA trial, patients receiving Radium-223 had only modest side effects, principal of which included myelosuppression, which was expected in a patient population with significant marrow infiltration. In addition, the slightly increased risk of myelosuppression in these patients seemed to be correlated with prior chemotherapy use19.
Next-generation anti-androgens such as abiraterone and enzalutamide appear to be attractive candidates for combination therapy with Radium-223. In previous studies utilizing abiraterone and enzalutamide in the pre-chemotherapy setting, no significant hematological toxicity was reported 8,11. As there appear to be non-overlapping toxicities, a novel treatment strategy with concurrent combination therapy may be a reasonable option. In addition, due to the relatively modest impact of Radium-223 on PSA control, it may be even preferable to include a second agent to address non-osseous disease. Given this, a number of patients at our institution have begun to receive combination treatment as per the discretion of their treating medical oncologist. Here, we report on treatment toxicity findings from our institution in patients receiving concurrent Radium-223 and next generation anti-androgen therapies with either enzalutamide or abiraterone.
Methods
This is a retrospective chart review that was conducted under an Institutional Review Board-approved outcomes protocol. From December 2012 to September 2014, 25 consecutive patients with metastatic castrate resistant prostate cancer received Radium-223 treatment at a single institution. Patients initially received Radium-223 treatment as part of an early access trial (NCT01516762), then following FDA approval in May 2013, all patients received Radium-223 treatment as part of standard of care. Indications for Radium-223 treatment included patients with castration-resistant prostate cancer, ≥ 2 symptomatic bone metastases, and no known visceral metastatic disease. Fourteen patients who received Radium-223 also received concurrent enzalutamide or abiraterone during prescribed monthly injections as indicated by their treating medical oncologist. Patient demographics are listed on Table 1. Complete blood counts from patients receiving Radium alone and concurrently with next-generation anti-androgens were compared.
Table 1.
Initial Patient Characteristics
| Age | Radium alone | Concurrent |
|---|---|---|
| Median | 78 | 69 |
| % >75 years | 28.0 (7) | 20.0 (5) |
| Prior Docetaxel use | ||
| Yes | 64.1 (9) | 63.6 (7) |
| No | 35.9 (5) | 36.4 (4) |
| KPS | ||
| Median | 80 | 80 |
| Median transfusions required | 3 | 2 |
| Median prior treatments | 3.5 | 4 |
| Concurrent Enzalutamide | 8 | |
| Concurrent Abiraterone | 6 | |
| Initial ANC (x 103/L) | 4.55 | 3.56 |
| Initial Hemoglobin (g/L) | 10.95 | 11.2 |
| Initial Platelet count (x 106/L) | 215.5 | 251 |
| Initial PSA (μg/L) | 45 | 106.9 |
Radium-223
Prior to the first administration, absolute neutrophil count (ANC) was required to be ≥ 1.5 × 109/L, platelet count was required to be ≥ 10.0 × 109/L, and hemoglobin ≥ 10 g/dL. Five patients received a blood transfusion prior to treatment. Following initial treatment, subsequent treatment required ANC to be ≥ 1.0 × 109/L and platelet count to be ≥ 50 × 109/L. Radium-223 was given at standard dosing of 50 kBq/kg every 4 weeks for 6 total cycles. Patients were followed with monthly CBCs and routine follow up visits to assess for toxicity.
Systemic treatment
Patients were heavily pretreated prior to beginning radium treatment. The average number of systemic treatments prior to initiating Radium-223 was 3.5. Sixty-four percent of patients received prior Docetaxel, with median of 6 cycles (range 1–16). Eight patients were enrolled in experimental trials including use of an aurora kinase inhibitor, anti-prolactin receptor antibody, androgen biosynthesis inhibitor (orteronel), or mTOR inhibitor. Abiraterone was given 1,000 mg (four 250 mg tablets) administered orally once daily in combination with prednisone 5 mg administered orally twice daily. Enzalutamide was given 160 mg (four 40 mg capsules) administered orally once daily. Dose was adjusted based on patient tolerability.
Toxicity
The primary goal of this study was to evaluate the incidence of hematologic adverse events with concurrent administration of Radium-223 and either enzalutamide or abiraterone. Hematologic adverse events were defined by grade 2–4 anemia, neutropenia, and thrombocytopenia according to Common Terminology Criteria for Adverse Events (CTCAE) v4.020. Complete blood counts were performed prior to treatment monthly and following each injection. Initial baseline labs and nadir levels during and after treatment were also analyzed. Mean counts amongst patients receiving Radium alone and concurrently with next-generation anti-androgens were compared using match-paired analysis with student’s t-test. Fisher’s exact test was used for binomial data. (GraphPad Prism, version 5.00, GraphPad Software, San Diego, CA).
Results
Twenty five total patients receiving Radium-223 were evaluated. Median follow up with laboratory complete blood count was six months. Fourteen patients received concurrent treatment (8 patients received concurrent abiraterone and 6 patients received concurrent enzalutamide) and 11 patients received Radium alone. Median pretreatment absolute neutrophil count, hemoglobin, and platelets for patients receiving radium alone were 4.55 ×109 cells/L (range: 2.1–9.5), 10.95 gm/dL (range: 9.5–14.6), and 215,500/ul (range: 138–325), respectively. Median pretreatment absolute neutrophil count, hemoglobin, and platelets for patients receiving concurrent treatment were 3.56 ×109 cells/L (range: 2.4–9.5), 11.2 gm/dL (range: 9.9–13.9), and 251,000/ul (range: 134–467), respectively.
Six patients expired due to disease progression. One patient receiving Radium-223 alone discontinued Radium-223 due to Grade 3 anemia and one patient receiving concurrent abiraterone discontinued Radium-223 due to Grade 3 thrombocytopenia. Enzalutamide was dose-reduced in two patients due to fatigue and in one patient due to nausea and vomiting. Abiraterone was discontinued in one patient receiving concurrent treatment due to fatigue.
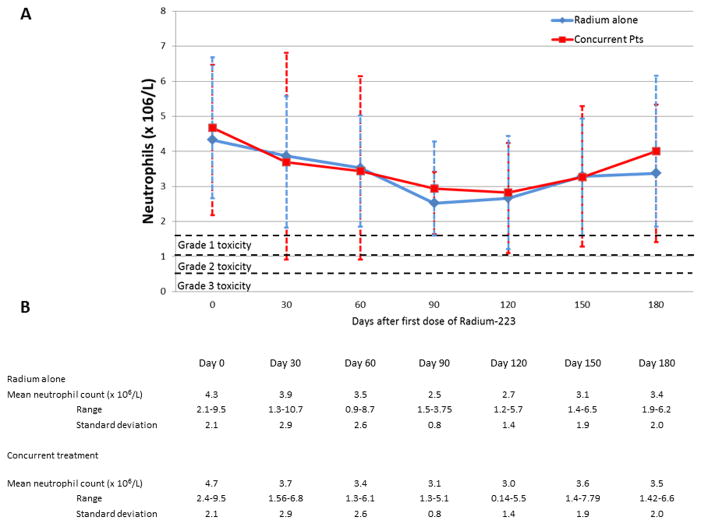
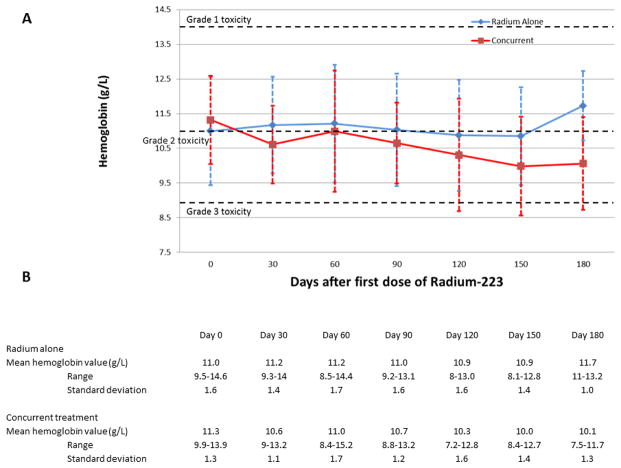
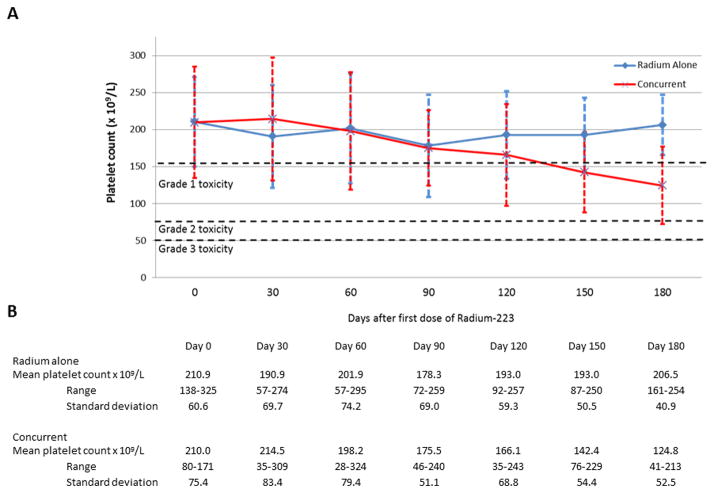
For patients receiving either Radium alone and with concurrent next generation anti-androgen therapy, there did not appear to be any statistically significant differences between initial and nadir blood counts. Figure 1 demonstrates mean absolute neutrophil counts over time following administration of Radium-223. Mean change from initial neutrophil count to nadir was 1.9 × 106/L in patients receiving Radium alone, vs. 2.3 × 106/L in patients receiving concurrent therapy (p= 0.77). Figure 2 demonstrates mean hemoglobin values over time following administration of Radium-223. Mean change from initial hemoglobin value to nadir was 1.5 g/L in patients receiving Radium alone, vs. 1.8 g/L in patients receiving concurrent therapy (p= 0.31). Figure 3 demonstrates mean platelet counts over time following administration of Radium-223. Mean change from initial platelet count to nadir was 52.3 ×109 cells/L in patients receiving Radium alone vs. 70.6 ×109 cells/L in patients receiving concurrent therapy (p= 0.39). There did appear to be a trend in thrombocytopenia in the concurrent group that did not reach statistical significance. Three of four patients experiencing grade 3 thrombocytopenia had previous history of docetaxel use and had associated advanced disease. PSA was stable or decreased in 22% of patients receiving Radium alone vs. 35% of patients receiving combination treatment (p=0.24). Individual blood counts for each measured laboratory are included in the Supplemental Digital Content. Supplemental Figure 1 demonstrates individual absolute neutrophil count over time. Supplemental Figure 2 demonstrates individual hemoglobin values over time. Supplemental Figure 3 demonstrates individual platelet values over time.
Figure 1.
Absolute Neutrophil count following administration of Radium-223.
Figure 2.
Hemoglobin level following administration of Radium-223.
Figure 3.
Platelet count following administration of Radium-223.
Hematologic adverse events are reported in Table 2. A number of patients began treatment with pre-existing toxicity, with almost all patients having at least Grade 1 anemia. Incidence of adverse events appears to be similar in both groups with no Grade 4 toxicities in either group.
Table 2.
Hematologic Toxicity based on Concurrent treatment vs. Radium alone
| Concurrent Treatment | Radium alone | |||||||
|---|---|---|---|---|---|---|---|---|
| Percent Toxicity (absolute number) | ||||||||
| CTCAE | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Neutrophils (x 109/L) | 21 (3) | (0) | 7 (1) | 0 | 18 (2) | 9 (1) | 0 | 0 |
| Hemoglobin (g/L) | 43 (6) | 43 (6) | 14 (2) | 0 | 45 (5) | 54 (6) | 0 | 0 |
| Platelet count (x 106/L) | 29 (4) | 14 (2) | 14 (2) | 0 | 18 (2) | 9 (1) | 18 (2) | 0 |
Hematologic adverse events based on prior history of chemotherapy are reported in Table 3. Over one-third of patients with a prior history of docetaxel use experienced a Grade 3 hematologic toxicity while there was only one patient who experienced a Grade 3 hematologic toxicity who had no history of Docetaxel exposure.
Table 3.
Hematologic Toxicity based on prior chemotherapy use
| Prior treatment with Docetaxel | No prior Chemotherapy | |||||||
|---|---|---|---|---|---|---|---|---|
| Percent Toxicity (absolute number) | ||||||||
| CTCAE | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Neutrophils (x 109/L) | 19 (3) | 0 | 6 (1) | 0 | 22 (2) | 0 | 0 | 0 |
| Hemoglobin (g/L) | 25 (4) | 63 (10) | 13 (2) | 0 | 78 (7) | 22 (2) | 0 | 0 |
| Platelet count (x 106/L) | 19 (3) | 19 (3) | 19 (3) | 0 | 33 (3) | 33 (3) | 11 (1) | 0 |
Discussion
In this single-institution retrospective study, concurrent administration of Radium-223 and next generation anti-androgen therapies appears to be well tolerated with similar toxicities to standard administration of Radium-223 alone. This particular cohort of patients represents a high-risk, heavily pretreated group of patients with advanced metastatic disease and significant marrow burden. Despite these risk factors, hematologic toxicity was modest and was in the range expected for this risk group based on previous trials 13.
As treatment options in the metastatic prostate landscape continue to expand, there is a growing interest in using rational combinations of agents to improve the therapeutic ratio. Due to unique mechanisms of action for many of these agents, the addition of combination therapy may improve outcomes without a corresponding increase in toxicity. The use of cytotoxic therapy and radioisotope treatment has previously been explored with early generation radiopharmaceuticals.
In a randomized phase II study, bone-targeted therapy for advanced prostate cancer using Sr-89 plus doxorubicin weekly was associated with improved survival vs doxorubicin alone 21. Authors from this study suggested that the combination of systemic therapy with bone targeted treatment improved outcomes due to dual-targeting of both epithelial and the stromal components of the disease. By targeting the primary tumor as well as the metastatic niche, a synergistic treatment response was obtained. Similarly, newer trials have reported promising data with regard the usage of Radium-223 with concurrent taxane-based chemotherapy, although data has not yet matured22.
Next-generation anti-androgen therapies with abiraterone and enzalutamide represent unique opportunity for combination therapy as the side effect profile from these therapies tends to be mild for most patients. Currently there are a number of clinical trials underway investigating the use of concurrent anti-androgen and radiopharmaceutical treatment. In one of the largest of these trials, patients will be randomized between Radium-223 alone, Radium-223 with abiraterone, or Radium-223 with enzalutamide (NCT02034552). Accrual is currently ongoing with an estimated study completion date in June 2018. While final results of this and other similar trials complete accrual, there is currently limited published data regarding the safety and efficacy of this treatment combination.
There are a number of limitations of this study. This study represents a relatively small, single institution retrospective experience. Patients were not prospectively stratified by pre-treatment characteristics according to treatment group. Furthermore, a majority of patients in this study were heavily pretreated and may have demonstrated higher toxicity rates as a consequence of advanced marrow infiltration or residual chemotherapy toxicity rather than treatment effect. In addition, usage of concurrent treatment was not uniform amongst patients and was at the discretion of the treating medical oncologists. Finally, as Radium-223 was only recently approved, the long term data regarding treatment related toxicity is still being evaluated.
To the authors knowledge, this report represents one of the first single institution experiences with concurrent treatment with Radium-223 and next generation anti-androgen therapy. Findings from this study suggest combination treatment is well tolerated with minimal toxicity. Data from this study is hypothesis-generating and future studies investigating safety and efficacy with combination treatment are needed.
Supplementary Material
1) Supplemental Figure 1 - individual absolute neutrophil count over time
2) Supplemental Figure 2 - individual hemoglobin values over time
3) Supplemental Figure 3 - individual platelet values over time
Acknowledgments
This study is funded in part by the National Cancer Institute Cancer Center with support grant P30 CA56036-11.
Footnotes
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Den, Dr. Lin and Dr. Dicker are speakers for the Bayer Oncology speaker Bureau. Dr. Den and Dr. Gomella serve on the Bayer advisory board.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 3.Cookson MS, Roth BJ, Dahm P, et al. Castration-Resistant Prostate Cancer: AUA Guideline. J Urol. 2013;190(2):429–38. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Basch E, Loblaw DA, Oliver TK, et al. Systemic Therapy in Men With Metastatic Castration-Resistant Prostate Cancer: American Society of Clinical Oncology and Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol. 2014 doi: 10.1200/JOP.2014.001701. JCO.2013.54.8404. [DOI] [PubMed] [Google Scholar]
- 5.Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N Engl J Med. 2004;351(15):1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 8.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med. 2014;371(5):424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. N Engl J Med. 2012;367(13):1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 10.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–92. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 11.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N Engl J Med. 2013;368(2):138–48. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 13.Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N Engl J Med. 2013;369(3):213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 14.Den RB, Doyle LA, Knudsen KE. Practical guide to the use of radium 223 dichloride. Can J Urol. 2014;21(2 Supp 1):70–6. [PubMed] [Google Scholar]
- 15.Nilsson S, Larsen RH, Fosså SD, et al. First Clinical Experience with α-Emitting Radium-223 in the Treatment of Skeletal Metastases. Clin Cancer Res. 2005;11(12):4451–9. doi: 10.1158/1078-0432.CCR-04-2244. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson S, Strang P, Aksnes AK, et al. A randomized, dose–response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48(5):678–86. doi: 10.1016/j.ejca.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Dreicer R. How to approach sequencing therapy in patients with metastatic castration resistant prostate cancer. Can J Urol. 2014;21(2 Supp 1):93–7. [PubMed] [Google Scholar]
- 18.Keizman D, Maimon N, Gottfried M. Metastatic Hormone Refractory Prostate Cancer: Recent Advances in Standard Treatment Paradigm, and Future Directions. J Clin Oncol. 2014 Jun;37(3):289–96. doi: 10.1097/COC.0b013e318248dc1e. [DOI] [PubMed] [Google Scholar]
- 19.Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014;15(12):1397–406. doi: 10.1016/S1470-2045(14)70474-7. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services. Natl Inst Health Natl Cancer Inst. 2009. Common terminology criteria for adverse events (CTCAE) version 4.0. [Google Scholar]
- 21.Tu S-M, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. The Lancet. 2001;357(9253):336–41. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 22.Morris MJ, Hammers HJ, Sweeney C, et al. Safety of radium-223 dichloride (Ra-223) with docetaxel (D) in patients with bone metastases from castration-resistant prostate cancer (CRPC): A phase I Prostate Cancer Clinical Trials Consortium Study. J Clin Oncol [Internet] 2013;31(suppl):abstr 5021. [cited 2014 Aug 8] Available from: http://meetinglibrary.asco.org/content/115274-132. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1) Supplemental Figure 1 - individual absolute neutrophil count over time
2) Supplemental Figure 2 - individual hemoglobin values over time
3) Supplemental Figure 3 - individual platelet values over time